Abstract
Low 25(OH) vitamin D levels have been associated with several autoimmune diseases and recently with autoimmune thyroid disease (AITD). The aim of the study was to investigate the association of AITD with 25(OH) vitamin D levels in women with polycystic ovary syndrome (PCOS). Fifty women with PCOS were consecutively enrolled and underwent routine health checkups, which included measurements of 25(OH) vitamin D, anti-thyroid peroxidase (TPO-Ab), anti-thyreoglobulin (TG-Ab) antibodies, FT3, FT4, and TSH. Selecting 50 nmol/L as cut-off point, low 25(OH) vitamin D levels were detected in 23 of 50 patients (46 %). AITD was diagnosed when TPO-Ab levels exceeding 80 U/ml and/or TG-Ab levels exceeding 70 U/ml. AITD was detected in 12 of 50 patients (24 %). The levels of 25(OH) vitamin D were significantly lower in women with PCOS and AITD when compared with women with PCOS and without AITD (p = 0.02). In women with AITD no correlation was found between 25(OH) vitamin D and TG-Ab (r = 0.48; p = 0.16), TPO-Ab (r = 0.43; p = 0.21), TSH (r = 0.38; p = 0.27), FT3 (r = −0.40; p = 0.25) and FT4 levels (r = −0.54; p = 0.10). These findings suggest that low levels of 25(OH) vitamin D were significantly associated with AITD in women with PCOS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovary syndrome (PCOS) is a complex syndrome characterized by reproductive and metabolic implications [1, 2]. Several risk factors for cardiovascular disease (CVD), including obesity, diabetes, insulin resistance (IR), hypertension, dyslipidemia, cardiopulmonary impairment, increased serum plasminogen activator inhibitor 1 (PAI-1) levels, and intima-media thickness (IMT) are frequent in PCOS [2–7]. Further, PCOS is characterized by chronic anovulatory, olighomenorrea, or amenhorrea and signs of hyperandrogenism and it is currently considered the most common cause infertility in women in reproductive age [1, 2].
The exact pathogenetic mechanism of PCOS is still debated although it is considered as a heterogeneous disorder with both genetic and environmental components. Recently vitamin D deficiency has been considered as a novel risk factor that could take part to the pathogenesis of PCOS. Vitamin D has been hypothesized to have a role in AMH production patterns in ovarian granulose cells and to blunt follicle stimulating hormone (FSH) sensitivity, probably playing a role in ovarian follicle development [8]. An inverse association between vitamin D status and metabolic disturbances has been reported in PCOS patients [9, 10]. Vitamin D deficiency has been found to be associated with an increased risk to develop thyroid autoimmunity [11–13]. The latter has been found to be very common in PCOS patients [14]. This association may lie on the fact that vitamin D may be able to trigger both innate and adaptive immune responses and to switch the immune system in a tolerogenic sense, avoiding the autoimmune response [15]. Thus, vitamin D deficiency may be associated to the increase risk to develop autoimmune disease [16]; providing scientific evidence on the association between vitamin D deficiency and thyroid autoimmune disease (AITD) could represent a promising therapeutic approach to improve and/or prevent AITD in women with PCOS.
Based on these considerations, the aim of the current study was to investigate the association of AITD with low 25(OH) vitamin D levels in women with PCOS.
