Abstract
The diagnostic accuracy of dehydroepiandrosterone sulfate (DHEAS) to predict subclinical Cushing’s syndrome (sCS) has been a matter of debate. The primary objective of this study was to assess the diagnostic power of DHEAS in predicting sCS. This retrospective study was conducted in a tertiary referral center and based on subjects referred between 2004 and 2014. Data of 249 subjects with adrenal incidentalomas were evaluated. We also reviewed 604 DHEAS measurements from adults, which were performed during the same period in our laboratory (LB group). Adrenocortical function, tumor size, and clinical characteristics were assessed. We diagnosed sCS in 15.2 % of the participants in the presence of ≥2 of the following; 1 mg dexamethasone suppression test >3.0 μg/dl, urinary free cortisol >70 μg/24 h, and corticotrophin (ACTH) <10 pg/ml. DHEAS levels were significantly reduced in patients with sCS (n = 38) compared to sCS (−) (n = 141) and LB groups (n = 604) (27.95, 65.90, and 66.80 µg/dl, respectively, p < 0.001) while age was comparable. The ROC curve analysis showed that the cut-off of the DHEAS with the best diagnostic accuracy for detecting sCS was 40.0 μg/dl (SN, 68 %; SP, 75; PPV, 43 %; NPV, 90 %, AUC: 0.788, p < 0.001). Logistic regression assessed the impact of age, BMI, low DHEAS (<40 μg/dl), bilateral tumors, and tumor size on the likelihood of having sCS. The strongest predictor was low DHEAS, recording an OR of 9.41. DHEAS levels are inversely associated with the extent of cortisol excess. In subjects with intermediate laboratory findings, detection of low DHEAS could be advantageous for distinguishing sCS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Subclinical Cushing’s syndrome (sCS) is the most frequent endocrine dysfunction detected in subjects with incidentally discovered adrenal tumors [1, 2]. This term refers to autonomous cortisol secretion which is not associated with typical signs and symptoms of hypercortisolism, as in the overt Cushing’s syndrome (CS) [3]. However, an increased frequency of several metabolic problems including glucose intolerance, hypertension, central obesity, or osteoporosis has been described in patients with sCS since patients are exposed to slight albeit chronic cortisol excess [4, 5].
The diagnosis of sCS is difficult due to the lack of a single gold standard test. Many authors have proposed a variety of different criteria including 1 mg dexamethasone suppression test (DST) > 3 or 5 μg/dl, increased urinary free cortisol (UFC), suppressed corticotrophin (ACTH), or blunted response to corticotrophin-releasing hormone [6–9]. The current uncertainty on which diagnostic test(s) is best suited to define sCS depends on several factors including no or very mild signs of cortisol excess, the lack of sufficient sensitivity of the tests to recognize mild cortisol excess and the lack of a specific clinical picture of sCS.
Dehydroepiandrosterone sulfate (DHEAS) is an androgen precursor secreted by the zona reticularis under the dominant regulation of ACTH. It has been postulated that a low DHEAS level could indicate several hypothalamus-pituitary-adrenal (HPA) axis disturbances like adrenal insufficiency, chronic glucocorticoid exposure, or sCS due to the chronic suppression of ACTH [10]. However, data regarding the diagnostic accuracy of DHEAS to predict sCS have been perplexing. While several publications proposed low DHEAS as a predictor for sCS [11–13], some authors disagreed because of the inconsistent findings [14, 15] .
In this retrospective study, we sought to evaluate DHEAS measurements in patients with CS, sCS, and sCS (−) patients, and 604 consecutive adult individuals were tested in our laboratory to determine the association between DHEAS levels and the extent of cortisol excess. We also aimed to evaluate the diagnostic accuracy of DHEAS level to predict sCS.
Patients and methods
Patients
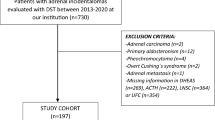
We retrospectively evaluated 249 subjects referred to Endocrinology Division of Dokuz Eylul University between January 2004 and March 2014 (Fig. 1). We excluded patients with pheochromocytoma (n = 7), adrenal cysts or adrenal myelolipomas (n = 18), adrenal metastasis (n = 12), primary hyperaldosteronism (n = 9), adrenocortical carcinoma (n = 5), missing data (n = 4), alcoholism (n = 1), malignancy requiring active therapy (n = 1), and those taking glucocorticoid drugs (n = 3).
