Abstract
Aim
To analyze if the 1mg-dexamethasone suppression test (DST) is a reliable marker of glucocorticoid excess and cardiometabolic risk in patients with adrenal incidentalomas (AIs).
Methods
Cross-sectional study of patients with nonfunctioning adrenal incidentalomas (NFAIs, defined by cortisol post-DST ≤ 1.8 µg/dL) and patients with autonomous cortisol secretion (ACS, defined by cortisol post-DST > 1.8 µg/Dl). The urinary steroid profile (USP) was determined by gas chromatography coupled to mass spectrometry. Both groups were matched by sex, age and body mass index.
Results
Forty-nine patients with AIs (25 with ACS and 24 with NFAI) were included. As a whole, AIs showed a high excretion of β-cortolone, tetrahydro-11-deoxycortisol (THS), α-cortolone, α-cortol, tetrahydrocortisol (THF) and tetrahydrocortisone (THE). A positive yet modest correlation between post-DST cortisol and total excretion of glucocorticoid metabolites (r = 0.401, P = 0.004) was observed, with the stronger being observed with total THS (r = 0.548, P < 0.001) and THF (r = 0.441, P = 0.002). Some of the metabolites that were elevated in patients with AIs, were higher in patients with ACS-related comorbidities than in those without comorbidities. Post-DST cortisol showed a fair diagnostic accuracy for the prediction of ACS-related comorbidities (AUC 0.767 [95% CI 0.634–0.882]). However, post-DST diagnostic accuracy improved when combined with urinary cortisone, α-cortol, THS and serum DHEAS (0.853 [0.712‒0.954]).
Conclusion
The DST has a positive, but modest, correlation with urinary glucocorticoid excretion. Similarly, the diagnostic accuracy of the DST for the prediction of ACS-related comorbidities is only fair, but it may be improved if combined with the results of the USP and serum DHEAS.
Significance statement
This is the first study aimed to evaluate if 1mg-dexamethasone suppression test (DST) is a reliable marker of glucocorticoid excess and cardiometabolic risk in patients with adrenal incidentalomas (AIs) and if urinary steroid profile was measured by GS-MS could improve such a prediction. We found a positive yet modest correlation between post-DST cortisol and total excretion of glucocorticoid metabolites, with the stronger being observed with total tetrahydro-11-deoxycortisol (THS) and tetrahydrocortisol. Post-DST cortisol showed a fair diagnostic accuracy for the prediction of ACS-related comorbidities (AUC 0.767). However, post-DST diagnostic accuracy improved when combined with urinary cortisone, α-cortol, THS and serum DHEAS (0.853).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adrenal incidentalomas (AIs) are one of the most frequent reasons for consultation in Endocrinology, and due to the growing use of imaging techniques, their prevalence has increased markedly [1]. The prevalence of AIs is age-dependent, ranging from 3% at age 50–10% in adults over age 70 [2].
Most AIs are classified as nonfunctioning adrenal incidentalomas (NFAIs) or as AIs with associated autonomous cortisol secretion (ACS) [3]. Both conditions, ACS and NFAI, have been associated with an increased cardiometabolic risk, including a higher risk of developing diabetes mellitus, hypertension, obesity, osteoporosis, and mortality [4,5,6]. Serum cortisol concentrations after the 1 mg-dexamethasone suppression test (DST) is considered the best test to differentiate patients without apparent glucocorticoid excess [NFAI] and those with subtle overproduction of glucocorticoid [ACS] [3]. Although some authors described a linear positive association between mortality and post-DST cortisol (at values below 200 nmol/L) [6], other studies suggested that the post-DST cortisol is an unreliable marker of comorbidities in patients with AIs [7, 8]. Thus, other biomarkers such as urinary steroid profile (USP) could be used to identify subtle grades of glucocorticoid excess that might associate increased cardiometabolic risk in patients with AIs [9]. To our best knowledge, no previous study has analyzed if post-DST cortisol concentrations correlate with USP, in particular with urinary glucocorticoid excretion as measured by gas chromatography-mass spectrometry (GC-MS).
Hence, we here aimed to evaluate if the DST is a reliable marker of glucocorticoid excess in patients with AIs—and may also predict ACS-related comorbidities—in patients with AIs, and if USP could improve such a prediction.