Materials and methods
Subjects
A total of 50 women with PCOS were enrolled from May to July 2014 after obtaining written consent. The protocol was approved by Local Ethical Committee. In order to avoid seasonal influences on 25(OH) vitamin D levels, the study has been carried out in the same time-period (May–July 2014). The duration of exposure to sunlight (number of hours per day) was noted. Dietary vitamin D and/or calcium intake was assessed. Qualitative and quantitative aspects of food intake were assessed using an oral freely usable semi-quantitative food frequency questionnaire. The diagnosis of PCOS was based on the Rotterdam criteria that required two out of three of the following criteria to fulfill the diagnosis: oligo-and/or anovulation; clinical and/or biochemical signs of hyperandrogenism and polycystic ovaries by ultrasound. Oligo- and/or anovulation were defined by the presence of oligomenorrhea or amenorrhea. Hyperandrogenism was defined by the clinical presence of hirsutism (Ferriman–Gallwey score >8, acne or alopecia, and/or elevated androgen levels). Transvaginal ultrasonographic examinations had been performed by the same experienced operator during the early follicular phase (2nd–3rd day) of a spontaneous or progesterone-induced bleedings, and ovarian dimension and morphology were noted bilaterally in each subject [17]. Exclusion criteria included: age <18 or >40 years, body mass index (BMI) higher than 30 and lower than 18, pregnancy, glucose intolerance as screened by a 2-h oral glucose tolerance test (OGTT) and diabetes, hypothyroidism, hyperprolactinemia, Cushing’s syndrome, nonclassical congenital adrenal hyperplasia, and previous use of oral contraceptives, glucocorticoids, antiandrogens, ovulation induction agents, antidiabetic or antiobesity drugs, or other hormonal drugs within the previous 6 months. Subjects with cancer, metabolic glucose derangements (fasting glucose ≥100 mg/dl), hepatic, respiratory, and any cardiovascular disorder, a history of any thyroid disease or thyroid surgery or other concurrent medical illness (i.e., renal disease, cephalea, or malabsorptive disorders) were also excluded from the study.
Clinical and biochemical evaluation
Hirsutism was quantified by the Ferriman–Gallwey score [18]. We selected 50 nmol/l as cut off of low 25(OH)D because it was closest to median value of our population [19]. Patients were asked not to depilate for at least 1 month before each evaluation. After fasting overnight for 10–12 h, blood samples were collected for the following assays: FSH, luteinizing hormone (LH), testosterone, androstenedione, 17-hydroxyprogesterone, dehydroepiandrosterone sulfate (DHEAS), 25(OH) vitamin D, FT3, FT4, TSH, TG-Ab, TPO-Ab, hepatic, and renal function.
Biochemical assays
Serum levels of 25(OH)D were determined by chemiluminescence immunoassay radioimmunoassay (CLIA; Diasorin, Liaison) (intra- and inter-assay coefficients of variations were 5.8 and 7.8 %, respectively). All hormone concentrations were determined using the same commercial RIA kits (Diagnostic Products Los Angeles, CA). TPO-Ab and TG-Ab were determined using a BRAHMS Radioimmunoassay kit (Limburg, Germany). AITD was diagnosed when TPO-Ab levels exceeding 80 U/ml and/or TG-Ab levels exceeding 70 U/ml.
Statistical analysis
Statistical analysis was carried out using SPSS 9.0. Data are expressed as mean ± SD. Kolmogorov—Smirnov test was used to examine for normal distribution of the variables and, where necessary, log transformation was performed. After log transformation, the normal distribution of the variables was checked. Pearson correlations were used to determine relationships between variables. Depending on the distribution of the data, the Student’s t test for independent samples and the non-parametric Manne–Whitney U test for independent samples were applied to test for differences between groups.
Statistical significance was defined as p value lower than 0.05.
Results
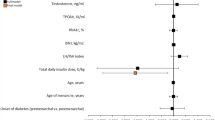
Clinical and biochemical variables of the all cohort of women with PCOS and AITD (study group) and controls are shown in Table 1. The mean age was 26.8 ± 8.4 years and the mean BMI was 28.1 ± 7.1 in the all cohort of women with PCOS.
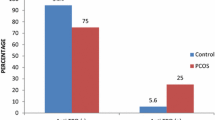
Selecting 50 nmol/L as cut-off point of vitamin D deficiency (9), low 25(OH) vitamin D levels were detected in 23 women (46 %) of all patients. Twelve women (24 %) with PCOS were affected by AITD. We compared the serum 25(OH)vitamin D levels between patients with AITD and controls. 25(OH) vitamin D levels were significantly lower in women with AITD when compared with (32.0 ± 22.6 vs. 49.6 ± 19.9 nmol/L; p = 0.02) (Fig. 1).