Demonstration of the study group. 1 mg DST indicates 1 mg dexamethasone suppression test. Subclinical Cushing Syndrome is diagnosed in the presence of two out of the following three parameters: ACTH < 10 pg/; 1 mg DST > 3 µg/dL, and UFC > 70 µg/day. aExcluded due to alcoholism (n = 1), malignancy requiring active therapy (n = 1), treatment with glucocorticoid drugs (n = 3)
All adrenal masses were detected by ultrasonography, computed tomography (CT), or magnetic resonance imaging (MRI). Ultrasound findings were confirmed with CT or MRI.
Anthropometric characteristics included body weight, height, and blood pressure. Body mass index was calculated as [body weight (kg)]/[height (m2)]. Non-diabetic patients underwent an oral glucose tolerance (OGT) test with 75 g glucose and blood taken for measurements of glucose at 0 and 2 h.
All subjects underwent 24-h urine collections for the assessment of metanephrine and normetanephrine excretion. Aldosterone/plasma renin activity (PRA) was measured in subjects with hypokalemia or hypertension. Initial evaluation of cortisol secretion included baseline morning serum cortisol, ACTH, and DHEAS. All patients underwent a standard 1 mg DST.
Adrenal Cushing syndrome was defined as the ACTH-independent overt cortisol excess in a patient with multiple signs or symptoms of classic Cushing syndrome (e.g., striae rubrae, moon face, buffalo hump, skin atrophy, proximal myopathy, hypogonadism, or osteoporotic vertebral compression fractures). Pheochromocytoma was defined as the presence of significantly increased catecholamine excretion (higher than three times the upper reference limits). Primary hyperaldosteronism was diagnosed when PAC (ng/dl)/PRA (ng/ml/h) >25 and when intravenous salt loading (saline infusion test) revealed a non-suppressed PAC level (>10 ng/dl).
Adenomas were defined as well-circumscribed homogenous masses with a diameter of 10 mm or more. A Hounsfield unit cut-off of <10 in unenhanced CT scan or rapid washout of contrast medium in enhanced CT scan was used as indicating a benign imaging phenotype. The loss of signal on out-of-phase images in relation to spleen differentiated adenomas in MRI. The diagnosis of an adrenal myelolipoma was based on the presence of macroscopic fat on CT and T1-hyperintense signal that suppressed with frequency-selective fat saturation on MRI. An adrenal cyst was identified by fluid characteristics (≤20 HU on CT and/or high T2 signal on MRI) with thin walls that show no enhancement. Adrenal metastasis and 4 of 5 ACCs were identified by pathological examination. In 1 subject, ACC was diagnosed after the pathological examination of liver metastasis.
The diagnosis of sCS was made on the basis of at least two of the following criteria: 1 mg DST > 3.0 μg/dl, UFC > 70 μg/24 h, and ACTH < 10 pg/ml. The DST–UFC–ACTH criterion seems to be the reasonable combination to diagnose sCS because it was validated on a clinical basis [16, 17]. In our study, UFC was measured in subjects when the results of 1 mg DST and ACTH were discordant (1 mg DST > 3.0 μg/dl and ACTH > 10 pg/ml or 1 mg DST < 3.0 μg/dl and ACTH < 10 pg/ml). On the basis of these criteria, sCS was defined in 38 subjects.
Adrenalectomy was performed in patients with overt hormone excess, radiological aspects compatible with malignancy or significantly enlarging tumors. Additionally, adrenalectomy was recommended to patients with sCS when medical therapy did not reach treatment goals of associated diseases potentially linked to hypercortisolism.
