Abstract
To compare the rate of positive thyroid peroxidase antibodies (TPO Ab) between women with different polycystic ovary syndrome (PCOS) phenotypes and women without PCOS. This is a retrospective cohort study. Women with PCOS at My Duc Hospital between June 1, 2020, and March 27, 2021, were matched with non-PCOS women by age. TPO Ab (cut-off: 34 IU/mL) and thyroid-stimulating hormone (TSH) levels were measured as markers of Hashimoto thyroiditis and thyroid function, respectively. One thousand eight hundred eight infertile women were included, 904 with PCOS (mean age 29.0 ± 3.58 years) and 904 without PCOS (29.1 ± 3.4 years; controls). Women with PCOS had a higher body mass index (22.8 ± 3.84 vs. 19.9 ± 2.23 kg/m2, p < 0.001), but most were not overweight/obese. Rates of positive TPO Ab in women with versus without PCOS were 8.2% and 8.4%, respectively (p = 0.932). Rates of positive TPO Ab in patients with PCOS phenotype A, B, C, or D were not statistically different (7.5%, 2.9%, 20.0%, and 7.8%, respectively). Median TSH concentrations were similar in the PCOS and control groups (1.84 mIU/L vs. 1.78 mIU/L, respectively; p = 0.194). Based on a linear regression model, there was no correlation between either BMI or the estradiol to progesterone ratio and TPO Ab status. In a large population of infertile women with PCOS who were mostly lean patients, rates of positive TPO Ab across all four PCOS phenotypes did not differ significantly from those in women without PCOS. These findings did not support the hypothesis that PCOS is a risk factor for Hashimoto thyroiditis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of autoimmune thyroid diseases (AITDs) is 4% and up to 15% in women of childbearing age [1]. AITDs cover a spectrum of disorders ranging from thyrotoxicosis to hypothyrodism which is caused by infiltration of lymphocytes to the thyroid gland, production of antibodies against self-structures, and cytotoxic action of T cells on thyrocytes. Thyroid autoimmunity and subsequent thyroid dysfunction induce abnormal sex hormone metabolism and menstrual irregularities and contribute to infertility [2, 3].
The etiology of AITDs is thought to include a combination of genetic susceptibility and environmental factors. Either thyroid specific (TSHR, thyroglobulin) or immunoregulatory genes (FOXP3, CD25, CD40, CTLA-4, and HLA) play essential roles in the development of AITDs, e.g., Graves’ disease and Hashimoto’s thyroiditis (HT) [4]. However, the development of AITDs cannot be solely predicted from susceptibility genes. Environmental factors contributing to the development of AITDs include viral infections, stress, body mass index, and sex steroid hormones. Compared with men, women had a five to tenfold higher chance of acquiring AITDs, which has been correlated with their higher estrogen levels. Estrogen is implicated as an enhancer of humoral immunity, while androgens and progesterone are thought to be protective as natural immune suppressors. Thyroid peroxidase antibody (TPO Ab) and thyroglobulin antibody (Tg Ab) are fundamental markers of HT, which is one of the most common types of AITDs leading to hypothyroidism. A study by Poppe et al. [2] showed that TPO Ab is significantly higher in infertile patients compared with controls (18% vs. 8%, respectively). In a Danish population, Feldthusen et al. [5] also reported impaired fertility associated with TPO Ab. However, population-based study conducted in Iran showed no association between infertility and TPO Ab [6]. These studies had several limitations, e.g., not focusing on the analysis of different metabolic disorders or on the correlation between TPO Ab and body mass index (BMI) or sex hormones. Moreover, thyroid autoimmunity is also associated with miscarriage, thyroid disorders during pregnancy, and in the postpartum period [3]. Ning et al. [7] found a strong association between TPO Ab positivity and preterm birth in euthyroid women with a female fetus.
