Abstract
The purpose of this study was to analyze our experience with surgically treated isolated adrenal metastases in order to find those factors which can significantly affect survival. This method includes a retrospective single-center chart review. We evaluated how overall survival and disease-free survival (DFS) were influenced by demographic, tumor, and procedure-related variables. Thirty-seven adrenalectomies were performed in 34 patients. Procedures included 25 laparoscopic and 12 open adrenalectomies. Median follow-up was 49 months. Median overall survival was 63 months. Patients submitted to laparoscopic approach had a median survival of 57 months while it was 65 months for those who underwent open procedure (p = 0.67). DFS was 30 months, and these were 35 and 25 months after laparoscopic approach and open approach, respectively (p = 0.59). The concurrent resection of the adrenal metastasis with the primary tumor was the only factor influencing DFS (HR 6.8 95 % CI 1.2–37.3, p = 0.02). Patients with non-small cell lung cancer (n = 15) had a median survival of 63 months and DFS of 35 months. Our experience confirms that adrenalectomy, regardless of the surgical approach, can offer durable disease-free and overall survival outcomes for surgical candidates with isolated adrenal metastases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adrenal metastases are not uncommon, with prevalence in patients with a history of cancer ranging between 10 and 25 % [1–3].
Even though their presence represents an advanced stage of the primary malignancy, long-term survival has been reported for patients undergoing adrenalectomy for isolated adrenal metastasis [2, 4, 5].
Series of adrenal resections in these particular cases show median survivals longer than 30 months with 5-year survivals of 22.5–54 % [3, 4, 6–13].
It seems that minimally invasive surgery can offer the same oncologic outcomes as open surgery. However, only a few series supporting these results have been published [5, 9, 14].
The analysis of prognostic factors in this subgroup of patients is difficult due to the rarity of this condition, and thus, data regarding this topic are limited.
In this report, we aim to add useful data regarding our experience with surgically treated isolated adrenal metastases and to analyze the most recently published series to elucidate clinically relevant prognostic factors.
Materials and methods
From a prospectively collected institutional database, we identified records of patients with a diagnosis of adrenal metastasis in the period between January 2000 and November 2014. Data were collected and managed according to institutional rules. The diagnosis of adrenal metastasis was obtained by computed tomography staging and positron emission tomography. Magnetic resonance imaging was employed only in order to assess suspicious hepatic lesions.
Patients with direct invasion of the primary tumor into the adrenal gland, treated for oligometastatic disease, or who had undergone non-radical (R1 or R2) resections were excluded.
Open adrenalectomies were performed via an anterior median or subcostal approach while laparoscopic procedures were performed via a lateral trans-abdominal approach as described [15, 16].
Open procedure was performed in those patients with tumors greater than 8 cm at preoperative imaging or when the neoplasm involved the renal vein or inferior vena cava.
Disease-free interval (DFI) was defined as the interval between the resection of the primary tumor and the detection of adrenal metastasis.
Metastases were defined synchronous when detected within 6 months after treatment of the primary tumor.
Primary outcome variables were overall survival (OS) and disease-free survival (DFS).
OS was defined as the period from adrenalectomy to the date of death. DFS was defined as the period from adrenalectomy to the date of disease progression.
Risk factors analyzed for OS and DFS included sex, age, ASA score, body mass index (BMI), tumor-related variables (side, size, type of primary, and DFI), and surgery-related variables (approach and intraoperative complication).
Statistical analysis
Mann–Whitney U-test was used to compare continuous variables which were presented as median and interquartile range (IQR). Kaplan–Meier survival analysis was used to determine survival variables. Cox proportional hazards regression was performed to determine predictors of OS and DFS. Hazard ratios (HR) and 95 % confidence interval (95 % CI) were calculated when required. Logistic regression analysis was used to find correlations between the type of recurrence and treatment- and tumor-related variables. Analyses were performed with SPSS 14.0 for Windows (SPSS inc., Chicago, USA).
