Abstract
Purpose of Review
We conducted a systematic review to evaluate the current evidence for screening and treatment for early-onset gestational diabetes mellitus (GDM)
Recent Findings
Many of the women with early GDM in the first trimester do not have evidence of hyperglycemia at 24–28 weeks’ gestation.
Summary
A high proportion (15–70%) of women with GDM can be detected early in pregnancy depending on the setting, criteria used and screening strategy. However, there remains no good evidence for any of the diagnostic criteria for early-onset GDM. In a meta-analysis of 13 cohort studies, perinatal mortality (relative risk (RR) 3.58 [1.91, 6.71]), neonatal hypoglycemia (RR 1.61 [1.02, 2.55]), and insulin use (RR 1.71 [1.45, 2.03]) were greater among early-onset GDM women compared to late-onset GDM women, despite treatment. Considering the high likelihood of benefit from treatment, there is an urgent need for randomized controlled trials that investigate any benefits and possible harms of treatment of early-onset GDM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) affects up to 25% pregnancies worldwide [1]. According to World Health Organization (WHO) 2013 criteria, GDM is hyperglycemia first recognized at any time in pregnancy that is below the diagnostic threshold for undiagnosed diabetes in pregnancy (DIP) [2••]. GDM affects outcomes for both mother and baby during and beyond pregnancy [3••,4••,5•]. Treatment is effective after 24 weeks when the diabetogenic effect of pregnancy is most marked [6••,7••].
Early screening for DIP is recommended for pregnant women with risk factors to identify undiagnosed type 2 diabetes [8••]. This also detects women with lesser degrees of hyperglycemia that would be diagnostic of GDM at 24–28 weeks based on WHO 2013 criteria [2••], but the diagnostic criteria for such “early” or “booking” GDM remain uncertain [9••], particularly as many of those with “early GDM” in the first trimester, do not have evidence of hyperglycemia at 24–28 weeks [10••]. It is unknown whether the evidence for treating GDM, and the intensity of treatment, can be applied to women diagnosed < 24 weeks’ gestation, as this is derived from studies between 24 and 34 weeks’ gestation [3••, 6••, 7••]. Because of the heterogeneous nature of GDM, this early group may represent a different range of phenotypes, compared with women diagnosed later in pregnancy. Furthermore, if there are benefits from identifying and treating early GDM, then the “test characteristics” of using risk factor screening for DIP need to be considered. Risk factor screening is unreliable later in pregnancy [11] and is unlikely to fare better earlier in pregnancy.
We conducted this systematic review to evaluate the available screening and diagnostic approaches for detecting GDM in early pregnancy (early-onset GDM) and compare the clinical characteristics and pregnancy outcomes of women with GDM who were diagnosed and treated early in pregnancy (< 24 weeks of gestation) with women who were diagnosed and treated late in the pregnancy (24–28 weeks of gestation). During this review, we aimed to identify the highest quality studies, use them for a pooled meta-analysis to draw conclusions, and thereby assess relevant knowledge gaps.
Materials and Methods
Data Sources and Searches
This systematic review and meta- analysis was performed according to Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines [12]. A title and abstract search of five electronic databases (PubMed, CINAHL, EMBASE, Cochrane Library, and Scopus) was performed using MeSH (Medical subject headings) terms such as “gestational diabetes,” “pregnancy induced diabetes,” “hyperglycemia,” “first trimester pregnancy,” “early screening,” “early diagnosis,” and “early treatment.” Keywords such as “booking gestational diabetes mellitus,” “early gestational diabetes,” and “early pregnancy” were also used if MeSH terms were not found. A hand search was then conducted using the reference list of retrieved articles. The search strategy is summarized in Supplementary Table 1.
Study Selection
Any studies that assessed the screening, diagnostic thresholds, treatment, and neonatal or obstetric outcomes of early-onset GDM women were eligible for inclusion, regardless of maternal characteristics, research design, publication year, setting, or test method. Studies that included only a subgroup of the population, a small sample size of < 10, and those that were published in non-English languages were excluded. Studies with inadequate description of research methodology, abstract-only studies, and those studies that used non-fasting oral glucose tolerance test (OGTT) for diagnosis were also excluded from the review. The primary pregnancy outcome evaluated in this review was large for gestational age (LGA), and the secondary outcomes were as follows: macrosomia, neonatal hypoglycemia, small for gestational age (SGA), hypertensive disorders in pregnancy, perinatal mortality, hyperbilirubinemia, neonatal intensive care unit (ICU) admission, cesarean delivery, respiratory distress syndrome, preterm delivery, shoulder dystocia, or insulin use.
Articles retrieved from the literature search were screened for duplicates. Titles and abstracts were screened for potential eligibility for inclusion. The selected articles were further analyzed by a full-text review to assess their eligibility for final inclusion. The reasons for final inclusion were reviewed by the second author, and disagreements were resolved by further discussion.