Methods
In addition to the data of patients, we also retrospectively reviewed our laboratory’s DHEAS measurements performed between January 2004 and March 2014 with the same immunoassay (LB group). There were 604 relevant tests from adult subjects (505 females, 99 males, median age: 54 (19–89 years)). The leading indications for DHEAS measurements were assessment of adrenocortical function in suspected adrenocortical deficiency, evaluation of hirsutismus, routine premenopausal or climacteric hormonal evaluation or adrenal incidentaloma work-up. This data were utilized as a control group (LB group) for the comparison of DHEAS levels with sCS (+), sCS (−), and CS subjects.
Type 2 Diabetes Mellitus (T2DM) was defined using World Health Organization criteria [18] and/or when the patient was on anti-diabetic therapy. Arterial hypertension (AHT) was defined as the presence of systolic blood pressure ≥140 mm Hg, and/or diastolic blood pressure ≥90 mm Hg, or when the patient was on antihypertensive drug treatment [19].
The CT examination was performed using a 64-slice CT, and MR imaging was performed on a 1.5T scanner equipped with commercially available body coils in the prone position. All image data sets were evaluated by one experienced radiologist who was blinded to patients’ clinical status.
Serum cortisol levels were measured by a commercially available chemiluminescent microparticle immunoassay (Architect System, Abbott, USA). Reference range for morning cortisol level: 3.7−19.4 μg/dl; inter-assay and intra-assay coefficients of variation (CV) <10 %; and analytical sensitivity was <0.8 μg/dl. Plasma ACTH levels were measured by a commercially available chemiluminescent immunometric assay (Immulite 2000, Diagnostic Products Corporation, USA). Reference range for morning ACTH level: 10−60 pg/ml; inter-assay and intra-assay CVs <10 %; and analytical sensitivity was 5 pg/ml. Serum DHEAS levels were measured by a commercially available solid-phase, competitive, chemiluminescent enzyme immunoassay (Immulite 2000, Diagnostic Products Corporation, USA). Reference range for morning DHEAS level for females: 35–430 μg/dl, for males: 80–560 μg/dl; inter-assay and intra-assay CVs <10 %; and analytical sensitivity was 3 μg/dl. Urine free cortisol (normal: <70 μg/24 h), metanephrine (normal range: 52.0–341.0 μg/24 h), and normetanephrine (normal range: 88.0–444.0 μg/24 h) were measured by high-performance liquid chromatography.
Statistical analysis was performed by SPSS version 15.0 (SPSS Inc). The results are expressed as mean ± SD or median (min–max). Distribution of the variables was assessed by Kolmogorov–Smirnov test. Categorical variables were compared by χ 2 test. Continuous variables were compared among two groups using Independent Samples t test or Mann–Whitney U test according to the distribution of the variable. Among three or more groups, continuous variables were compared using Kruskal–Wallis test and Mann–Whitney U test with Bonferroni adjustment or one-way ANOVA. Standard multiple regression analysis was performed to identify the predictors of DHEAS levels in sCS (+) and sCS (−) subjects. The model included age, 1 mg DST cortisol level, and tumor size as independent variables. Direct logistic regression was performed in sCS (+) and sCS (−) subjects to assess the impact of age, BMI, low DHEAS (<40 μg/dl), bilateral tumors, and tumor size on the likelihood of having sCS. The receiver operating characteristic (ROC) curve analysis assessed the cut-off of DHEAS with the best diagnostic accuracy for detecting sCS.
Results
Characteristics of the subjects in the database
General characteristics of the subjects in adrenal incidentaloma database are shown in Table 1. The majority of the referred patients were females (74.9 %). Ratio of male to female was significant in subjects with adrenal metastasis. Patients with ACCs had larger masses compared to those with benign tumors. Patients with pheochromocytomas or non-adenomatous benign tumors were younger at presentation compared to subjects with other tumor types.
Retrospective evaluation of relevant DHEAS measurements in adults
The number of relevant tests performed between January 2004 and March 2014 was 604. DHEAS levels showed a non-normal distribution (Kolmogorov–Smirnov, p < 0.001). Median of DHEAS was 66.80 μg/dl (2.6–822.1 μg/dl), and age was 54 y (19–89y). There was a strong negative correlation between age and DHEAS levels (r:−0.504, p < 0.001). There were 99 tests from male subjects and 505 tests from female subjects. Males were significantly older than females (58.29 ± 12.27 vs. 49.67 ± 13.80, p < 0.001, Independent Samples t test). DHEAS measurements from male subjects were slightly lower than those from females (56.7 μg/dl (2.6–419.9 μg/dl) vs. 68.4 μg/dl (2.8–822.1 μg/dl), p = 0.103, Mann–Whitney U test).