Results
Patients’ baseline characteristics are reported in Table 1. Thirty-seven adrenalectomies were performed in 34 patients. Procedures included 25 laparoscopic and 12 open adrenalectomies. Two laparoscopic adrenalectomies (two pulmonary adenocarcinomas) were concomitant resection of the primary tumor and the adrenal metastases. Difficult dissection due to inflammatory peritumoral reaction required conversion to laparotomy in two cases. Conversion was also required in one case of uncontrolled bleeding due to splenic injury and in one case of hemorrhage from the right adrenal vein owing to impaired functioning of the disposable clip applier. One splenic injury treated with hemostatic agents occurred during one open adrenalectomy. No postoperative complications regardless of the surgical approach occurred. Median operative times were 120 (IQR 88–161), 270 (IQR 221–293), and 293 (IQR 216–395) min for laparoscopic, open, and converted procedures, respectively. Median lengths of hospital stay were 4 (IQR 3–5) and 6 (IQR 5–8) days for patients submitted to laparoscopic approach and open procedure, respectively (p = 0.0035). For those cases which had conversion to laparotomy median hospital stay was 6 days (range 5–9 days).
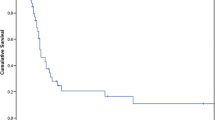
Median follow-up for the entire cohort was 49 months. Median OS was 63 months (Fig. 1). Patients who had undergone laparoscopic approach had a median survival of 57 months, while this was 65 months for those who underwent open procedure (p = 0.67).
Median DFS was 30 months (Fig. 1); these were 35 and 25 months after laparoscopic approach and open approach, respectively (p = 0.59).
Median OS and DFS of patients with DFI >6 months were 63 and 35 months, respectively; they were 18 and 8 months for those with DFI <6 months (p = 0.63; p = 0.73).
Recurrence occurred in 18 patients: 3 in the site of the primary tumor, 3 recurrences in the adrenal cavity, and 12 cases of hematogenous distant metastasis. Logistic regression analysis found no significant association between the considered variables (sex, age, ASA score, BMI, tumor-related, and surgery-related variables) and the development of local recurrence or hematogenous distant metastases.
An early local recurrence (≤8 months) in the adrenal cavity was seen in one case of hepatocarcinoma and in one of bladder cancer. In both the cases, rupture of the tumor capsule occurred during adrenectomy (1 laparoscopic and 1 open). Both the patients underwent laparotomy to remove the recurrent mass. The patient with metastasis from hepatocarcinoma is alive at 24 months with metastatic disease in the liver and the lungs, while the one with metastasis from bladder cancer is disease-free after 14 months.
None of the considered variables affected OS upon Cox proportion hazards regression. The same analysis demonstrated that the concomitant resection of the primary tumor and adrenal metastasis can influence DFS significantly (HR 6.8 95 % CI 1.2–37.3, p = 0.02). Patients who had undergone concurrent resection of primary tumor and synchronous adrenal metastasis had a DFS of 8 months, while it was 35 for those who had adrenalectomy for metachronous metastasis (p = 0.001).
Patients with non-small cell lung cancer (NSCLC) (n = 15) had a median survival of 63 months and DFS of 35 months (Fig. 2). No variables influencing either OS or DFS were found at Cox proportion-hazards regression for this subgroup of patients.
Discussion
Metastases to the adrenal gland are not necessarily associated with poor survival. Even in inoperable disease, acceptable survivals can be achieved with adrenal-directed local treatment, stereotactic ablative body radiotherapy, and radiofrequency ablation with a 2-year local control and OS of 63 and 19 %, respectively. Recent data demonstrated that surgery may be a reasonable treatment option for isolated adrenal metastasis in selected patients. A recent systematic review showed that the 2-year OS for adrenalectomy was 46 % [17].
As shown in Table 2, adrenalectomy can offer median survivals ranging from 7 to 48 months with 5-year survival of 20–45 % [6–13]. It must be highlighted that the majority of these papers, like the present study, are characterized by extremely varied study groups with a limited number of patients which make results difficult to compare.