Data Extraction and Quality Assessment
The data from the selected articles were extracted using two data extraction forms: one for screening studies (consisting of author, year, setting, study design, selection criteria, evaluated test, study population, diagnostic criteria, and study results) and one for treatment outcome studies (consisting of author, year, research design, aim of the study, population, setting, period of study, selection criteria, screening test, treatment provided, ethnicity, age, prepregnancy body mass index (BMI), gestational BMI, family history of diabetes, bad obstetric history, multiparity, gestational weight gain (GWG), glycemic profile on diagnosis, and study results). The methodological quality of the cohort studies was analyzed using the Newcastle Ottawa scale, which assesses three main domains: selection, comparability, and outcome [13]. The Cochrane risk of bias assessment tool was used to evaluate the included randomized controlled trials (RCTs), which covers six domains of bias: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias [14]. The quality assessment of cohort studies and RCTs are summarized in Supplementary Tables 2 and 3. The Grading of recommendations, Assessment, Development and Evaluation (GRADE) approach was used to assess the quality of the body of evidence for each outcome in the meta-analysis [15].
Meta-analysis
A meta-analysis was performed using Review Manager (RevMan 5.3) statistical software provided by the Cochrane Collaboration. The risk ratio and 95% confidence interval were used to measure the effect size of the dichotomous outcomes, and mean difference was used to measure the effect size of the continuous outcome. A random-effects model was employed to pool data across studies. An I 2 statistic was used to measure the heterogeneity of the studies; values of 25, 50, and 75% were considered as low, moderate, and high heterogeneities, respectively. Publication bias was not determined, as there were only 13 studies included for the meta-analysis. A subgroup analysis was performed, considering the potential effects of obstetric care in a lower resource setting, by stratifying the studies into developed and developing countries. Inequality in maternity care has been found between developed and developing countries [16].
Results
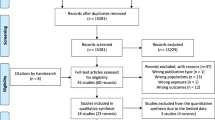
A literature search identified 1495 articles, of which 35 met the inclusion criteria (21 based on early screening and 14 based on treatment outcomes) and were included in the systematic review (Supplementary Fig. 1). The meta-analysis included 13 cohort studies that were based on treatment outcomes. Most studies were observational besides two that were RCTs: one comparing the diagnostic performance of three tests: fasting plasma glucose (FPG), two-step 50-g glucose challenge test (GCT)/OGTT, and 75-g OGTT in early pregnancy [17•] and the second study examining the benefits of treating pregnant women with prediabetes (defined by hemoglobin AIc (HbA1c)) early in pregnancy [18]. Studies were conducted in different settings, with seven conducted in the USA.
Diagnostic Criteria for Early GDM
There is consensus that DIP is diagnosed if one or more of the following criteria is met: Fasting plasma glucose (FPG) ≥ 7.0 mmol/l (126 mg/dl) or HbA1c ≥ 6.5% (48 mmol/mol) or random plasma glucose (RPG) ≥ 11.1 mmol/l (200 mg/dl) with a confirmatory FPG or HbA1c [8••]. Most national/international organizations make no distinction between GDM criteria at or up to 24–28 weeks’ gestation besides the International Association of Diabetes in Pregnancy Study Group (IADPSG) which has recently recommended not to use their criteria [9••] but have recommended no alternative.
HbA1c for Diagnosis in Early Pregnancy
We identified three observational studies (Supplementary Table 4) that investigated the association of higher first trimester HbA1c (5.9–6.4%; 41–46 mmol/mol) and adverse pregnancy outcomes [19••, 20•, 21•]. The largest and the highest quality study among these was by Hughes et al. [19••] who studied Europid women to determine the optimal threshold for diabetes in early pregnancy but excluded GDM treated women in the analysis. The study reported greater than 2-fold increased risk of preeclampsia, shoulder dystocia, major congenital anomaly, and greater than 3-fold increased risk of perinatal death associated with a higher first trimester HbA1c threshold. Mañé et al. [20•] studied a multiethnic group to identify women without diabetes at increased risk of adverse pregnancy outcomes. Women with GDM diagnosed at 24–28 weeks’ gestation were also included in the analysis. The study found a 3-fold increased risk of macrosomia and preeclampsia associated with an early HbA1c of 5.9–6.4% (41–46 mmol/mol). Sweeting et al. [21•] studied a multiethnic group of early- and late-onset GDM women to examine the relationship between antenatal HbA1c at GDM diagnosis with adverse pregnancy outcome. The early-onset GDM women were preselected from a group of high-risk women. The study reported significantly increased risk of adverse pregnancy outcomes such as macrosomia, hypertensive disorders, and cesarean section among women with HbA1c of 5.9–6.4% (41–46 mmol/mol). However, unlike in late-onset GDM, the association between the higher HbA1c level and adverse pregnancy outcomes was less distinct in early-onset GDM. The higher HbA1c did not adequately capture all the risks associated with early-onset GDM such as LGA, SGA, and neonatal hypoglycemia. Only one study [20•] included hemoglobin(Hb) and mean corpuscular volume (MCV) levels in the analysis to adjust for the presence of microcytic anemia. None of the studies used electrophoresis for identifying potential hemoglobinopathies.