Material and methods
Patients
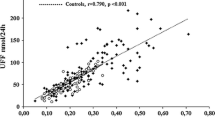
This was a cross-sectional study of patients with NFAIs and patients with AIs and ACS. We prospectively enrolled 25 adult patients in each group. These patients were evaluated at the Endocrine outpatient clinic between November 2019 and January 2020. Both groups were matched by age (±5 years old), sex (±10% in frequency in each group) and body mass index (BMI) (±5 kg/m2). A previous study on this cohort plus 25 healthy-adrenal controls evaluated differences in USP between groups [10].
Patients with unilateral and/or bilateral AIs ≥1 cm were recruited, provided that no exclusion criteria were present. As described earlier [10], the following exclusion criteria were applied: i) known diagnosis of hereditary syndromes associated with adrenal conditions; ii) chronic treatment with glucocorticoids or drugs that might affect dexamethasone metabolism; iii) treatment with oral hormonal contraceptives; iv) impossibility to collect 24-h urine specimens and/or creatinine clearance <45 ml/min/1.73 m2 (estimated by the MDRD-4 formula); v) active malignant disease or overt hormonal hyper or hyposecretory disorders; vi) AIs identified during the extension study of an extra-adrenal primary cancer [11]; vii) patients with Cushing´s syndrome; primary aldosteronism; hyperandrogenism; pheochromocytoma; or congenital adrenal hyperplasia, viii) adrenocortical carcinoma; ix) adrenal metastasis from extra-adrenal tumors; and x) radiological features suspicious for malignancy (necrosis, irregular margins or tumors larger than 6 cm without characteristics of typical adenoma or myelolipoma); and xi) AIs not meeting the ACS or NFAI criteria [11].
The study was approved by Hospital Universitario Ramón y Cajal’s Ethics Committee (date of approval on November 12th, 2019, ACTA 374). All participants signed written informed consent.
Clinical and hormonal evaluation
At inclusion, demographic information (age and sex) and the presence/absence of ACS-related comorbidities were registered. We considered as ACS-related comorbidities the presence of hypertension, type 2 diabetes (T2DM), obesity, dyslipidemia, cerebrovascular and cardiovascular disease. Physical examination included BMI, systolic and diastolic blood pressure. Comorbidities were defined following the same criteria that we previously described [10].
The hormonal evaluation was based on the determination of 24h-urinary free cortisol (UFC), catecholamines and metanephrines concentrations, serum post-DST cortisol concentrations, DHEA-S,,early morning plasma ACTH concentrations, late night salivary cortisol concentrations and serum aldosterone and renin concentrations. Cortisol was measured in serum and urine by immunochemiluminescence assays in an Architect i2000 Abbott Diagnostics platform, with an intra-assay coefficient of variation (CV) < 10%; the reference range was 102–535 nmol/L (3.7–19.4 µg/dl) for serum cortisol and less than 3862 nmol/24 h (140 µg/24 h) for 24-h urine cortisol. Plasma ACTH was measured by immunochemiluminescence assays (Liaison XL, Diasorin), with an intra-assay CVs < 10%, with a reference range of 1.0–10.7 pmol/L (4.7–48.8) pg/ml. DHEAS was measured by chemiluminescence assay in Immulite 2000 Siemens system before March 2020, and then by Advia Centaur XP Siemens; with intra-assay CV < 15%. Reference ranges for DHEAS were age- and sex-specific. Women: 18–24 years 150–3402 ng/ml; 25–34 years 150–2982 ng/ml; 35–49 years 150–2582 ng/ml; 50–59 years 260–2000 ng/ml; 60–69 years 130–1300 ng/ml; and 70–79 years 170–900 ng/ml. Men: 20–29 years 2800–6400 ng/ml; 30–39 years 1200–5200 ng/ml; 40–49 years 950–5300 ng/ml; 50–59 years 700–3100 ng/ml; 60–69 years 420–2900 ng/ml; and 70–79 years 280–1750 ng/ml. Late night-salivary cortisol was measured by electroimmunochemiluminescence in a Cobas 6000 Roche autoanalyzer, with an intra-assay CV < 10% and was considered abnormal if ≥5.74 µg/dL (158.34 nmol/L).