Our study confirmed that adrenalectomy for isolated adrenal metastasis can result in long survivals.
The survival figures presented in our series are higher than those reported in other studies. There are a number of possible explanations for this. Firstly, our series did not include patients treated for oligometastatic disease or those who underwent palliative or partial (R1 or R2) resections as these factors were demonstrated to be negative predictors of long survival [8, 10, 12]. In addition, 82 % of patients in the present study had a DFI longer than 6 months. A short DFI is another well-established risk factor for poor survival [6, 7] (Table 2). In a recent series, Hwang et al. [7] showed a median survival of only 10.4 months, and this can be partly explained by the extremely high number (almost 60 % of patients) of cases with DFI <6 months. These results were also confirmed by the authors at multivariate analysis where they found that DFI <6 months and inflammation-based prognostic score are significant predictors of poor survival (Table 2).
Of late, Howell et al. [6] found that patients with DFI >12 months had a longer median survival than those with DFI <12 months (41 vs. 13 months). Similar results were also published by Muth et al. [10] who demonstrated that DFI >12 months was a positive prognostic factor with a median survival of 57 months compared with 21 months for patients who had a DFI <12 months.
A European multicenter study including 317 patients undergoing adrenalectomy for metastatic disease showed improved survival for DFI >6 months only at bivariate analysis (synchronous vs. metachronous) but could not confirm the same result at multivariate analysis [9].
In our analysis, we could not find any statistical significance in demonstrating DFI as a prognostic factor. However, it should be underlined that 3/5 patients with DFI <6 months were recruited in the last 12 months of the study period.
We identified synchronous resection of the adrenal metastasis and the primary tumor as a negative prognostic factor for DFS. No report analyzed and identified this variable as a predictor of survival. However, this finding does support the hypothesis that short DFI is associated with poor prognosis.
Four of the largest published series [8, 11–13] (Table 2) demonstrated that the size of the metastasis could also have an impact on prognosis. Strong et al. [11] showed that metastases <45 mm were associated with better prognosis. Interestingly, in this subgroup of patients, survivals similar to our study were achieved. Therefore, we have analyzed tumor size within our study group; finding that relatively small size of tumors (median 5 cm; IQR 3.5–8.7 cm) influenced favorably long-term outcomes.
The hematogenous diffusion of adrenal metastasis lends itself to laparoscopic resection.
Our group has already demonstrated that laparoscopic adrenal surgery for metastasis is more likely to be converted than minimally invasive surgery performed for other adrenal tumors [16]. However, in the present paper, we did not find any correlation between conversion to open surgery and any survival variables. In addition, other authors show no differences in survival between the laparoscopic and the open approaches [10–13], confirming the oncologic appropriateness of laparoscopic surgery. This, in combination with the well-documented advantages of laparoscopy, has increased the number of patients eligible for adrenalectomy for metastases.
In our series, we registered three cases of local recurrence. The approach to recurrent disease is usually non-surgical. No cases of surgery for recurrent disease after adrenalectomy for metastasis have been reported in literature. In the present paper, two patients were successfully treated through laparotomy 8 and 4 months after adrenalectomy. The positive outcome for these patients with isolated recurrent disease in the adrenal cavity should be taken into account when treating others with a similar pattern of disease. However, further studies should be designed to understand which selection criteria should be used in patients who could benefit from a second surgical approach in case of loco-regional recurrence.
NSCLC metastasis
NSCLC is the most common primary in the majority of the published series and in the present paper. As shown in Table 3, survivals after adrenalectomy range between 12 and 42 months with 5-year survivals of 20–35 % [6–13, 18–20]. Most of these data are extracted from heterogeneous series of adrenalectomies for metastasis from various primary tumors. Reporting of patients’ inclusion criteria and characteristics in the existing NSCLC literature are often unclear, and this makes it difficult to define the real value of NSCLC as a prognostic factor. However, dedicated series demonstrated the benefits of the surgical approach over medical treatment for isolated adrenal metastasis [20, 21].