While it is inevitable that there will be women with abnormal HbA1c and normal OGTT and vice versa, Balaji et al. [22] described a subset of GDM women in the first trimester population, with positive OGTT values but with low HbA1c levels (< 6% (42 mmol/mol)): it was suggested that the period of hyperglycemia in this category of women was not long enough to influence HbA1c. Mañé et al. [20•] reported that only half of the women with first trimester HbA1c ≥ 5.9% (≥ 41 mmol/mol) developed GDM in later pregnancy, whereas Hughes et al. [19••] found that 74% of the women with first trimester HbA1c of ≥ 5.9% (≥ 41 mmol/mol) developed abnormal OGTT at some stage during gestation and over two thirds of these women were detected during early pregnancy.
We identified 6 studies (Supplementary Table 5) that investigated the prognostic values of first trimester HbA1c for developing GDM, using different glycemic thresholds. Osmundson et al. [23] reported a 50% excess risk of GDM (adjusted relative risk 1.48 (1.15–1.89), p < 0.001) among women with HbA1c threshold of 5.7–6.4% (39–46 mmol/mol) compared with women with a normal HbA1c (< 5.7% (39 mmol/mol). In a study conducted in Switzerland, all high-risk women with a value ≥ 6.0% (42 mmol/mol) developed GDM, while no GDM was found among women with HbA1c < 4.5% (26 mmol/mol) [24]. Another study conducted in a high-risk ethnic population reported a cutoff value of 6.0% (42 mmol/mol) for GDM and 5.3% (34 mmol/mol) for normal pregnant women [22]. Although a threshold of ≥ 5.7% (39 mmol/mol) to ≥ 5.9% (≥ 41 mmol/mol) showed high specificity (94–100%) for GDM, four studies reported low sensitivity (13–28.6%) of first trimester HbA1c for predicting GDM at a level usually recommended for identification of “prediabetes” outside of pregnancy [19••, 20•, 23, 25•].
OGTT for Diagnosis in Early Pregnancy (Supplementary Table 6)
The DALI (Vitamin D And Lifestyle Intervention for GDM prevention) pilot and lifestyle pan-European multicenter trial reported the use of 75-g OGTT with IADPSG criteria in identifying high-risk obese women obese women (BMI ≥ 29.0 kg/m2) with significant metabolic disturbances in early pregnancy [26•]. The RCT comparing the diagnostic performance of the three tests: FPG, 50-g two-step GCT/OGTT, and 75-g GTT reported better performance of the 75-g GTT for predicting GDM in the first trimester. The 75-g GTT showed the highest sensitivity (87%) and specificity (100%) for GDM prediction and larger area under the curve (AUC) (0.792) than FPG (sensitivity 47%, specificity 77%, AUC 0.623) and two-step GCT (sensitivity 68%, specificity 100%, AUC 0.708) [17•]. Another study in a high-risk Arab population found that early multiple screening using 75-g OGTT at two monthly intervals helped to detect majority of GDM cases (> 88%) before seventh month of pregnancy [27]. A retrospective study that evaluated the diagnostic performance of an early 75-g OGTT in predicting late-onset GDM reported that morbidly obese women (BMI ≥ 40 kg/m2) with a 2-h value of < 6 mmol/l can be excluded from a repeat OGTT at 28 weeks, avoiding a second OGTT in > 50% of women [28].
FPG for Diagnosis in Early Pregnancy
In the nine identified studies (Supplementary Table 7), Riskin-Mashiah et al. [29••] reported a strong graded association between higher first trimester FPG values, below DIP, and adverse pregnancy outcomes (e.g., LGA, macrosomia, primary cesarean section). Higher FPG was an independent risk factor for developing GDM, with a 1.5-fold increase in GDM for every 0.27 mmol/l (5 mg/dl) rise in FPG [30]. However, opinions have varied regarding the utility of FBG for detecting hyperglycemia and the optimal FPG level that would best predict GDM. Zhu et al. [10••] recommended a threshold of 6.1 mmol/l (110 mg/dl), providing 100% specificity. Two studies evaluated the diagnostic value of first trimester FPG of ≥ 5.1 mmol/l and found a lack of complete agreement between the first trimester FPG values and the third trimester OGTT results [10••, 31•] with only 39.8% of women with FPG of ≥ 5.1 mmo/l in the first trimester diagnosed with GDM at 24–28 weeks [10••]. A study in a high-risk ethnic population found no correlation between first trimester FPG levels and GDM development at 24–28 weeks [32]. Although a first trimester FPG of ≥ 5.1 mmol/l is highly predictive of GDM (adjusted odd ratio (aOR) of 9.32 [5.07–17.14] [29], aOR of 7.1 [3.8–13.1] [31••]), two studies [33, 34] reported low specificity and high false positive rate of first trimester FPG for diagnosing GDM.
Screening Tests in Early Pregnancy
RPG in Early Pregnancy
In the two comparative studies identified (Supplementary Table 8), one reported RPG as a better predictor for GDM than maternal age or BMI [35] while the second reported poor performance of RPG as a screening tool, comparing its test characteristics with 50-g GCT [25•].