Impact of hypercortisolism on DHEAS levels
At first, we compared age and DHEAS levels between CS, sCS (+), sCS (−), and LB groups to evaluate the impact of different levels of cortisol excess on DHEAS levels. Subjects in CS (n = 10) and sCS (+) (n = 38) groups had significantly lower levels of DHEAS when compared to those in sCS (−) (n = 141) and LB (n = 604) while age was comparable (median DHEAS 16.10 (12.0–89.1) μg/dl, 27.95 (9.4–128.0) μg/dl, 69.90 (14.0–363.0) μg/dl and 66.8 (2.6–822.1) μg/dl, respectively; χ 2 (3, n = 793) = 44.64, p < 0.001, Kruskal–Wallis Test). There was no significant difference between CS and sCS (+) groups or sCS (−) and LB groups in terms of DHEAS levels (Fig. 2).
a Association between cortisol excess and DHEAS levels. Median DHEAS level is represented by black bar, and median age is represented by gray bar (age and DHEAS had non-normal distribution, Kolmogorov–Smirnov test, p < 0.001). Error bars represent 95 % confidence interval. DHEAS levels are significantly and inversely related with the level of cortisol excess while age was comparable between groups; CS (n = 10), sCS (+) (n = 38), sCS (−) (n = 141), and LB (n = 604). (Kruskal–Wallis test and Mann–Whitney U test with Bonferroni adjustment were used) *p < 0.001 versus LB # p < 0.001 versus sCS(−). CS Cushing syndrome, sCS subclinical Cushing syndrome, LB laboratory DHEAS data b Distribution of subjects in each group with respect to DHEAS levels. Reference line indicates 40 μg/dl
Characteristics of the subjects in sCS (+) and sCS (−) groups are presented in Table 2. No significant difference in age, male–female ratio, number of bilateral adenomas, or BMI was observed between patients with sCS and those without. Patients with sCS had significantly larger adenomas compared to sCS (−) subjects (30 (20–60) mm vs. 21 (10–70) mm, p < 0.001). Significant differences were observed in ACTH, 1 mg DST, and UFC levels, related with sCS definition. The presence of sCS was associated with a tendency for morning cortisol to be increased but the difference was not significant (16.0 ± 4.6 vs. 14.3 ± 5.5, p = 0.078). In patients with sCS, prevalence of T2DM (31.5 %) and AHT (67.5 %) was increased when compared to subjects without (17.8 %, p = 0.074, 43.8 %, p = 0.01, respectively). DHEAS levels were significantly reduced in sCS (+) patients compared to sCS (−) subjects (27.95 (9.4–128.0) μg/dl vs. 65.90 (14.0–363.0) μg/dl, p < 0.001, Mann–Whitney U test).
Additionally, we stratified the subjects according to suppression of 1 mg DST cortisol level as follows: Group S (<1.8 μg/dl), n = 115; Group I (1.8–3.0 μg/dl), n = 24 and Group N (≥3.0 μg/dl), n = 40. Subjects in Group I and N had significantly low DHEAS levels when compared to Group S (p < 0.001, Kruskal–Wallis test and Mann–Whitney U test with Bonferroni adjustment), while age was comparable (p = 0.063, one-way ANOVA and post Hoc analysis with Tukey) (Fig. 3).