Our survival rates are slightly longer than those reported in literature. This can be ascribed to the long DFI, as discussed above for the whole cohort, which may indicate more indolent behavior of the primary tumor in these patients.
In conclusion, our experience confirms that adrenalectomy is associated with good survival and acceptable morbidity. A long DFI can be considered a positive prognostic factor, but larger prospective series are needed to broaden our knowledge of factors influencing survival.
References
H.L. Abrams, R. Spiro, N. Goldstein, Metastases in carcinoma: analysis of 1000 autopsied cases. Cancer 3(1), 74–85 (1950)
C.T. Bradley, V.E. Strong, Surgical management of adrenal metastases. J. Surg. Oncol. 109(1), 31–35 (2014). doi:10.1002/jso.23461
K.Y. Lam, C.Y. Lo, Metastatic tumours of the adrenal glands: a 30-year experience in a teaching hospital. Clin. Endocrinol. 56(1), 95–101 (2002)
J.J. Sancho, F. Triponez, X. Montet, A. Sitges-Serra, Surgical management of adrenal metastases. Langenbecks Arch. Surg. 397(2), 179–194 (2012). doi:10.1007/s00423-011-0889-1
J. Uberoi, R. Munver, Surgical management of metastases to the adrenal gland: open, laparoscopic, and ablative approaches. Curr. Urol. Rep. 10(1), 67–72 (2009)
G.M. Howell, S.E. Carty, M.J. Armstrong, M.T. Stang, K.L. McCoy, D.L. Bartlett, L. Yip, Outcome and prognostic factors after adrenalectomy for patients with distant adrenal metastasis. Ann. Surg. Oncol. 20(11), 3491–3496 (2013). doi:10.1245/s10434-013-3050-2
E.C. Hwang, I. Hwang, S.I. Jung, T.W. Kang, D.D. Kwon, S.H. Heo, J.E. Hwang, S.G. Kang, S.H. Kang, J.G. Lee, J.J. Kim, J. Cheon, Prognostic factors for recurrence-free and overall survival after adrenalectomy for metastatic carcinoma: a retrospective cohort pilot study. BMC Urol. 14, 41 (2014). doi:10.1186/1471-2490-14-41
X. Ma, H. Li, X. Zhang, Q. Huang, B. Wang, T. Shi, D. Hu, Q. Ai, S. Liu, J. Gao, Y. Yang, J. Dong, T. Zheng, Modified anatomical retroperitoneoscopic adrenalectomy for adrenal metastatic tumor: technique and survival analysis. Surg. Endosc. 27(3), 992–999 (2013). doi:10.1007/s00464-012-2553-4
P. Moreno, A. de la Quintana Basarrate, T.J. Musholt, I. Paunovic, M. Puccini, O. Vidal, J. Ortega, J.L. Kraimps, E. Bollo Arocena, J.M. Rodriguez, O. Gonzalez Lopez, C.D. Del Pozo, M. Iacobone, E. Veloso, J.M. Del Pino, I. Garcia Sanz, D. Scott-Coombes, J. Villar-Del-Moral, J.I. Rodriguez, J. Vazquez Echarri, C. Gonzalez Sanchez, M.T. Gutierrez Rodriguez, I. Escoresca, J. NunoVazquez-Garza, E. Tobalina Aguirrezabal, J. Martin, M.F. Candel Arenas, K. Lorenz, J.M. Martos, J.M. Ramia, Adrenalectomy for solid tumor metastases: results of a multicenter European study. Surgery 154(6), 1215–1222 (2013). doi:10.1016/j.surg.2013.06.021. discussion 1222–1213
A. Muth, F. Persson, S. Jansson, V. Johanson, H. Ahlman, B. Wangberg, Prognostic factors for survival after surgery for adrenal metastasis. Eur. J. Surg. Oncol. 36(7), 699–704 (2010). doi:10.1016/j.ejso.2010.04.002