In addition to AITDs, polycystic ovary syndrome (PCOS) is another common endocrine disorder that includes irregular menstrual cycles, hyperandrogenism, and infertility. Obesity, dyslipidemia, type 2 diabetes mellitus, metabolic syndrome, and cardiovascular disorders are the most common comorbidities related to PCOS. PCOS was subdivided into four clinical phenotypes [8]. Phenotype A is characterized by clinical and/or biochemical hyperandrogenism (HA), menstrual dysfunction (oligo/amenorrhea) (OA), and polycystic ovarian morphology (PCOM) in ultrasonography. Phenotype B is recognized when HA and OA are present, phenotype C is characterized by HA and PCOM, and the fourth phenotype, D, is defined by the presence of OA and PCOM. It is still unclear whether metabolic problem are the same in PCOS patients with the milder versus more severe phenotypes. Some studies claimed that phenotype A results in the most severe endocrine and metabolic abnormalities [9], while these were less extreme in phenotype D (the normoandrogenic phenotype) [10].
Several evidences suggested that PCOS is an independent risk factor for HT. In 2018, a meta-analysis of data from 1210 patients with PCOS and 987 non-PCOS women reported that the prevalence of positive TPO Ab was 26% and 9.7%, respectively (odds ratio [OR] 3.27, 95% confidence interval [CI] 2.32–4.63) [11]. Of note, the risk of AITDs in women with PCOS persisted for Asians (OR 4.56). However, it has also been reported that the rate of positive TPO Ab does not differ significantly between women with and without PCOS from Korea and Poland [12, 13]. Until now, only one single study explored the rate of TPO Ab positivity between PCOS phenotypes with clinical and/or biochemical hyperandrogenism and the control group [13]. In the current study, we reported the prevalence of TPO Ab in a large cohort of Vietnamese women with different phenotypes of PCOS compared with non-PCOS women. We also examined the correlation between BMI or the estradiol to progesterone ratio with TPO Ab status using a linear regression model.
Materials and Methods
Study Design
This retrospective cohort study was conducted at IVFMD center (My Duc Hospital), Ho Chi Minh City, Vietnam, between June 1, 2020, and March 27, 2021. The study was approved by the Institutional Ethics Committee of My Duc Hospital (02/2021/MĐ-HĐĐĐ).
Study Population
Women (aged ≥ 18 years) who sought infertility treatment and had at least two of the following diagnostic criteria of PCOS were eligible for the study: irregular menstrual cycles (> 35 days or < 21 days); clinical and/or biochemical signs of HA; and/or PCOM on ultrasound, after exclusion of other disorders that mimic PCOS. Biochemical HA was defined as elevated testosterone (1.8 nmol/L) or free testosterone index > 6. For PCOM, using endovaginal ultrasound transducers with a frequency bandwidth that includes 8 MHz, the threshold for PCOM on either ovary was > 20 follicles per ovary, ensuring no corpora lutea, cysts, or dominant follicles were present. The four different PCOS phenotypes were A (OA + HA + PCOM), B (OA + HA), C (HA + PCOM), and D (OA + PCOM). Age-matched controls were female patients who visited the hospital because of problems unrelated to PCOS, had normal menses, and did not seek advice for thyroid dysfunction. Individuals with hyperprolactinemia, congenital adrenal hyperplasia, androgen-secreting tumors, Cushing syndrome, hypertension, hepatic or renal insufficiency, and diabetes mellitus and those who had used preparations containing selenium in the previous 3 months were excluded.
Assessments
For all participants, at the first time of visit, thyroid-stimulating hormone (TSH) and free thyroxine (fT4) levels were quantified using the Access 2 Immunoassay System (Beckman Coulter, Inc., USA). Chemiluminescent competitive immunoassay (Roche Cobas® E 411, Roche Diagnostics, German) was utilized for measurement of TPO Ab, estradiol, and progesterone levels. The reference range for TPO Ab was < 34 IU/mL; level above this threshold was considered positive.
Outcome Measures
The primary outcome was the rate of TPO Ab positivity or median antibody levels between patients with and without PCOS and among different PCOS phenotypes.
Statistical Analysis
Data were analyzed using descriptive statistics (mean and standard deviation for normally distributed variables or median and interquartile range for skewed variables). Between-group differences were analyzed using one-way ANOVA with post hoc Tukey HSD test or Kruskal Wallis test for normally distributed or skewed variables, respectively, and the chi-square test for categorical variables. Univariable and multivariable logistic regression analyses were performed to identify factors associated with TPO Ab positivity. All variables with a p value < 0.25 in the univariate analysis were included in the multivariable analysis. All analyses were performed using the R statistical package (R version 3.3.3). Statistical significance was defined as p < 0.05.