Risk Factor-Based Screening
There are multiple publications relating to risk factor-based screening for GDM in late pregnancy; however, evidence is limited for women at booking in early pregnancy (Supplementary Table 6). In current practice, screening for DIP is recommended to be based on risk factors alone [8••]. However, early universal screening, especially in populations with a high prevalence of type 2 diabetes helps to detect GDM women with no risk factors (21%) [36], and is more likely to identify women including those with auto-immune diabetes [37]. More GDM is diagnosed before 24 weeks’ gestation in “early universal” compared with risk factor based screening (27.7 vs 6.3%) [38].
GDM Found Early in Pregnancy: Proportions and Prevalence
The prevalence of GDM found in early pregnancy ranged between 0.8 and 22.9% (Supplementary Table 9). The proportion of GDM women diagnosed before 24 weeks’ gestation varied widely (15–70%) depending on the setting, criteria used and screening strategy. (Supplementary Table 10 describes various GDM criteria used in the selected studies).
The proportions and prevalence of early-onset GDM estimated in this review may not represent the true prevalence of early GDM due to the limitations of the studies involved in this review. One of the challenges in determining the true prevalence of early-onset GDM was the presence of biased study populations due to selective screening of high-risk women and/or mixing of DIP with the early GDM group.
Maternal Characteristics of Those Found Early vs Late
Table 1 summarizes the comparison of maternal characteristics of early- and late-onset GDM women in the treatment studies. Compared with late-onset GDM, women with early GDM were older, more likely to be multiparous, with a higher pregestational BMI and diabetes family history, HbA1c and fasting glucose on OGTT, and features of the metabolic syndrome.
Insulin therapy is often, but not always, required.
A Japanese study [39] that evaluated the effectiveness of nutritional therapy among early-onset GDM women, diagnosed as per IADPSG criteria, reported less frequency of post-partum type 2 diabetes among those who had glucose tolerance normalized during midgestation with diet therapy alone. This subset of women was more likely to be younger primipara, with greater first trimester weight gain compared to those women who retained GDM in midgestation. Beta cell function (ISSI-2) was significantly higher in this subgroup in the post-partum period who also showed an upward trend in insulin secretion over gestation.
Treatment Outcomes of Early-Onset GDM
The selected studies (Supplementary Table 11) were categorized into the following four groups.
-
(1)
Early treatment vs usual prenatal care: We identified one RCT that evaluated the treatment outcomes among women with prediabetes (HbA1c 5.7–6.4% (39–46 mmol/mol)) in early pregnancy [18]. The study found no significant difference in outcomes except a lower overall mean HbA1c, and a 50% risk reduction for GDM among non-obese women, in the treatment group. The study was underpowered due to its small sample size.
-
(2)
Comparison of pregnancy outcomes between early- and late-onset GDM: We identified 13 cohort studies that compared the treatment outcomes of early and late-onset GDM women [40, 41•, 42,43,44,45,46,47, 48•, 49, 50•, 51, 52•]. All studies used OGTT for GDM diagnosis except one [49] which used HbA1c. In our meta-analysis, the early-onset GDM women had significantly higher likelihood of perinatal mortality (relative risk (RR) 3.58 [1.91, 6.71]), neonatal hypoglycemia (RR 1.61 [1.02, 2.55]), and insulin use (RR 1.71 [1.45, 2.03]) compared to late-onset GDM women (Fig. 1). There was no significant difference between early- and late-onset GDM in mean birth weight, LGA, or SGA (Table 2).
Subgroup Analysis
In the subgroup analyses, the early-onset GDM women in the developed countries had significantly higher likelihood of neonatal ICU admission (RR1.12 [1.04, 1.22]) compared to late-onset GDM women (Fig. 1).
In the three identified studies [49, 51, 52•] that investigated post-partum screening rates, a significant follow-up loss (46–49%) for the post-partum OGTT was reported [49, 52•]. Compared to late GDM women, a higher rate of impaired glucose tolerance (23 vs 14%) and diagnosis of type 2 diabetes (5 vs 1%) were found in early GDM women [52•].
-
(3)
Comparison of outcomes between women with early treated GDM and normal glucose tolerance (NGT): Of the two studies [42, 50•], one reported that the birth weight of babies born to early treated GDM women were comparable to that of NGT women [50•]. In the other, there was no significant difference in LGA, hyperbilirubinemia or perinatal mortality; however, hypertensive disorders (preeclampsia 14.7 vs 5.4%, p < 0.001, chronic hypertension 11.8 vs 3.8%, p < 0.001), preterm delivery (19.6 vs 7.6%, p < 0.057), RDS (3.0 vs 0.5%, p = 0.02), and neonatal hypoglycemia (4.0 vs 1.0%, p = 0.02) were significantly higher in the early treated GDM group compared to women in the NGT group [42].
-
(4)
Comparison of treatment outcomes between women with early-onset GDM and preexisting diabetes: In the two identified studies [46, 52•] that compared pregnancy outcomes between women with early-onset GDM and preexisting diabetes, one [46] reported a significantly higher rate of macrosomia and insulin need in women with preexisting diabetes while the second study showed comparable rates of pregnancy outcomes between women with preexisting diabetes and those with GDM diagnosed in < 12 weeks of gestation [52•].