Although thyroid hormones were within the normal range (TSH: 0.35–5.50 mcUI/ml; FT3: 2.3–4.2 pg/ml; FT4: 0.89–1.76 ng/dl), women with AITD displayed a higher values of TSH compared to women without AITD (2.8 ± 1.0 vs. 1.9 ± 0.8 mcUI/ml; p = 0.02) whereas there was no difference regarding to the FT3 (3.5 ± 0.2 vs. 3.4 ± 0.4 pg/ml; p = 0.30) and FT4 levels (1.24 ± 0.1 vs. 1.26 ± 0.1 ng/dl; p = 0.25). Similarly, no difference was found in terms of TSH (2.7 ± 1.1 vs. 2.8 ± 1.1 mcUI/ml; p = 0.7), FT3 (3.5 ± 0.2 vs. 3.1 ± 0.3 pg/ml; p = 0.06), and FT4 levels (1.2 ± 0.1 vs. 1.0 ± 0.07 ng/dl; p = 0.06) between women with AITD and vitamin D deficiency (25(OH) vitamin D <50 nmol/L) and women with AITD without vitamin D deficiency, respectively.
In women with AITD Pearson coefficient analyses revealed no correlation between 25(OH) vitamin D and TG-Ab (r = 0.48; p = 0.16), TPO-Ab (r = 0.43; p = 0.21), TSH (r = 0.38; p = 0.27), FT3 (r = −0.40; p = 0.25), and FT4levels (r = −0.54; p = 0.10).
Discussion
The main finding of this study was that women with PCOS and AITD had significantly lower 25(OH) vitamin D concentrations compared to women with PCOS without AITD.
Both AITD and low 25(OH) vitamin D concentration has been reported to be associated with PCOS [8–10]. In our study the prevalence of AITD in women with PCOS was 24 % and this was in agreement with a previous study performed by Jannsen et al. [20] that reported a prevalence of around 25 % of AITD in women with PCOS. Although the pathogenesis of the association of PCOS with AITD is not clear, one of the possible hypotheses could be the defective progesterone secretion which leads to an imbalance between estrogens and progesterone. Estrogen could increase the expression of interleukin-6 in T cells and the lack of inhibitory action of progesterone may cause an overstimulation of the immune system, thus leading to an increased prevalence of autoimmune disorders in these patients [21]. However, the development of autoimmune disease could be determined by several risk factors. Recently, vitamin D deficiency has been identified as an environmental trigger of AITD [17–21]. Taking into account human studies, lower 25(OH) vitamin D levels has been found in patients with AITD compared to healthy subjects by Orbach et al. [22]. This finding was subsequently confirmed by Tamer et al. [23] who demonstrated an increased prevalence of vitamin D deficiency [25(OH) vitamin D ≤10 ng/ml] in patients with AITD. Vitamin D deficiency has been reported to increase the risk to develop metabolic derangements in women with PCOS [9, 10]. In our study the prevalence of vitamin D deficiency in PCOS was 46 % and this was in agreement with previous study performed by Li et al. [24] reporting a prevalence of 45 % in women with PCOS. The association of AITD with low 25(OH) vitamin D levels has been already reported in pre-menopausal women but not in post-menopausal women, thus suggesting a possible cross-talk mechanism between vitamin D and estrogens in the pathogenesis of AITD [25].
The degree of vitamin D deficiency has been reported to have a tight relationship with antibody levels and with thyroid hormones, thus suggesting that vitamin D levels may have a direct role in determining the severity of autoimmune disease and of the consequent hypothyroidism [26, 27]. In our study, we failed to find any correlation between 25(OH) vitamin D levels and antibody titer and thyroid function markers. This could be due to the fact that our patients were euthyroid and did not show any alterations of thyroid function that could be related to vitamin D levels. There are limitations of the current study. First, we only evaluated cross-sectional data and a follow-up of this cohort may provide more information about the role of vitamin D in determining the onset of AITD in PCOS. Second, the assessment of AITD did not include thyroid ultrasound and this may prevent to assess the association of vitamin D with the morphological parameters associated with AITD. Third, all the enrolled women with PCOS were euthyroid and this may prevent to assess the association between the degree of vitamin D deficiency and the severity of thyroid dysfunction. Latter, a limitation of our study is represented by the lack of a control group. For this reason, we performed subgroup analyses of AITD and no AITD women with PCOS to overcome this limitation. Further, the AITD group was much smaller than non AITD group, although this did not prevent to reach a statistical significance.