Association between 1 mg DST cortisol and DHEAS levels in sCS (+) and sCS (−) patients. Median DHEAS level is represented by black bar, and median age is represented by gray bar. DHEAS levels are significantly related with the magnitude of cortisol suppression after DST while age was comparable between groups; Group N (n = 40), Group I (n = 24), and Group S (n = 115). Age and DHEAS had non-normal distribution, Kolmogorov–Smirnov test, p < 0.001; Kruskal–Wallis test and Mann–Whitney U test with Bonferroni adjustment were used. *p < 0.001 versus Group S, error bars represent 95 % confidence interval
Standard multiple regression analysis was performed to identify the predictors of DHEAS levels in sCS (+) and sCS (−) subjects. The model included age, 1 mg DST cortisol level, and tumor size as independent variables (model r2 = 0.145, p < 0.001). 1 mg DST cortisol (beta = −0.324, p < 0.001, 95 % CI for beta:−8.244–3.122) recorded a higher beta value when compared to age (beta = −0.216, p = 0.002, 95 % CI for beta:−1.926–0.409) and tumor size (beta = −0.044, p = 0.494, 95 % CI for beta:−1.028–0.553).
The ROC curve analysis (Fig. 4) including sCS(+) and sCS(−) subjects showed that the cut-off of the DHEAS level with the best diagnostic accuracy for detecting sCS was 40.0 μg/dl (sensitivity, 68 %; specificity, 75 %; positive predictive value, 43 %; negative predictive value, 90 %; AUC: 0.788, p < 0.001).
Direct logistic regression was performed in sCS (+) and sCS (−) subjects to assess the impact of age, BMI, low DHEAS (<40 μg/dl), bilateral tumors, and tumor size on the likelihood of having sCS. As shown in Table 3, only low DHEAS and tumor size made a significant contribution to the model. The strongest predictor of having sCS was low DHEAS, recording an odds ratio of 9.41.
Discussion
In our population of subjects with adrenal adenomas, we observed that DHEAS level was negatively associated with the extent of hypercortisolism. DHEAS level in sCS (+) patients was significantly decreased compared to age-matched sCS (−) subjects and to those from our laboratory database. Low DHEAS level (<40 μg/dl) predicted sCS significantly (Fig. 4).
The reduction of DHEAS was described as the most frequent HPA axis abnormality in subjects with adrenal incidentalomas. However, the inverse association between DHEAS level and cortisol excess has been demonstrated in several [1, 7, 8, 20, 21], but not all [14, 22] studies. Therefore, the predictive value of low DHEAS for sCS has been a matter of debate. The physiological decline of DHEAS with aging contributes to this uncertainty. Consistent with the literature, age was an important predictor of DHEAS level in our study. Nevertheless, subtle but autonomous cortisol production and the resulting inhibition of ACTH appear to be responsible for the reduction in DHEAS levels in sCS (+) subjects. Three findings accentuated the impact of autonomous cortisol production on DHEAS levels beyond the well-known effect of aging. First, subjects with sCS had significant low levels of DHEAS when compared to age-matched sCS (−) and LB individuals. Second, patients with CS featured with the lowest DHEAS levels despite younger age. Finally, 1 mg DST cortisol level predicted DHEAS better than age.
In our study, some patients classified as sCS (−) might have subtle cortisol excess because of the uncertainty in sCS definition. Therefore, we stratified the sCS (+) and sCS (−) subjects into three groups with respect to 1 mg DST cortisol level. Despite the arbitrary criteria of sCS, excluding cortisol hypersecretion is more straightforward. 1 mg DST cortisol level <1.8 μg/dl can clearly exclude autonomous cortisol secretion. We observed that subjects with non-suppressed 1 mg DST cortisol levels (both >3 μg/dl and 1.8–3.0 μg/dl) had significantly low DHEAS levels. This was independent from the impact of age. Subjects with subtle cortisol excess (1 mg DST cortisol between 1.8 and 3.0 μg/dl) but not classified as sCS had also lower DHEAS levels. This finding also supported the impact of cortisol excess on DHEAS levels.
To the best of our knowledge, data regarding the diagnostic accuracy of DHEAS for the prediction of sCS have been inadequate and obscure. Several guidelines from different associations have indicated that low DHEAS could support the diagnosis of sCS [11, 13]. However, because of the diversity in sCS criteria, the small number of patients enrolled or age discrepancies in study groups; a cut-off value has not been established. In this study, we defined sCS according to the DST–UFC–ACTH combination criterion as it has been validated on a clinical basis [16, 17]. DHEAS cut-off value <40 μg/dl showed the best balance between sensitivity (68 %) and specificity (75 %), reaching an acceptable accuracy (73.7 %) in predicting sCS. Our results demonstrated that low DHEAS had comparable sensitivity, specificity, and accuracy when compared to the DST–UFC–ACTH combination.