Urinary steroid profile
The USP was analyzed both in total and free (unconjugated) fractions of 24 h urine specimens. The procedures applied are based on the methodology described by Shackleton et al. [12, 13]. The reference and internal standards, as well as other details about the determination of USP, have been described earlier [14,15,16]. Gas chromatography-mass spectrometry (GC-MS) analyses were performed on a Shimadzu GCMS-QP2010 Ultra instrument (Kyoto, Japan). The reference ranges for total steroids are described in Table 1. The reference range for GC-MS analysis for urinary free cortisol was 13–64 µg/24 h and for free cortisone was 9–80 µg/24 h.
Adrenal enzyme activity was quantified based on the calculation of the ratios of the ratios of the different steroid metabolites.
Data management and statistical analysis
Data were collected and managed using REDCap® electronic data capture tools hosted at Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS) [17, 18].
Statistical analyses used STATA 15.0. The quantitative differences between two groups were analyzed by Student’s t. Qualitative differences were analyzed with the χ2 test and/or logistic regression. Linear correlations between post-DST cortisol and USP used Pearson’s correlation coefficient (r). The diagnostic accuracy of post-DST cortisol, USP and the combination of post-DST cortisol and USP, was determined using receiver operating characteristics curve (ROC) analysis. Statistical significance required a two-tailed p < 0.05.
Results
Patients
Of the 50 patients initially enrolled in the study, 1 patient in the NFAI group was excluded after a diagnosis of non-classical congenital adrenal hyperplasia was made during follow-up. The baseline characteristics of both groups are described in Table 2 as we have previously described [10]. Although we found a higher prevalence of T2DM in the group of patients with ACS than NFAI, no differences were detected in HbA1c and glucose levels since 85.7% of the patients with diabetes had HbA1c < 7% and all of them were under hypoglycemic treatment.
Urinary glucocorticoid excretion in patients with NFAI and patients with ACS
When considering all patients with AIs as a whole, the percentages of patients with increased excretion of urinary steroids were 65.3% for β-cortolone, 55.1% for THS, 53.1% for α-Cortolone, 28.6% for α-cortol, 24.5% for THF, 20.4% for THE, 16.3% for 11-oxo-Etiocolanolone, 10.4% for total cortisol, 10.2% for cortisone, 8.2% for β-cortol and 8.2% for 11β-OH-Etiocholanolone. Regarding urinary free fractions, 15.7% of patients showed increased UFC and 56.3% of them showed increased free cortisone levels. The proportion of patients with increased concentrations of these metabolites was similar in patients with ACS and in patiens with NFAI, except for β-cortol concentrations that were more commonly elevated in patients with ACS than in those with NFAI (16% vs 0%, P = 0.041) (Table 3).
Correlation between serum post-DST cortisol concentrations and urinary glucocorticoid metabolites
Overall, we did not find any correlation between post-DST cortisol and the excretion of total glucocorticoids. However, positive correlations were found between serum post-DST cortisol levels and urinary THF, THE, cortisol and cortisone levels. These correlations were stronger in patients with ACS, whereas some of them lost statistical significance when NFAI patients were considered separately from those with ACS (Table 4).
We did find a positive yet modest correlation between post-DST cortisol levels and total urinary concentrations of precursors of glucocorticoids. This correlation was due to the specific metabolite THS. Again, the correlation of serum post-DST cortisol with urinary THS was stronger in patients with ACS (r = 0.562; p < 0.004) than in patients with NFAI (r = 0.391; p = 0.059) (Table 4).
When we tested the correlations between serum post-DST cortisol and the activity of the main enzymes involved in adrenal steroidogenenic pathways, we found a modest positive correlation between serum post-DST cortisol and 11β-hydroxylase activity (r = 0.387, P = 0.006). The total excretion of precursors of glucocorticoids presented a strong correlation with the activity of 11β-hydroxylase (r = 0.711, P < 0.001) and with those of other enzymes involved in the glucocorticoid synthesis pathway (Table 5).