Results
Study Population
Of 3182 women seeking fertility treatment at IVFMD over the study period, 904 had confirmed PCOS. The same number of non-PCOS women were randomly matched with PCOS patients by age (mean age 29 years) (Table 1). Although mean BMI was significantly higher in women with versus without PCOS women (22.8 vs. 19.9 kg/m2; p < 0.05), the majority of women with PCOS were lean. As expected, the anti-Müllerian hormone (AMH) level, luteinizing hormone (LH) to follicle-stimulating hormone (FSH) ratio, and the free testosterone index (FTI) were significantly higher in the women with versus without PCOS (Table 1). Duration of infertility was comparable in the two groups, but most of the patients with PCOS had primary infertility (p < 0.05) (Table 1).
Thyroid Peroxidase Antibody and Thyroid Hormonal Status
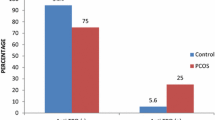
There were no significant differences between patients with and without PCOS or between different PCOS phenotypes with respect to rates of TPO Ab positivity or median antibody levels (Table 2, Fig. 1). Although the median concentration of fT4 was slightly lower in the PCOS group than in the non-PCOS group, TSH levels were not statistically different between groups (Table 2).
Influence of PCOS Phenotype
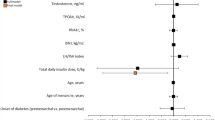
The prevalence of TPO Ab was slightly higher in PCOS patients with phenotype C, but between-group differences did not reach statistical significance (Table 3). Neither BMI nor estradiol to progesterone ratio had any significant association with TPO Ab status on univariate analyses. Multivariate analyses also confirmed that PCOS was not correlated to TPO Ab positivity (Table 4).
Discussion
This was a large-scale retrospective study. Based on the large cohort of women included, our study provides robust data showing that neither the prevalence nor median level of TPO Ab increased in women with versus without PCOS. In addition, a PCOS diagnosis was not a risk factor for positive TPO Ab on multivariate analysis. In terms of clinical studies, plenty of authors reported the presence of a higher anti-TPO, and TSH levels as well as a higher frequency of anti-TPO positivity in women with PCOS than in age-matched healthy women [14, 15]. However, these studies usually had relatively small sample sizes (the largest included 210 patients) and the mean BMI of PCOS patient were higher than ours (24.9 ± 3.6 and 30.0 ± 7.9) [14, 15]. A meta-analysis study also confirmed the association between PCOS and AITDs (OR 3.27, 95% CI 2.32–4.63) [11]. Nevertheless, AITD diagnosis was made according to the authors’ chosen criteria, so these criteria were not identical, and the study also found the large heterogenity between the included studies [11]. Some studies on the Asian population showed that AITD was not more prevalent in women with PCOS than in controls, and subjects with AITD showed significantly higher adiposity than those without AITD [12, 13]. Moreover, the mean BMI of those PCOS population was similar to our population (20.4 ± 2.8 and 22.8 ± 3.84). Although the median BMI in the PCOS group was significantly higher than that in the non-PCOS group in our study, the majority of our patients had a normal BMI (Table 1). Other studies attributed an increase in the prevalence of HT to obesity, which is usually seen in women with PCOS. In one meta-analysis, obesity was significantly correlated with 93% increased risk of detection of positive TPO Ab [16]. Adipocyte-derived leptin, which serves as a cytokine polarizing T cells towards the T helper 1 cell phenotype and suppressing regulatory T cell activity, triggers and perpetuates the autoimmune process [16]. Furthermore, reciprocal crosstalk between estradiol and leptin may occur potentiating immune T cell responses [17]. This contrasts with Caucasian populations of PCOS patients, who are usually overweight or obese.