In conclusion, the present study shows that low 25 (OH) vitamin D levels were associated with AITD in PCOS. Further, study after study, utilizing varying designs in both human subjects and the laboratory, has reported that adequate vitamin D levels is substantially associated with a lower risk of developing AITD. The A.B. Hill criteria have been largely satisfied, providing a compelling case for a causal, inverse relationship between vitamin D status and risk of AITD [28]. Based on this association, the supplementation with vitamin D could represent a promising therapeutic approach to prevent or improve the development of AITD. This is of crucial importance in PCOS; as well known, PCOS is characterized by metabolic derangements and AITD may contribute to further worsen metabolic dysfunction in these subjects. Thus, a future longitudinal cohort studies along with prospective interventional trials may contribute to better clarify the role of vitamin D in the pathogenesis of AITD in women with PCOS.
References
S.M. Sirmans, K.A. Pate, Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin. Epidemiol. 6, 1–13 (2001)
R. Azziz, E. Carmina, D. Dewailly, E. Diamanti-Kandarakis, H.F. Escobar-Morreale, W. Futterweit, O.E. Janssen, R.S. Legro, R.J. Norman, A.E. Taylor, S.F. Witchel, Androgen excess society: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an androgen excess society guideline. J. Clin. Endocrinol. Metab. 91, 4237–4245 (2006)
A. Ricardo, Diagnosis of polycystic ovarian syndrome: the rotterdam criteria are premature. J. Clin. Endocrinol. Metab. 91, 781–785 (2006)
R.L. Thomson, S. Spedding, J.D. Buckley, Vitamin D in the aetiology and management of polycystic ovary syndrome. Clin. Endocrinol. (Oxf.) 77, 343–350 (2012)
M. Irani, H. Minkoff, D.B. Seifer, Z. Merhi, Vitamin D increases serum levels of the soluble receptor for advanced glycation end products in women with PCOS. J. Clin. Endocrinol. Metab. 99, E886–E890 (2014)
M. Irani, Z. Merhi, Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertil. Steril. 102, 460–468 (2014)
S.K. Patra, H. Nasrat, B. Goswami, A. Jain, Vitamin D as a predictor of insulin resistance in polycystic ovarian syndrome. Diabetes Metab. Syndr. 6, 146–149 (2012)
F.A. Dabrowski, B. Grzechocinska, M. Wielgos, The role of vitamin D in reproductive health–a Trojan Horse or the Golden Fleece? Nutrients 7(6), 4139–4153 (2015)
G. Muscogiuri, J. Mitri, C. Mathieu, K. Badenhoop, G. Tamer, F. Orio, T. Mezza, R. Vieth, A. Colao, A. Pittas, Mechanisms in endocrinology: vitamin D as a potential contributor in endocrine health and disease. Eur. J. Endocrinol. 171(3), R101–R110 (2014)
G. Muscogiuri, C. Policola, A. Prioletta, G. Sorice, T. Mezza, A. Lassandro, S. Della Casa, A. Pontecorvi, A. Giaccari, Low levels of 25(OH)D and insulin-resistance: 2 unrelated features or a cause-effect in PCOS? Clin Nutr 31(4), 476–480 (2012)
M. Feng, H. Li, S.F. Chen, W.F. Li, F.B. Zhang, Polymorphisms in the vitamin D receptor gene and risk of autoimmune thyroid diseases: a meta-analysis. Endocrine 43(2), 318–326 (2013)
T. Yasuda, Y. Okamoto, N. Hamada, K. Miyashita, M. Takahara, F. Sakamoto, T. Miyatsuka, T. Kitamura, N. Katakami, D. Kawamori, M. Otsuki, T.A. Matsuoka, H. Kaneto, I. Shimomura, Serum vitamin D levels are decreased in patients without remission of Graves’ disease. Endocrine 43(1), 230–232 (2013)
M. Rotondi, L. Chiovato, Vitamin D deficiency in patients with Graves’ disease: probably something more than a casual association. Endocrine 43(1), 3–5 (2013)