V.E. Strong, M. D’Angelica, L. Tang, F. Prete, M. Gonen, D. Coit, K.A. Touijer, Y. Fong, M.F. Brennan, Laparoscopic adrenalectomy for isolated adrenal metastasis. Ann. Surg. Oncol. 14(12), 3392–3400 (2007). doi:10.1245/s10434-007-9520-7
B.J. Vazquez, M.L. Richards, C.M. Lohse, G.B. Thompson, D.R. Farley, C.S. Grant, M. Huebner, J. Moreno, Adrenalectomy improves outcomes of selected patients with metastatic carcinoma. World J. Surg. 36(6), 1400–1405 (2012). doi:10.1007/s00268-012-1506-3
C. Zerrweck, R. Caiazzo, B. Clerquin, G. Donatini, A. Lamblin, Z. El Khatib, L. Arnalsteen, B. Carnaille, F. Pattou, Renal origin and size are independent predictors of survival after surgery for adrenal metastasis. Ann. Surg. Oncol. 19(11), 3621–3626 (2012). doi:10.1245/s10434-012-2464-6
J.T. Adler, E. Mack, H. Chen, Equal oncologic results for laparoscopic and open resection of adrenal metastases. J. Surg. Res. 140(2), 159–164 (2007). doi:10.1016/j.jss.2006.08.035
L. Solaini, L. Arru, G. Merigo, M. Tomasoni, F. Gheza, G.A. Tiberio, Advanced sealing and dissecting devices in laparoscopic adrenal surgery. JSLS 17(4), 622–626 (2013). doi:10.4293/108680813X13693422520350
G.A. Tiberio, L. Solaini, L. Arru, G. Merigo, G.L. Baiocchi, S.M. Giulini, Factors influencing outcomes in laparoscopic adrenal surgery. Langenbecks Arch. Surg. 398(5), 735–743 (2013). doi:10.1007/s00423-013-1082-5
A. Gunjur, C. Duong, D. Ball, S. Siva, Surgical and ablative therapies for the management of adrenal ‘oligometastases’—A systematic review. Cancer Treat. Rev. 40(7), 838–846 (2014). doi:10.1016/j.ctrv.2014.04.001
M. Lucchi, P. Dini, M.C. Ambrogi, P. Berti, G. Materazzi, P. Miccoli, A. Mussi, Metachronous adrenal masses in resected non-small cell lung cancer patients: therapeutic implications of laparoscopic adrenalectomy. Eur. J. Cardiothorac. Surg. 27(5), 753–756 (2005). doi:10.1016/j.ejcts.2005.01.047
O. Mercier, E. Fadel, M. de Perrot, S. Mussot, F. Stella, A. Chapelier, P. Dartevelle, Surgical treatment of solitary adrenal metastasis from non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 130(1), 136–140 (2005). doi:10.1016/j.jtcvs.2004.09.020
D.J. Raz, M. Lanuti, H.C. Gaissert, C.D. Wright, D.J. Mathisen, J.C. Wain, Outcomes of patients with isolated adrenal metastasis from non-small cell lung carcinoma. Ann. Thorac. Surg. 92(5), 1788–1792 (2011). doi:10.1016/j.athoracsur.2011.05.116. discussion 1793
P. Sastry, A. Tocock, A.S. Coonar, Adrenalectomy for isolated metastasis from operable non-small-cell lung cancer. Interact. Cardiovasc. Thorac. Surg. 18(4), 495–497 (2014). doi:10.1093/icvts/ivt526
Acknowledgments
The authors are grateful to Mr. Richard Humphies (BA Hons English Literature) for his precious help in revising the English language of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Solaini, L., Ministrini, S., Tomasoni, M. et al. Adrenalectomy for metastasis: long-term results and predictors of survival. Endocrine 50, 187–192 (2015). https://doi.org/10.1007/s12020-015-0596-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-015-0596-8