Abstract
Background
Indications and survival benefit for adrenalectomy (ADX) in the setting of metastasis are not clearly defined. We aimed to determine which patients with primary malignancies may benefit from ADX performed for metastasis. Mayo Clinic institutional outcomes in patients with metastatic disease to the adrenal(s) treated by adrenalectomy were compared to stage-matched historical controls from the Surveillance Epidemiology and End Results (SEER) database.
Methods
A retrospective review (1992–2010) was conducted to identify patients treated with ADX for metastatic cancer at Mayo Clinic, Rochester. Associations of clinical, surgical, and pathologic features with overall survival (OS) were evaluated using Cox proportional regression models. OS for those treated with ADX was compared with that for SEER database stage-matched patients who underwent primary resection without resection of distant disease using log-rank tests.
Results
A total of 166 patients underwent ADX for metastatic primaries involving the kidney 60, lung 24, sarcoma 19, colon 15, pancreas 13, and other-35. Patients with sarcoma and kidney, lung, and pancreatic tumors who underwent ADX had better OS at 1, 2, and 3 years than did the SEER-matched controls. Respectively, the rates were for sarcoma (100, 93, 86% vs. 57, 36, 30%), kidney (86, 80, 72% vs. 55, 37, 27%), lung (91, 69, 52% vs. 52, 34, 25%), and pancreas (79, 56, 45% vs. 33, 20, 12%). Univariate analysis identified primary diagnosis <2 years before ADX, other distant site, pancreatic primary, palliative operation, and persistent disease as risk factors for death.
Conclusions
An aggressive surgical approach results in improved OS in patients with metastatic disease arising from soft tissues, kidney, lung, and pancreas. Other tumors may benefit, but larger study cohorts are needed for a meaningful comparison.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The adrenal glands are commonly a site for metastatic disease, with postmortem series reporting the incidence as high as 38% for patients diagnosed with extraadrenal cancers [1–4]. Metastatic disease to the adrenal glands is rarely found in patients without a history of cancer [5]. The prevalence of metastatic lesions among incidentally discovered adrenal masses has been reported to be 2.5% [5–7]. Patients with adrenal metastasis are frequently deemed inoperable, and supportive-palliative therapy is often adopted.
Despite small series reporting improved survival [8–12] among those with metastatic melanoma, renal cell carcinoma, and lung cancer, among others, the indications for adrenalectomy (ADX) in the setting of metastatic cancer have not been clearly defined. The lack of randomized controlled trials represents the biggest challenge to developing treatment guidelines for this specific subset of patients. The current American Association of Clinical Endocrinologists/American Association of Endocrine Surgeons (AACE/AAES) practice guidelines for the management of incidental adrenal masses suggests that an adrenal metastatic lesion can be resected for symptomatic palliation [5]. Furthermore, their guidelines state that surgical resection can improve disease-free survival in a very select group of patients with good control of their extraadrenal disease and a good performance status [5]. This study aimed to review our experience retrospectively to identify prognostic factors for overall survival (OS) and determine which malignancies may benefit from ADX in the setting of metastatic disease.
Materials and methods
Protocol
A retrospective chart review, from August 1992 through October 2010, was conducted with the purpose of identifying patients treated with ADX in the setting of metastatic cancer. Patient medical records were reviewed for demographics, timing of diagnosis of primary disease and metastasis, type of primary tumor, operative interventions (i.e., open, laparoscopic, en bloc). surgical intent and findings, presence of persistent disease, recurrence, and length of follow-up. Recurrence-free survival (RFS) and OS were determined using the Kaplan-Meier method. Associations of clinical, surgical, and pathologic features with OS were evaluated using Cox proportional hazards regression models. The duration of follow-up was calculated from the time of ADX to the time of recurrence, death, or date of last follow-up. Statistical analyses were performed using the SAS software package (SAS Institute, Cary, NC, USA). All tests were two-sided, and p < 0.05 were considered statistically significant.