Association between serum post-DST cortisol and urinary glucocorticoid excretion with cardiometabolic comorbidities
Dyslipidemia was more prevalent in patients with increased urinary THS levels than in those showing normal THS levels (81.5% vs. 54.6%, respectively, P = 0.042). Also, patients presenting with increased urinary total cortisol excretion showed a larger prevalence of diabetes compared with patients with normal urinary total cortisol excretion (60.0% vs. 18%, respectively, P = 0.037). No other differences were observed in the prevalence of comorbidities according to USP.
We found that some of the metabolites usually elevated in patients with AIs (THS and cortisone) were also increased in patients with ACS-related comorbidities compared with those lacking these comorbidities (Table 6). In addition, serum post-DST cortisol levels were higher in the group of patients with comorbidities than in those without any comorbidity (3.3 ± 2.55 vs. 1.5 ± 0.75 µg/dL, respectively, P = 0.001). Patients with comorbidities were older than those without them (71.0 ± 7.92 vs. 60.3 ± 6.96, P < 0.001). However, no statistically significant correlation was observed between age and the excretion of total glucocorticoids (data not shown).
Among the single analytes studied here, serum post-DST cortisol concentrations showed the best diagnostic accuracy for the prediction of ACS-related comorbidities (AUC 0.767 [95CI 0.634‒0.882]), followed by urinary cortisone (AUC 0.671 [95CI 0.525‒0.801]) and THS (AUC 0.622 [95CI 0.462‒0.748]). However, the combination of serum post-DST cortisol concentrations with urinary cortisone, α-cortol and THS had the highest diagnostic accuracy for the diagnosis of ACS-related comorbidities (AUC 0.813 [95CI 0.680‒0.912]), although no statistically significant differences were found between the areas under the ROC curves of both models (P = 0.291) (Fig. 1). The AUC-ROC when DHEAS levels were included in the model increased to 0.853 [0.712‒0.954].
Discussion
The main findings of this study were the following: i) both, ACS and NFAI were characterized by a high urinary excretion of some glucocorticoid metabolites, although a high b-cortol excretion was more frequent in patients with ACS but not in patients with NFAI; ii) serum post-DST cortisol concentrations and glucocorticoid excretion presented modest positive correlations; iii) some of the metabolites that are usually elevated in patients with AIs were higher in patients with ACS-related comorbidities than in those without comorbidities; and iv) the diagnostic accuracy of serum post-DST cortisol concentrations increased from 0.77 when used as a single test, to 0.81 when combined with urinary cortisone, α-cortol and THS levels.
The urinary excretion pattern observed in patients with AIs revealed a large percentage of patients with elevated THS, a metabolite of 11-deoxycortisol—which is the immediate precursor of cortisol synthesis—and also large percentages of patients showing elevations of the most abundant inactive cortisol catabolites (THFs, THE, cortols and cortolones). This is in contrast with a lower proportion of patients presenting with elevated total cortisol excretion.
Overall, the observed pattern suggests an activation in both the synthesis and catabolism of cortisol. This is further supported by the ratios of steroid precursors that may indicate an increase in the activities of the main enzymes involved in cortisol synthesis (21-hydroxylase, 11β-hydroxylase and 3β-hydroxysteroid dehydrogenase). These findings are in accordance with the reports of other authors [19, 20] suggesting that both ACS and NFAI associate excessive glucocorticoid excretion. Hence, these results further supports, a change in the current terminology of “non-functioning adrenal incidentaloma”, i.e. to “Adrenal Lesions of Undetermined Secretion of Adrenal Steroids” as we have recently proposed [21].
Of note, we did not observe a different USP in patients with NFAI compared to those with ACS, although in the latter the proportion of patients with elevation of the different metabolites was generally larger. β-cortol was the only metabolite that was excreted in excess more frequently in patients with ACS compared with NFAI patients. Although this may only reflect a higher glucocorticoid excretion of ACS patients, further studies should define whether this is a specific finding of ACS among patients with AIs. The applicability of the USP is not limited to the evaluation of glucocorticoid excess in AIs, as it has been also described as a useful tool to differentiate being and malignant adrenal tumors [22], to predict adrenocortical carcinoma recurrence, for subtyping unilateral and bilateral forms of primary aldosteronism, and could predict the outcomes of surgical treatment of primary aldosteronism [9].