Discussion
Evidence is growing that many (15 to 70%) women with GDM have evidence of hyperglycemia before 24 weeks’ gestation. Our meta-analysis shows that such hyperglycemia is associated with significantly increased risk for perinatal mortality, neonatal hypoglycemia, and insulin therapy (based upon the glycemic thresholds in use). These women also are at increased risk of neonatal ICU admission in developed countries. Our systematic review, however, reveals multiple knowledge gaps, with insufficient data to identify how to diagnose early GDM, what therapeutic targets should be used, and how best to screen for early GDM. Early GDM appears to represent at least four different phenotypes: those with one or more features of the metabolic syndrome [26•], those with autoimmune etiology [53], those with impaired beta cell function [26•], and those with monogenic diabetes [54]. Although most of these women do not appear to have glycaemia elevated sufficiently to have impaired fasting glucose (IFG) or impaired glucose tolerance (IGT) outside of pregnancy, they could be said to have “prevalent” GDM, while those developing GDM by the time of the 24–28-week OGTT could be said to have “incident” GDM. The situation is made more complex by the variation in glycemic profile through pregnancy, with a very early increase in glycaemia after implantation, followed by reduced glycaemia with increased insulin sensitivity to 18–20 weeks, and then increasing glycaemia with the growing insulin resistance from placental hormonal secretion (HPL, PGH) [55]. Though the current IADPSG criteria have a high “detection rate,” capturing high-risk women with metabolic disturbances early in pregnancy (as shown by the DALI study (26•)), it is unknown whether early treatment will prevent adverse pregnancy outcomes. The first trimester HbA1c has limited utility in diagnosing early-onset GDM with inconsistent evidence for a proportionate increase in adverse pregnancy outcomes with higher values. Its low sensitivity in capturing GDM makes it a less desirable tool in early pregnancy. Although, an HbA1c of 5.9–6.4% (41–46 mmol/mol) is considered as prediabetes in the general population and is associated with increased risk of adverse pregnancy outcomes [19••], this threshold has not been evaluated among women of reproductive age outside of pregnancy. Approximately 7 to 66% of women of reproductive age are affected by anemia worldwide [56] with various etiologies such as insufficient iron intake, inability to absorb iron, vitamin B12 or folate deficiency, and menstrual blood loss [57]. While a higher first trimester HbA1c at a prediabetes range is highly specific for GDM, there remain concerns over the influence of minor changes in red cell turnover which can falsely lower HbA1c values [58, 59]. Therefore, further evidence is required to assess whether HbA1c is reliable enough in early pregnancy and, if so, to define an appropriate HbA1c threshold for diagnosing GDM in early pregnancy. The efficacy of FPG as a screening tool in early pregnancy is limited by its high false positive rate. The gestational week at which the women are screened and referred to treatment is crucial in determining the benefits of screening. This review was unable to determine the most appropriate gestational week for early screening.
Not only is there uncertainty whether women with early GDM benefit from treatment, there are concerns about risks from over-treatment. Currently, there is no evidence of the optimal glucose threshold for glycemic management in early pregnancy. If the fetus is exposed to relative undernutrition from over treatment, fetal programming for future metabolic disease could increase [60]. This over-treatment/undernutrition paradigm has emerged with excess number of SGA babies in GDM studies [61] and in clinical treatment of women with Mature Onset Diabetes of the Young (MODY 2), whose MODY 2 offspring may be SGA from over-vigorous treatment of the maternal elevated glycemic set point [62]. More recently, bariatric surgical registries have shown increased SGA in pregnancy, and others have shown undernutrition with reduced lean body mass in offspring of obese women who have lost weight during pregnancy [63].
Limitations
One of the limitations in this review was the heterogeneity of the studies. The review involved a mix of studies with different study designs and various screening and diagnostic approaches. There was a lack of uniformity among studies in relation to the interventions provided. Interventions were carried out with various glycemic targets. Some studies were associated with sampling bias as they were carried out with purposive or selective sampling of high-risk women. A few studies combined GDM and DIP. No study was identified that provided a cost-benefit analysis of early treatment. And finally, no studies demonstrated the long-term effects of early treatment on mothers and offspring. One of the key strengths of this study is that it includes a comprehensive search for the best possible evidences that were currently available. To our knowledge, this is the first study of its kind.
Conclusion and Recommendations
Women with relative hyperglycemia early in pregnancy are at increased risk of some adverse pregnancy outcomes despite early treatment. Such early, or “prevalent GDM,” is common and appears to have a range of etiologies. There is no good evidence for appropriate diagnostic criteria, optimal screening procedures, or approaches to management, and suitable RCTs are urgently required. At this point in time, pending RCTs before 24 weeks’ gestation, a pragmatic approach to “diagnose” GDM may be to use the 24–28 week GDM criteria in the second trimester. In the first trimester, we recommend the use of a fasting glucose of 6.1–6.9 mmol/l, but there are insufficient data to recommend 1- or 2-h glucose or HbA1c criteria to diagnose GDM currently. Pragmatically, 24–28-week GDM criteria could be used with the 1- or 2-h OGTT results. Glucose targets should be comparable to those for pregnant women with preexisting diabetes.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Simmons D. Epidemiologic context of diabetes in pregnancy. In: McCance D, Maresh M, Sacks DA, editors. A practical manual of diabetes in pregnancy. London: Blackwell Publishing; 2010.