SEER data
The OS for those treated with ADX was compared to the OS of the National Institutes of Health Surveillance Epidemiology and End Results (NIH-SEER) [13] database of stage IV patients who underwent primary resection without removal of distant disease. Overall survival data for patients with stage IV kidney, lung, or pancreatic cancer diagnosed between 2004 and 2007 were retrieved from the SEER database. Patients whose primary tumors were not treated surgically and those whose distant metastases were treated surgically were excluded. The OS for the SEER patients was compared to the OS for the patients treated with ADX for distant metastases arising from the kidney, lung, and pancreas described above using log-rank tests. For these analyses, the duration of follow-up was calculated from the primary tumor diagnosis date to the death or last follow-up date. There were 60, 24, and 13 patients treated with ADX for distant metastases arising from the kidney, lung, and pancreas, respectively, of which 59, 21, and 11 had primary diagnosis dates available.
To ensure that this project met federal and state regulations and institutional policies for the protection of the rights, privacy, and welfare of all participants, it was reviewed and committee-approved by the Mayo Clinic’s institutional review board.
Results
Diagnosis
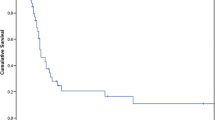
A total of 166 patients (61 women, 105 men) underwent ADX to resect metastatic disease. The mean age at ADX was 60.4 years. The ADX was performed a mean of 2.3 years from diagnosis of the primary malignancy, with 40% of patients undergoing ADX within 6 months of diagnosis. The primary malignancies were kidney (n = 60), lung (n = 24), sarcoma (n = 19), colon (n = 15), pancreas (n = 13), and other sites (n = 35) (Table 1). Intent of surgery was cure in 45% and palliation in 55% (n = 161). Mean size for the metastatic mass was 4.5 cm with adrenal metastatic disease unilateral in 36%, bilateral in 2%, and to the adrenal and another organ in 62%. In all, 45 patients (30%) were diagnosed as having persistent disease after surgery for adrenal disease; and 58 of 81 patients with recurrence data recurred at a mean of 1.3 years. Estimated RFS rates at 1, 5, and 10 years were 51, 19, and 14%, respectively (median RFS 1.1 years). At last follow-up, 101 (61%) patients had died at a mean of 1.9 years after ADX. The mean follow-up for the 65 patients alive was 3.1 years. Estimated OS rates at 1, 5, and 10 years following ADX were 68, 31, and 17%, respectively, with a median OS of 2.2 years (Fig. 1).
Univariate analysis identified primary diagnosis <2 years before ADX, metastasis size, other distant site, pancreatic primary, palliative operation, and persistent disease as risk factors for death (p < 0.05). There were no statistically significant relations between age, sex, open versus laparoscopic resection, or tumors of the lung and kidney or colon and kidney with death (Table 2).
Comparisons with SEER data
Sarcoma
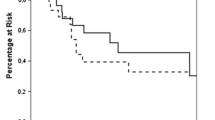
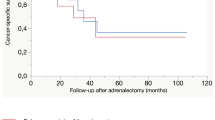
Estimated OS [95% confidence interval (CI), number still at risk] at 1, 2, and 3 years following diagnosis of the primary tumor were 100% (100–100; 15), 93% (82–100; 13), and 86% (70–100; 12), respectively, for the 19 patients treated with ADX for metastasis arising from a sarcoma compared with 57% (52–61; 245), 36% (31–41; 110), and 30% (26–35; 110), respectively, for the 564 patients with stage IV sarcoma from the SEER database (p < 0.001). The median OS for the two groups occurred at 5.8 and 1.3 years, respectively (Table 3; Fig. 2).
Kidney
Estimated overall survival rates (95% CI, number still at risk) at 1, 2, and 3 years following the primary kidney tumor diagnosis were 86% (78–96; 52), 80% (70–91; 45), and 72% (62–85; 38), respectively, for the 59 patients treated with ADX for metastasis arising from the kidney cancer compared with 55% (53–57; 1209), 37% (35–39; 530), and 27% (25–29; 200), respectively, for the 2869 patients with stage IV kidney cancer from the SEER database (p < 0.001). The median OS for the two groups occurred at 8.4 and 1.3 years, respectively (Fig. 3).
Lung
Estimated overall survival rates (95% CI, number still at risk) at 1, 2, and 3 years following the primary lung tumor diagnosis were 91% (79–100; 19), 69% (51–93; 12), and 52% (33–81; 9), respectively, for the 21 patients treated with ADX for metastasis arising from the lung cancer compared with 52% (49–54; 984), 34% (32–37; 430), and 25% (23–28; 162), respectively, for the 2420 patients with stage IV lung cancer from the SEER database (p = 0.002). The median OS for the two groups occurred at 3.5 and 1.1 years, respectively (Fig. 4).