Interestingly, the estradiol to progesterone ratio in our lean patients with PCOS did not differ from that in the study showing a positive correlation between this ratio and the frequency of positive TPO Ab [14]. Median values for AMH and AFC in our PCOS population were also in the typical ranges for these patients. Therefore, we postulated that sex hormone imbalance per se did not elicit HT in the absence of obesity.
Phenotype D was the most common among our infertile PCOS women as well as in China [18], and type D was considered to be more common in East Asian patients than in Caucasian patients [19]. Although the highest rate of positive TPO Ab was seen in phenotype C group which had only a small number of patients, there were no statistically significant differences between other phenotypes or between the different phenotypes and the control group. Because PCOS is a very heterogeneous disorder, it is essential to analyze the relationship between PCOS and HT in different phenotypes. Until now, only one previous study reported TPO Ab-positive frequency in the four different PCOS phenotypes [13]. In that study, the frequency of positive TPO Abs was the highest in phenotype C and lowest in phenotype D, but differences between phenotypes and the control group were not statistically significant. However, it is hard to explain the frequency of TPOAbs in these phenotypes observed in our study.
Some authors suggested that higher TSH level was associated with hyperandrogenism in euthyroid patients with PCOS and that this relationship was independent of age, BMI, and thyroid autoimmunity [20]. However, we did not observe any clinically significant increase in TSH level or decrease in fT4 level between HA PCOS phenotypes A, B, and C compared with phenotype D (Table 3).
Our study had few limitations. First, we did not measure Tg Ab, similar to other reports. Therefore, some HT may be misdiagnosed in the PCOS and control groups. Second, this study did not include the LH/FSH ratio among non-PCOS patients because the current practice at our hospital did not capture this information. Third, this is a retrospective study, and we could not determine the time from PCOS diagnosis to the occurrence of TPO Ab meaning that exposure time could not be included in our study.
In conclusion, our study provides substantial evidence that the prevalence of positive TPO Ab was not increased in infertile women with PCOS, most of whom were lean. The rate of TPO Ab did not differ significantly between PCOS phenotypes. Therefore, routine screening for thyroid disorders has limited value in infertile PCOS women.
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available due to patient privacy but are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Kennedy RL, Malabu UH, Jarrod G, Nigam P, Kannan K, Rane A. Thyroid function and pregnancy: before, during and beyond. J Obstet Gynaecol. 2010;30(8):774–83.
Poppe K, Glinoer D, Van Steirteghem A, Tournaye H, Devroey P, Schiettecatte J, et al. Thyroid dysfunction and autoimmunity in infertile women. Thyroid. 2002;12(11):997–1001.
Medenica S, Nedeljkovic O, Radojevic N, Stojkovic M, Trbojevic B, Pajovic B. Thyroid dysfunction and thyroid autoimmunity in euthyroid women in achieving fertility. Eur Rev Med Pharmacol Sci. 2015;19(6):977–87.
Lee HJ, Li CW, Hammerstad SS, Stefan M, Tomer Y. Immunogenetics of autoimmune thyroid diseases: a comprehensive review. J Autoimmun. 2015;64:82–90.
Feldthusen AD, Pedersen PL, Larsen J, ToftKristensen T, Ellervik C, Kvetny J. Impaired fertility associated with subclinical hypothyroidism and thyroid autoimmunity: the Danish General Suburban Population Study. J Pregnancy. 2015;2015:1–6.
Birjandi B, Ramezani Tehrani F, Amouzegar A, Tohidi M, BidhendiYarandi R, Azizi F. The association between subclinical hypothyroidism and TPOAb positivity with infertility in a population-based study: Tehran thyroid study (TTS). BMC Endocr Disord. 2021;21(1):108.
Yuan N, Sun J, Li Z, Chai S, Zhang X, Ji L. Relationship between anti-thyroid peroxidase antibody positivity and pregnancy-related and fetal outcomes in Euthyroid women: a single-center cohort study. BMC Pregnancy Childbirth. 2020;20(1):491.
Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106(1):6–15.
Zhao Y, Fu L, Li R, Wang LN, Yang Y, Liu NN, et al. Metabolic profiles characterizing different phenotypes of polycystic ovary syndrome: plasma metabolomics analysis. BMC Med. 2012;10(1):153.