•• World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy, WHO/NMH/MND/13.2. Geneva: World health Organization. 2013. Available from: http://apps.who.int/iris/bitstream/10665/85975/1/WHO_NMH_MND_13.2_eng.pdf. Accessed November 10, 2016. WHO recommendations for clinicians to detect hyperglycemia in pregnancy.
•• HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. The landmark study that demonstrated the association of lesser degrees of hyperglycemia and adverse pregnancy outcomes.
•• Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–9. A meta-analysis compiling evidences for increased risk of type 2 diabetes among women with gestational diabetes.
• Yessoufou A, Moutairou K. Maternal diabetes in pregnancy: early and long-term outcomes on the offspring and the concept of “metabolic memory”. Exp Diabetes Res. 2011. Doi:https://doi.org/10.1155/2011/218598. An article discussing the impact of maternal hyperglycemia on offspring’s life.
•• Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477–86. The Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) trial that showed the benefits of treating gestational diabetes in reducing the rate of serious perinatal outcomes.
•• Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339–48. A randomised controlled trial that showed the benefit of treating women with mild carbohydrate intolerence during pregnancy.
•• Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–82. The recommendations by the IADPSG Consensus Panel to guide clinicians to detect hyperglycemic disorders in pregnancy.
•• McIntyre HD, Sacks DA, Barbour LA, Feig DS, Catalano PM, Damm P, et al. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care. 2015;39(1):53–4. An important article from the IADPSG group discussing the issues with diagnosis of hyperglycemia in early pregnancy.
•• Zhu WW, Yang HX, Wei YM, Yan J, Wang ZL, Li XL, et al. Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in China. Diabetes Care. 2013;36(3):586–90. An important study that evaluates fasting plasma glucose values at booking for GDM diagnosis.
Simmons D, Devers MC, Wolmarans L, Johnson E. Difficulties in the use of risk factors to screen for gestational diabetes mellitus. Diabetes Care. 2009;32(1):e8.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12.
Wells GAS, O’Connell BD, Peterson J, Welch V, Losos M, Tugwell P. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed November 10, 2016.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6.
World Health Organization. Trends in maternal mortality: 1990 to 2010. Tech. Rep., WHO, UNICEF, UNFPA and The World Bank estimates. Geneva, Switzerland, 2012.
• Yeral MI, Ozgu-Erdinc AS, Uygur D, Seckin KD, Karsli MF, Danisman AN. Prediction of gestational diabetes mellitus in the first trimester, comparison of fasting plasma glucose, two-step and one-step methods: a prospective randomized controlled trial. Endocrine. 2014;46(3):512–8. A study that shows 75 g OGTT as the best method for GDM screening in the first trimester.
Osmundson SS, Norton ME, El-Sayed YY, Carter S, Faig JC, Kitzmiller JL. Early screening and treatment of women with prediabetes: a randomized controlled trial. Am J Perinatol. 2016;33(2):172–9.
•• Hughes RC, Moore MP, Gullam JE, Mohamed K, Rowan J. An early pregnancy HbA1c >/=5.9% (41 mmol/mol) is optimal for detecting diabetes and identifies women at increased risk of adverse pregnancy outcomes. Diabetes Care. 2014;37(11):2953–9. A large cohort study demonstrating the potential utility of HbA1c in early pregnancy.
• Mañé L, Flores-Le Roux JA, Benaiges D, Rodríguez M, Marcelo I, Chillarón JJ, et al. Role of first-trimester HbA1c as a predictor of adverse obstetric outcomes in a multiethnic cohort. J Clin Endocrinol Metab. 2017;102(2):390–7. A prospective study showing the utility of HbA1c in ealry pregnancy.
• Sweeting AN, Ross GP, Hyett J, Molyneaux L, Tan K, Constantino M, et al. Baseline HbA1c to identify high-risk gestational diabetes: utility in early vs standard gestational diabetes. J Clin Endocrinol Metab. 2017;102(1):150–6. A retrospective cohort study demonstrating the limited utility of HbA1c for early GDM among high-risk women.
Balaji V, Madhuri BS, Ashalatha S, Sheela S, Suresh S, Seshiah V. A1C in gestational diabetes mellitus in Asian Indian women. Diabetes Care. 2007;30(7):1865–7.
Osmundson SS, Zhao BS, Kunz L, Wang E, Popat R, Nimbal VC, et al. First trimester hemoglobin A1c prediction of gestational diabetes. Am J Perinatol. 2016;33(10):977–82.
Amylidi S, Mosimann B, Stettler C, Fiedler GM, Surbek D, Raio L. First-trimester glycosylated hemoglobin in women at high risk for gestational diabetes. Acta Obstet Gynecol Scand. 2016;95(1):93–7.
• Maegawa Y, Sugiyama T, Kusaka H, Mitao M, Toyoda N. Screening tests for gestational diabetes in Japan in the 1st and 2nd trimester of pregnancy. Diabetes Res Clin Pract. 2003;62(1):47–53. A study that indicates the higher prevalence of GDM in the first trimester.