Pancreas
Estimated overall survival rates (95% CI, number still at risk) at 1, 2, and 3 years following the primary pancreatic tumor diagnosis were 79% (56–100; 7), 56% (32–100; 5), and 45% (22–93; 4), respectively, for the 11 patients treated with ADX for metastasis arising from the pancreatic cancer compared with 33% (29–38; 129), 20% (16–25; 51), and 12% (9–17; 15), respectively, for the 490 patients with stage IV pancreatic cancer from the SEER database (p = 0.011). The median OS for the two groups occurred at 2.5 and 0.6 years, respectively (Fig. 5).
Discussion
The adrenal gland is one of the most common organs involved in metastatic disease. The increased used of computed tomography, magnetic resonance imaging, and positron emission tomography for routine surveillance of patients with known malignancy has led to an increased prevalence of asymptomatic adrenal masses [14]. The presence of adrenal metastasis purveys a poor prognosis to those patients treated nonsurgically. It continues to represent a significant challenge to physicians as the indications for ADX in cases of metastatic adrenal tumors remains a topic of controversy. The indications for surgery and a survival benefit have not been clearly defined because of small series of reported patients with many different primary malignancies.
Case reports and small series have suggested an increased survival for patients who undergo resection of solitary adrenal metastasis for primary tumors of the kidney, lung, gastrointestinal tract, and melanoma. Muth et al. [15] reported a recent series of 30 patients undergoing surgery for metastatic disease and identified the following as independent survival prognostics factors: ADX for potential cure, no previous metastasis surgery, tumor type. Better prognosis was identified in patients suffering from metastatic colorectal carcinoma and renal cell carcinoma [15]. Worse prognosis was found in patients with non-small-cell lung cancer and malignant melanoma [15].
In 1996 we published our experience with ADX for adrenal metastasis from 1983 to 1993. ADX was performed in 52 patients for different types of metastatic cancer, 32 of which were asymptomatic on initial evaluation [1]. A solitary adrenal metastasis was an indication for surgery in 60% of patients, and symptomatic adrenal pain relief was achieved in 11 of 13 patients [1]. Overall survival rates were 73 and 40% at 1 and 2 years, respectively [1]. In 1997, the Memorial Sloan Kettering Cancer Center reported their experience with 37 patients over a 10-year period. In all, 73% of whom were asymptomatic upon presentation [11]. All nine patients presenting with pain had symptomatic relief after surgery [11]. The OS was 24% at 5 years, with a survival median of 21 months.
Several series reporting on metastatic melanoma and metastases from lung and renal carcinomas to the adrenal gland(s) are encouraging. Significant survival improvement has been noted, and some authors even suggest the potential of surgical cure with aggressive interventions in a selected cohort with limited metastatic burden [9, 10]. Furthermore, symptomatic palliation has been achieved in most patients presenting with adrenal pain [1, 11]. Collinson et al. [9] reported increased survival in patients with melanoma, with a median survival of 16 months for patients who underwent ADX compared to 5 months for patients with documented adrenal metastases treated nonsurgically. A recent series reporting outcomes of ADX in cases of solitary metastatic disease originating from renal cell carcinoma showed median long-term survivals of 13.8 and 11.7 years for patients with no evidence of metastatic spread and those with a solitary intraadrenal metastatic lesion, respectively [10].
Thorough multidisciplinary oncologic evaluation, adequate preoperative imaging, and consideration of the patient’s functional status are imperative when selecting candidates for these interventions. There is an established role for ADX in the symptomatic palliation setting; surgery must be a consideration for patients presenting with a limited disease burden and adrenal pain [1, 5, 11]. We found no difference in risk of death between laparoscopic and open ADX in this setting. These findings support multiple recent series reports regarding the feasibility of adequate outcomes with a laparoscopic approach in selected patients with localized, resectable disease [16–19].