Guo M, Chen ZJ, Eijkemans MJE, Goverde AJ, Fauser BCJM, Macklon NS. Comparison of the phenotype of Chinese versus Dutch Caucasian women presenting with polycystic ovary syndrome and oligo/amenorrhoea. Hum Reprod. 2012;27(5):1481–8.
Romitti M, Fabris VC, Ziegelmann PK, Maia AL, Spritzer PM. Association between PCOS and autoimmune thyroid disease: a systematic review and meta-analysis. Endocr Connect. 2018;7(11):1158–67.
Kim JJ, Yoon JW, Kim MJ, Kim SM, Hwang KR, Choi YM. Thyroid autoimmunity markers in women with polycystic ovary syndrome and controls. Hum Fertil. 2022;25(1):128–34.
Adamska A, Łebkowska A, Krentowska A, Hryniewicka J, Adamski M, Leśniewska M, et al. Ovarian reserve and serum concentration of thyroid peroxidase antibodies in euthyroid women with different polycystic ovary syndrome phenotypes. Front Endocrinol. 2020;28(11):440.
Arduc A, AycicekDogan B, Bilmez S, ImgaNasiroglu N, Tuna MM, Isik S, et al. High prevalence of Hashimoto’s thyroiditis in patients with polycystic ovary syndrome: does the imbalance between estradiol and progesterone play a role? Endocr Res. 2015;40(4):204–10.
Janssen O, Mehlmauer N, Hahn S, Offner A, Gartner R. High prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Eur J Endocrinol. 2004;1:363–9.
Song Rh, Wang B, Yao Qm, Li Q, Jia X, Zhang Ja. The impact of obesity on thyroid autoimmunity and dysfunction: a systematic review and meta-analysis. Front Immunol. 2019;10:2349.
Matarese G, Sanna V, Giacomo AD, Lord GM, Howard JK, Bloom SR, et al. Leptin potentiates experimental autoimmune encephalomyelitis in SJL female mice and confers susceptibility to males. Eur J Immunol. 2001;31(5):1324–32.
Zhang HY, Guo CX, Zhu FF, Qu PP, Lin WJ, Xiong J. Clinical characteristics, metabolic features, and phenotype of Chinese women with polycystic ovary syndrome: a large-scale case–control study. Arch Gynecol Obstet. 2013;287(3):525–31.
Kim JJ, Choi YM. Phenotype and genotype of polycystic ovary syndrome in Asia: ethnic differences. J Obstet Gynaecol Res. 2019;45(12):2330–7.
Cai J, Zhang Y, Wang Y, Li S, Wang L, Zheng J, et al. High thyroid stimulating hormone level is associated with hyperandrogenism in euthyroid polycystic ovary syndrome (PCOS) women, independent of Age, BMI, and thyroid autoimmunity: a cross-sectional analysis. Front Endocrinol. 2019;10(10):222.
Acknowledgements
We are grateful to My Duc Hospital and HRC staffs for their contribution and supports in this study.
Author information
Authors and Affiliations
Contributions
The study was designed by LDL, VTTT, LNV, and TDP. Statistical analysis was performed by TDP and LTHT. The draft of this manuscript was first written by VTTT and LDL. The manuscript was reviewed and edited by all of the authors. VTTT, LDL, TDP, and LTHT had full access to all the data in the study. All authors had final responsibility for the decision to submission and assume responsibility for the accuracy and completeness of the analyses and the consistent adherence of this report to the research protocol.
Corresponding author
Ethics declarations
Ethics Approval
02/21/DD-BVMD.
Consent to Participate
Not applicable.
Consent for Publication
All the authors have consented to the publication.
Conflict of Interest
LNV has received grant, speaker, and conference fees from Merck Sharp and Dohme and grant, speaker, conference, and scientific board fees from Ferring. LDL has received speaker fees from Novo Nordisk. All other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tran, V.T.T., Ly, L.D., Nguyen, M.H.N. et al. Thyroid Peroxidase Antibodies in Infertile Women with Polycystic Ovary Syndrome. Reprod. Sci. 30, 3071–3076 (2023). https://doi.org/10.1007/s43032-023-01261-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01261-5