• Harreiter J, Simmons D, Desoye G, Corcoy R, Adelantado JM, Devlieger R, et al. IADPSG and WHO 2013 gestational diabetes mellitus criteria identify obese women with marked insulin resistance in early pregnancy. Diabetes Care. 2016;39(7):e90–2. A study that demonstrates the potential utility of IADPSG/WHO 2013 criteria in early pregnancy to identify high-risk women with metabolic disturbances.
Dashora U, Dashora V, Kennedy L. Two-hour 75-g oral glucose tolerance test early in pregnancy detects most cases of gestational diabetes. Diabetes Care. 2002;25(4):803.
Gandhi P, Farrell T. Gestational diabetes mellitus (GDM) screening in morbidly obese pregnant women. Eur J Obstet Gynecol Reprod Biol. 2011;159(2):329–32.
•• Riskin-Mashiah S, Younes G, Damti A, Auslender R. First-trimester fasting hyperglycemia and adverse pregnancy outcomes. Diabetes Care. 2009;32(9):1639–43. An important study that demonstrates the association of mild hyperglycemia in early pregnancy and increased risk of adverse pregnancy outcomes.
Riskin-Mashiah S, Damti A, Younes G, Auslender R. First trimester fasting hyperglycemia as a predictor for the development of gestational diabetes mellitus. Eur J Obstet Gynecol Reprod Biol. 2010;152(2):163–7.
•• Corrado F, D’Anna R, Cannata ML, Interdonato ML, Pintaudi B, Di Benedetto A. Correspondence between first-trimester fasting glycaemia, and oral glucose tolerance test in gestational diabetes diagnosis. Diabetes Metab. 2012;38(5):458–61. A study that evaluates first trimester fasting plasma glucose values for GDM diagnosis.
Bhattacharya SM. Fasting or two-hour postprandial plasma glucose levels in early months of pregnancy as screening tools for gestational diabetes mellitus developing in later months of pregnancy. J Obstet Gynaecol Res. 2004;30(4):333–6.
Agarwal MM, Dhatt GS, Punnose J, Zayed R. Gestational diabetes: fasting and postprandial glucose as first prenatal screening tests in a high-risk population. J Reprod Med. 2007;52(4):299–305.
Sacks DA, Chen W, Wolde-Tsadik G, Buchanan TA. Fasting plasma glucose test at the first prenatal visit as a screen for gestational diabetes. Obstet Gynecol. 2003;101(6):1197–203.
Meek CL, Murphy HR, Simmons D. Random plasma glucose in early pregnancy is a better predictor of gestational diabetes diagnosis than maternal obesity. Diabetologia. 2016;59(3):445–52.
Neelakandan R, Sethu PS. Early universal screening for gestational diabetes mellitus. J Clin Diagn Res. 2014;8(4):Oc12–4.
Murgia C, Berria R, Minerba L, Malloci B, Daniele C, Zedda P, et al. Gestational diabetes mellitus in Sardinia: results from an early, universal screening procedure. Diabetes Care. 2006;29(7):1713–4.
Bartha JL, Martinez-Del-Fresno P, Comino-Delgado R. Early diagnosis of gestational diabetes mellitus and prevention of diabetes-related complications. Eur J Obstet Gynecol Reprod Biol. 2003;109(1):41–4.
Horie I, Kawasaki E, Sakanaka A, Takashima M, Maeyama M, Ando T, et al. Efficacy of nutrition therapy for glucose intolerance in Japanese women diagnosed with gestational diabetes based on IADPSG criteria during early gestation. Diabetes Res Clin Pract. 2015;107(3):400–6.
Barahona MJ, Sucunza N, García‐Patterson A, Hernández M, Adelantado JM, Ginovart G, et al. Period of gestational diabetes mellitus diagnosis and maternal and fetal morbidity. Acta Obstet Gynecol Scand. 2005;84(7):622–7.
• Bartha JL, Martinez-Del-Fresno P, Comino-Delgado R. Gestational diabetes mellitus diagnosed during early pregnancy. Am J Obstet Gynecol. 2000;182(2):346–50. The first study that demonstrated women with early GDM as a high-risk subgroup.
Berkowitz GS, Roman SH, Lapinski RH, Alvarez M. Maternal characteristics, neonatal outcome, and the time of diagnosis of gestational diabetes. Am J Obstet Gynecol. 1992;167(4):976–82.
Boriboonhirunsarn D, Kasempipatchai V. Incidence of large for gestational age infants when gestational diabetes mellitus is diagnosed early and late in pregnancy. J Obstet Gynaecol Res. 2016;42(3):273–8.
De Muylder X. Perinatal complications of gestational diabetes: the influence of the timing of the diagnosis. Eur J Obstet Gynecol Reprod Biol. 1984;18(1–2):35–42.
Easmin S, Chowdhury TA, Islam MR, Beg A, Jahan MK, Latif T, et al. Obstetric outcome in early and late onset gestational diabetes mellitus. Mymensingh Med J. 2015;24(3):450–6.
Gupta S, Dolin C, Jadhav A, Chervenak J, Timor-Tritsch I, Monteagudo A. Obstetrical outcomes in patients with early onset gestational diabetes. J Matern Fetal Neonatal Med. 2016;29(1):27–31.