Conclusions
Our experience suggests that an aggressive surgical approach for adrenal metastasis results in statistically significant improvement in overall survival in selected patient cohorts with metastatic disease. Statistical significance was encountered in groups with primaries tumors arising from soft tissues (p < 0.001), kidney (p < 0.001), lung (p = 0.002), and pancreas (p = 0.011). Though a pancreatic primary purveyed increased risk of death for the patient, we did see improved survival with ADX when compared to SEER stage 4 cohort. Other tumors may benefit, but larger study cohorts are needed to obtain meaningful comparison data. Synchronous disease, tumor type, size, burden, and site are statistically significant risk factors for death and should be considered when selecting patients for ADX.
When establishing guidelines or treatment recommendations, one must be conscious of the lack of randomized controlled trials and the potential of referral bias to avoid misinterpretation of the data. Our biggest limitation and perhaps the caveat of our retrospective project was the lack of a cohort with localized and resectable metastasis treated nonsurgically. The SEER stage 4 cohort’s data provided us with the closest available nonsurgical comparison group.
References
Lo C, van Heerden J, Soreide J et al (1996) Adrenalectomy for metastatic disease to the adrenal glands. Br J Surg 83:528–531
Lam K, Lo C (2002) Metastatic tumours of the adrenal glands: a 30-year experience in a teaching hospital. Clin Endocrinol (Oxf) 56:95–101
Abrams H, Spiro R, Goldstein N (1950) Metastases in carcinoma: analysis of 1000 autopsied cases. Cancer 3:74–85
Brunt L, Moley J (2001) Adrenal incidentaloma. World J Surg 25:905–913. doi:10.1007/s00268-001-0029-0
Zeiger M, Thompson G, Duh Q et al (2009) The American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract 1:1–20
Young W (2000) Management approaches to adrenal incidentalomas: a view from Rochester, Minnesota. Endocrinol Metab Clin North Am 29:159–185
Barzon L, Sonino N, Fallo F et al (2003) Prevalence and natural history of adrenal incidentalomas. Eur J Endocrinol 149:273–285
Higashiyama M, Doi O, Kodama K et al (1994) Surgical treatment of adrenal metastasis following pulmonary resection for lung cancer: comparison of adrenalectomy with palliative therapy. Int Surg 79:124–129
Collinson F, Lam T, Bruijn W et al (2008) Long-term survival and occasional regression of distal melanoma metastasis after adrenal metastasectomy. Ann Surg Oncol 15:1741–1749
Kuczyk M, Wegener G, Jonas U (2005) The therapeutic value of adrenalectomy in case of solitary metastatic spread originating from primary renal cell cancer. Eur Urol 48:252–257
Kim S, Brennan M, Russo P et al (1998) The role of surgery in the treatment of clinically isolated adrenal metastasis. Cancer 82:389–394
Porte H, Siat J, Guibert B et al (2001) Resection of adrenal metastases from non-small cell lung cancer: a multicenter study. Ann Thorac Surg 71:981–985
National Cancer Institute Surveillance Epidemiology and End Results (SEER) database. http://seer.cancer.gov/statistics. Accessed 31 July 2011
Mitchell IC, Nwariaku FE (2007) Adrenal masses in the cancer patient: surveillance or excision. Oncologist 12:168–174
Muth A, Persson F, Jansson S et al (2010) Prognostic factors for survival after surgery for adrenal metastasis. Eur J Surg Oncol 36:699–704
Elashry OM, Clayman RV, Soble JJ et al (1997) Laparoscopic adrenalectomy for solitary metachronous contralateral adrenal metastasis from renal cell carcinoma. J Urol 157:1217–1222
Bonnet S, Gaujoux S, Leconte M et al (2008) Laparoscopic adrenalectomy for metachronous metastasis from renal cell carcinoma. World J Surg 32:1809–1814. doi:10.1007/s00268-008-9539-3
Moinzadeh A, Gill IS (2005) Laparoscopic radical adrenalectomy for malignancy in 31 patients. J Urol 173:519–525
Sturgeon C, Kebebew E (2004) Laparoscopic adrenalectomy for malignancy. Surg Clin North Am 84:755–774
Conflicts of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vazquez, B.J., Richards, M.L., Lohse, C.M. et al. Adrenalectomy Improves Outcomes of Selected Patients with Metastatic Carcinoma. World J Surg 36, 1400–1405 (2012). https://doi.org/10.1007/s00268-012-1506-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-012-1506-3