C. Duran, M. Basaran, O. Kutlu, Z. Kucukaydin, S. Bakdik, F.S. Burnik, U. Aslan, S.S. Erdem, S. Ecirli, Frequency of nodular goiter and autoimmune thyroid disease in patients with polycystic ovary syndrome. Endocrine 49(2), 464–469 (2015)
G. Muscogiuri, G. Tirabassi, G. Bizzaro, F. Orio, S.A. Paschou, A. Vryonidou, G. Balercia, Y. Shoenfeld, A. Colao, Vitamin D and thyroid disease: to D or not to D? Eur. J. Clin. Nutr. 69(3), 291–296 (2015)
T. Skaaby, L.L. Husemoen, B.H. Thuesen, A. Linneberg, Prospective population-based study of the association between vitamin D status and incidence of autoimmune disease. Endocrine 50(1), 231–238 (2015)
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 81, 19–25 (2004)
D. Ferriman, J.D. Gallawey, Clinical assessment of body hair growth in women. J. Clin. Endocrinol. Metab. 21, 1440e7 (1961)
L. Ovesen, R. Andersen, J. Jakobsen, Geographical differences in vitamin D status with particular reference to European countries. Proc. Nutr. Soc. 62, 813–821 (2003)
O.E. Janssen, N. Mehlmauer, S. Hahn, A.H. Offner, R. Gärtner, High prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Eur. J. Endocrinol. 150(3), 363–369 (2004)
J. Petríková, I. Lazúrová, S. Yehuda, Polycystic ovary syndrome and autoimmunity. Eur. J. Intern. Med. 21(5), 369–371 (2010)
H. Orbach, G. Zandman-Goddard, H. Amital, V. Barak, Z. Szekanecz, G. Szucs, K. Danko, E. Nagy, T. Csepany, J.F. Carvalho, A. Doria, Y. Shoenfeld, Novel biomarkers in autoimmune diseases: prolactin, ferritin, vitamin D, and TPA levels in autoimmune diseases. Ann. N. Y. Acad. Sci. 1109, 385–400 (2007)
G. Tamer, S. Arik, I. Tamer, D. Coksert, Relative vitamin D insufficiency in Hashimoto’s thyroiditis. Thyroid 21, 891–896 (2011)
H.W. Li, R.E. Brereton, R.A. Anderson, A.M. Wallace, C.K. Ho, Vitamin D deficiency is common and associated with metabolic risk factors in patients with polycystic ovary syndrome. Metabolism 60(10), 1475–1481 (2011)
Y.M. Choi, W.G. Kim, T.Y. Kim, S.J. Bae, H.K. Kim, E.K. Jang, M.J. Jeon, J.M. Han, S.H. Lee, J.H. Baek, Y.K. Shong, W.B. Kim, Low levels of serum vitamin D3 are associated with autoimmune thyroid disease in pre-menopausal women. Thyroid 24(4), 655–661 (2014)
N.C. Bozkurt, B. Karbek, B. Ucan, M. Sahin, E. Cakal, M. Ozbek, T. Delibasi, The association between severity of vitamin D deficiency and Hashimoto’s thyroiditis. Endocr. Pract. 19(3), 479–484 (2013)
A.M. Mackawy, B.M. Al-Ayed, B.M. Al-Rashidi, Vitamin d deficiency and its association with thyroid disease. Int. J. Health Sci. (Qassim) 7(3), 267–275 (2013)
A.B. Hill, The environment and disease: association or causation? Proc. R. Soc. Med. 58, 295–300 (1965)
Author contribution
GM is the guarantor of the manuscript. GM and SP carried out the studies and drafted the manuscripts. MC and DT performed statistical analyses. AC participated in the design of the study. FO conceived the study, participated in its design and coordination.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Disclosure
The authors have nothing to disclose.
Rights and permissions
About this article
Cite this article
Muscogiuri, G., Palomba, S., Caggiano, M. et al. Low 25 (OH) vitamin D levels are associated with autoimmune thyroid disease in polycystic ovary syndrome. Endocrine 53, 538–542 (2016). https://doi.org/10.1007/s12020-015-0745-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-015-0745-0