Hawkins JS, Lo JY, Casey BM, McIntire DD, Leveno KJ. Diet-treated gestational diabetes mellitus: comparison of early vs routine diagnosis. Am J Obstet Gynecol. 2008;198(3):287.e281–6.
• Most OL, Kim JH, Arslan AA, Klauser C. Maternal and neonatal outcomes in early glucose tolerance testing in an obstetric population in New York city. J Perinat Med. 2009;37(2):114–7. A study that demonstrates the greater severity of GDM in the early-onset subgroup.
Rowan JA, Budden A, Ivanova V, Hughes RC, Sadler LC. Women with an HbA1c of 41-49 mmol/mol (5.9-6.6%): a higher risk subgroup that may benefit from early pregnancy intervention. Diabet Med. 2016;33(1):25–31.
• Seshiah V, Cynthia A, Balaji V, Balaji MS, Ashalata S, Sheela R, et al. Detection and care of women with gestational diabetes mellitus from early weeks of pregnancy results in birth weight of newborn babies appropriate for gestational age. Diabetes Res Clin Pract. 2008;80(2):199–202. A study showing the benefit of early treatment of GDM.
Svare JA, Hansen BB, Molsted-Pedersen L. Perinatal complications in women with gestational diabetes mellitus: significance of a diagnosis early in pregnancy. Obstet Gynecol Surv. 2002;57:330–2.
• Sweeting AN, Ross GP, Hyett J, Molyneaux L, Constantino M, Harding AJ, et al. Gestational diabetes mellitus in early pregnancy: evidence for poor pregnancy outcomes despite treatment. Diabetes Care. 2016;39(1):75–81. A retrospective study that demonstrates women with early GDM as a high-risk subgroup with poorer pregnancy outcomes.
Murgia C, Orrù M, Portoghese E, Garau N, Zedda P, Berria R, et al. Autoimmunity in gestational diabetes mellitus in Sardinia: a preliminary case-control report. Reprod Biol Endocrinol. 2008;6(0):24.
Stoffel M, Bell KL, Blackburn CL, Powell KL, Seo TS, Takeda J, et al. Identification of glucokinase mutations in subjects with gestational diabetes mellitus. Diabetes. 1993;42(6):937–40.
Mills JL, Jovanovic L, Knopp R, Aarons J, Conley M, Park E, et al. Physiological reduction in fasting plasma glucose concentration in the first trimester of normal pregnancy: the diabetes in early pregnancy study. Metabolism. 1998;47(9):1140–4.
World Health Organization. Worldwide prevalence of anaemia 1993–2005: WHO global database on anaemia. Geneva: World Health Organization; 2008.
World Health Organization. Iron deficiency anaemia: assessment, prevention and control: a guide for programme managers. Geneva: World Health Organization; 2001.
Church D, Simmons D. More evidence of the problems of using HbA1c for diagnosing diabetes? The known knowns, the known unknowns and the unknown unknowns. J Intern Med. 2014;276(2):171–3.
Simmons D, Hlaing T. Interpretation of HbA1c: association with mean cell volume and haemoglobin concentration. Diabet Med. 2014;31(11):1387–92.
Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35(7):595–601.
Langer O, Levy J, Brustman L, Anyaegbunam A, Merkatz R, Divon M. Glycemic control in gestational diabetes mellitus-how tight is tight enough: small for gestational age versus large for gestational age? Am J Obstet Gynecol. 1989;161(3):646–53.
Hattersley AT, Beards F, Ballantyne E, Appleton M, Harvey R, Ellard S. Mutations in the glucokinase gene of the fetus result in reduced birth weight. Nat Genet. 1998;19(3):268–70.
Catalano PM, Mele L, Landon MB, Ramin SM, Reddy UM, Casey B, et al. Inadequate weight gain in overweight and obese pregnant women: what is the effect on fetal growth? Am J Obstet Gynecol. 2014;211(2):137.e131–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jincy Immanuel is supported by a post-graduate research scholarship from Western Sydney University. David Simmons declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Diabetes and Pregnancy
Electronic Supplementary Material
Supplementary Table 1
(DOCX 94 kb)
Supplementary Table 2
(DOCX 25 kb)
Supplementary Table 3
(DOCX 17 kb)
Supplementary Table 4
(DOCX 93 kb)
Supplementary Table 5
(DOCX 88 kb)
Supplementary Table 6
(DOCX 17 kb)
Supplementary Table 7
(DOCX 17 kb)
Supplementary Table 8
(DOCX 14 kb)
Supplementary Table 9
(DOCX 82.9 kb)
Supplementary Table 10
(DOCX 93 kb)
Supplementary Table 11
(DOCX 94 kb)
Supplementary Figure 1
(DOCX 78 kb)
Rights and permissions
About this article
Cite this article
Immanuel, J., Simmons, D. Screening and Treatment for Early-Onset Gestational Diabetes Mellitus: a Systematic Review and Meta-analysis. Curr Diab Rep 17, 115 (2017). https://doi.org/10.1007/s11892-017-0943-7
Published:
DOI: https://doi.org/10.1007/s11892-017-0943-7