It has been demonstrated that increased tumor size, bilateral tumors, and obesity were more common in patients with sCS [23, 24]. Therefore, we evaluated the predictive value of DHEAS and these clinical findings in the same regression model. Low DHEAS was the sole predictor of sCS in this model with a significant OR.
Our findings may contribute to the diagnostic work-up in subjects with adrenal incidentalomas. There is not a single gold standard test for sCS, and most of the proposed tests share similar accuracy problems. UFC cannot successfully reveal slight cortisol excess, and technical problems during collection, storage, or analysis could hamper diagnostic reliability [25, 26]. Several previous studies reported an altered circadian cortisol secretion rhythm in subjects with adrenal incidentalomas with high midnight cortisol levels [9, 12, 15, 25]. Midnight serum cortisol correlates with clinical conditions better than other tests, but its use is limited by the need of the hospital admission [9]. The use of midnight salivary cortisol seems to be less expensive and more feasible for screening Cushing’s Syndrome [27] but its routine use in sCS is still debated [28]. Some authors showed a low sensitivity in identifying sCS [29, 30], while others demonstrated that subjects with elevated midnight serum cortisol had sCS [31]. Besides, the various studies on the diagnostic accuracy of the different salivary cortisol assays are hardly comparable for differences in the patients’ and controls’ selection criteria and in the diagnostic performance and sample collection techniques of the various laboratory methods [16, 32]. Technical problems in ACTH assays [33] and the ongoing debate on the cut-off values of 1 mg DST cortisol level [34–39] complicate hormonal work-up. Because of the limitations of the proposed diagnostic methods, DHEAS measurement can be practical as a secondary test in selected patients who present with slight hormonal abnormalities (1 mg DST cortisol level between 1.8 and 3 μg/dl and ACTH level 10–15 pg/ml) or when DST–UFC–ACTH levels were discordant (1 mg DST cortisol level >3 μg/dl and ACTH level >10 pg/ml). Low DHEAS levels in such intermediate patients could be advantageous for the detection of autonomous cortisol secretion. DHEAS does not follow a circadian rhythm and has a long half life (10–20 h) [10]. These make a single measurement of DHEAS practical and reliable.
This study has several limitations. Although we used a reliable definition of sCS, the gold standard has yet to be determined. The accuracy of DHEAS may change related with the definition of sCS. The design of the study did not allow us to obtain prospective data about the change in DHEAS levels and HPA axis tests. Additionally, the small number of subjects with sCS may affect the power of results demonstrating the diagnostic accuracy of DHEAS.
In conclusion, this study provides two important findings. First, in subjects with adrenal incidentalomas, DHEAS levels are clearly associated with the levels of cortisol excess. Second, in subjects with intermediate laboratory findings, DHEAS measurements could be advantageous for distinguishing sCS.
References
M. Terzolo, G. Osella, A. Ali, Subclinical Cushing’s syndrome in adrenal incidentaloma. Clin. Endocrinol. (Oxf). 48, 89–97 (1998)
D.A. Vassiliadi, S. Tsagarakis, Subclinical hypercortisolism: debatable or visible on the lightbox? Endocrine 42, 7–8 (2012)
A. Colao, M. Boscaro, D. Ferone, F.F. Casanueva, Managing Cushing’s disease: the state of the art. Endocrine (2014). doi:10.1007/s12020-013-0129-2
V. Morelli, C. Eller-Vainicher, A.S. Salcuni, Risk of new vertebral fractures in patients with adrenal incidentaloma with and without subclinical hypercortisolism: a multicenter longitudinal study. J. Bone Miner. Res. 26, 1816–1821 (2011)
W.F. Young Jr, Management approaches to adrenal incidentalomas. A view from Rochester, Minnesota. Endocrinol. Metab. Clin. North Am. 29, 159–185 (2000)
I. Chiodini, V. Morelli, A.S. Salcuni, Beneficial metabolic effects of prompt surgical treatment in patients with an adrenal incidentaloma causing biochemical hypercortisolism. J. Clin. Endocrinol. Metab. 95, 2736–2745 (2010)
G. Osella, M. Terzolo, G. Borretta, Endocrine evaluation of incidentally discovered adrenal masses (incidentalomas). J. Clin. Endocrinol. Metab. 79, 1532–1539 (1994)
R. Rossi, L. Tauchmanova, A. Luciano, Subclinical Cushing’s syndrome in patients with adrenal incidentaloma: clinical and biochemical features. J. Clin. Endocrinol. Metab. 85, 1440–1448 (2000)
M. Terzolo, S. Bovio, A. Pia, Midnight serum cortisol as a marker of increased cardiovascular risk in patients with a clinically inapparent adrenal adenoma. Eur. J. Endocrinol. 153, 307–315 (2005)
S. Fischli, S. Jenni, S. Allemann, Dehydroepiandrosterone sulfate in the assessment of the hypothalamic-pituitary-adrenal axis. J. Clin. Endocrinol. Metab. 93, 539–542 (2008)
Y. Akehi, H. Kawate, K. Murase, Proposed diagnostic criteria for subclinical Cushing’s syndrome associated with adrenal incidentaloma. Endocr. J. 60, 903–912 (2013)
T. Katabami, R. Obi, N. Shirai, S. Naito, N. Saito, Discrepancies in results of low-and high-dose dexamethasone suppression tests for diagnosing preclinical Cushing’s syndrome. Endocr. J. 52, 463–469 (2005)
M.A. Zeiger, G.B. Thompson, Q.Y. Duh, The American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr. Pract. 15(Suppl 1), 1–20 (2009)
Z. Bencsik, I. Szabolcs, Z. Kovacs, Low dehydroepiandrosterone sulfate (DHEA-S) level is not a good predictor of hormonal activity in nonselected patients with incidentally detected adrenal tumors. J. Clin. Endocrinol. Metab. 81, 1726–1729 (1996)
A. Tanabe, M. Naruse, T. Nishikawa, Autonomy of cortisol secretion in clinically silent adrenal incidentaloma. Horm. Metab. Res. 33, 444–450 (2001)
I. Chiodini, Clinical review: diagnosis and treatment of subclinical hypercortisolism. J. Clin. Endocrinol. Metab. 96, 1223–1236 (2011)
V. Morelli, B. Masserini, A.S. Salcuni, Subclinical hypercortisolism: correlation between biochemical diagnostic criteria and clinical aspects. Clin. Endocrinol. (Oxf). 73, 161–166 (2010)
Diagnosis and classification of diabetes mellitus, Diabetes Care 27(Suppl 1), S5–S10 (2004)
J. Perk, G. De Backer, H. Gohlke, European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur. Heart J. 33, 1635–1701 (2012)
H. Morio, T. Terano, K. Yamamoto, Serum levels of dehydroepiandrosterone sulfate in patients with asymptomatic cortisol producing adrenal adenoma: comparison with adrenal Cushing’s syndrome and non-functional adrenal tumor. Endocr. J. 43, 387–396 (1996)
S. Tsagarakis, C. Roboti, P. Kokkoris, V. Vasiliou, C. Alevizaki, N. Thalassinos, Elevated post-dexamethasone suppression cortisol concentrations correlate with hormonal alterations of the hypothalamo-pituitary adrenal axis in patients with adrenal incidentalomas. Clin. Endocrinol. (Oxf). 49, 165–171 (1998)
R. Giordano, E. Marinazzo, R. Berardelli, Long-term morphological, hormonal, and clinical follow-up in a single unit on 118 patients with adrenal incidentalomas. Eur. J. Endocrinol. 162, 779–785 (2010)
H. Olsen, E. Nordenstrom, A. Bergenfelz, U. Nyman, S. Valdemarsson, E. Palmqvist, Subclinical hypercortisolism and CT appearance in adrenal incidentalomas: a multicenter study from Southern Sweden. Endocrine 42, 164–173 (2012)
D.A. Vassiliadi, G. Ntali, E. Vicha, S. Tsagarakis, High prevalence of subclinical hypercortisolism in patients with bilateral adrenal incidentalomas: a challenge to management. Clin. Endocrinol. (Oxf). 74, 438–444 (2010)
C. Eller-Vainicher, V. Morelli, A.S. Salcuni, Accuracy of several parameters of hypothalamic-pituitary-adrenal axis activity in predicting before surgery the metabolic effects of the removal of an adrenal incidentaloma. Eur. J. Endocrinol. 163, 925–935 (2010)
N. Valli, B. Catargi, N. Ronci, Biochemical screening for subclinical cortisol-secreting adenomas amongst adrenal incidentalomas. Eur. J. Endocrinol. 144, 401–408 (2001)
H. Raff, Update on late-night salivary cortisol for the diagnosis of Cushing’s syndrome: methodological considerations. Endocrine 44, 346–349 (2013)
T.L. Mazzuco, I. Bourdeau, A. Lacroix, Adrenal incidentalomas and subclinical Cushing’s syndrome: diagnosis and treatment. Curr. Opin. Endocrinol. Diabetes Obes. 16, 203–210 (2009)
B. Masserini, V. Morelli, S. Bergamaschi, The limited role of midnight salivary cortisol levels in the diagnosis of subclinical hypercortisolism in patients with adrenal incidentaloma. Eur. J. Endocrinol. 160, 87–92 (2009)
M.L. Nunes, S. Vattaut, J.B. Corcuff, Late-night salivary cortisol for diagnosis of overt and subclinical Cushing’s syndrome in hospitalized and ambulatory patients. J. Clin. Endocrinol. Metab. 94, 456–462 (2009)
S. Kidambi, H. Raff, J.W. Findling, Limitations of nocturnal salivary cortisol and urine free cortisol in the diagnosis of mild Cushing’s syndrome. Eur. J. Endocrinol. 157, 725–731 (2007)
M. Sereg, J. Toke, A. Patocs, Diagnostic performance of salivary cortisol and serum osteocalcin measurements in patients with overt and subclinical Cushing’s syndrome. Steroids 76, 38–42 (2010)
C. Invitti, F. Pecori Giraldi, M. de Martin, F. Cavagnini, Diagnosis and management of Cushing’s syndrome: results of an Italian multicentre study. Study Group of the Italian Society of Endocrinology on the Pathophysiology of the Hypothalamic-Pituitary-Adrenal Axis. J. Clin. Endocrinol. Metab. 84, 440–448 (1999)
I. Chiodini, V. Morelli, B. Masserini, Bone mineral density, prevalence of vertebral fractures, and bone quality in patients with adrenal incidentalomas with and without subclinical hypercortisolism: an Italian multicenter study. J. Clin. Endocrinol. Metab. 94, 3207–3214 (2009)
M.M. Grumbach, B.M. Biller, G.D. Braunstein, Management of the clinically inapparent adrenal mass (“incidentaloma”). Ann. Intern. Med. 138, 424–429 (2003)
L.K. Nieman, B.M. Biller, J.W. Findling, The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 93, 1526–1540 (2008)
M. Reincke, J. Nieke, G.P. Krestin, W. Saeger, B. Allolio, W. Winkelmann, Preclinical Cushing’s syndrome in adrenal “incidentalomas”: comparison with adrenal Cushing’s syndrome. J. Clin. Endocrinol. Metab. 75, 826–832 (1992)
A. Tabarin, S. Bardet, J. Bertherat, Exploration and management of adrenal incidentalomas French Society of Endocrinology Consensus. Ann. Endocrinol. (Paris) 69, 487–500 (2008)
M. Terzolo, A. Stigliano, I. Chiodini, AME position statement on adrenal incidentaloma. Eur. J. Endocrinol. 164, 851–870 (2011)
Disclosure
The authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yener, S., Yilmaz, H., Demir, T. et al. DHEAS for the prediction of subclinical Cushing’s syndrome: perplexing or advantageous?. Endocrine 48, 669–676 (2015). https://doi.org/10.1007/s12020-014-0387-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-014-0387-7