Overall, positive correlations were found between serum post-DST cortisol concentrations and 11β-hydroxylase activity, and between DST and the total excretion of precursors of glucocorticoids, although these correlations were modest with correlation coefficients around 0.4 (the stronger correlations were observed with total THS and THF).
However, the correlations between serum post-DST cortisol concentrations and metabolites in the USP lost statistical significance in patients with NFAI, suggesting that glucocorticoid excess in them was not enough to be detected by serum post-DST cortisol concentrations in this group of patients. In other words, serum post-DST cortisol concentrations might not be sensitive enough to detect subtle glucocorticoid excess in these AIs. Thus, other biomarkers such as USP could help for the proper hormonal classification of AIs [9]. In this way, our results suggest that the USP may be a useful and more precise tool to characterize the hormonal status of glucocorticoid pathway in patients with AIs. Nevertheless, the most important limitation of the USP is that it is not easily accessible in most centers, limiting its application for the routine evaluation of patients with AIs.
In this way, other studies proposed that the USP may be useful for the measurement of steroid hormones and metabolites which could be used as biomarkers for the early diagnosis of hypercortisolism [10, 20, 23, 24]. Moreover, it is known that serum post-DST cortisol concentrations have other limitations, including frequent false positives in patients with physiological or non-neoplastic hypercortisolism caused by conditions like alcoholism, obesity, insulin resistance and neuropsychiatric disorders [25], and conditions that cause alterations in the levels of cortisol binding globulin such as pregnancy, used of estrogens or even nephrotic syndrome, that may lead to spurious results [26]. In addition, CYP3A4 inducers or CYP3A4 inhibitors may decrease or increase the clearance of dexamethasone, risking false positive or negative results, respectively [27]. Thus, it is possible that all these factors explain why the correlation between serum post-DST cortisol concentrations and glucocorticoid excretion was only modest.
We observed that some of the metabolites that are usually elevated in patients with AIs—particularly THS and cortisone—were also higher in patients with ACS-related comorbidities than in those without comorbidities. As far as our knowledge goes, no other authors have evaluated the USP of patients with AIs according to the presence of not of comorbidities. Nevertheless, one recent study developed an LC-MS/MS method, that permitted the simultaneous quantification of thirteen steroid hormones in human serum, for the identification of patients with type 2 diabetes mellitus. These authors found that serum concentrations of 18-hydroxycortisol and cortisol were significantly increased in the patients with type 2 diabetes compared with a healthy control group, while corticosterone was significantly decreased [28]. The higher levels of cortisone in patients with comorbidities may be related to the differential regulation of 11βHSD enzymes in regulating the peripheral interconversion between cortisone and cortisol in patients with type 2 diabetes and/or obesity compared with those without them [28]. However, the expected finding would be increased levels of cortisol and decreased levels of cortisone in patients with these comorbidities because the active glucocorticoid in humans is cortisol, and not cortisone. Nevertheless, another study suggested that an enhanced glucocorticoid sensitivity, due to a sensitizing variant of the glucocorticoid receptor gene, is associated with a worse glucometabolic and lipid profile in patients with type 2 diabetes [29]. Thus, the different sensitivity to glucocorticoids in these patients might explain the worst cardiometabolic profile despite lower levels of cortisone.
Moreover, age is another factor that should be considered when evaluating the USP of patients with and without comorbidities, because an age-related increased 11β-HSD1 activity has been identified in human osteoblasts, skin dermal fibroblasts, the hippocampus, and brown adipose tissue [30]. In fact, as it would be expected, we found that patients with comorbidities were at least ten years older than those without ACS-related comorbidities; however, because age and the excretion of total glucocorticoids did not correlate in our series, we may speculate that alterations of the USP are more likely to be related to cardiometabolic risk than with older age.
Regarding the diagnostic accuracy of serum post-DST cortisol concentrations and the USP for the prediction of comorbidities, we found that the former showed a fair diagnostic accuracy for the prediction of ACS-related comorbidities, with an AUC of 0.77. The diagnostic accuracy turned to good (the AUC increased to 0.81) when serum post-DST cortisol concentrations were combined with urinary cortisone, α-cortisol and THS concentrations. In the same line, Morelli et al. [31] determined the cutoffs of the parameters of cortisol secretion and peripheral activation for predicting the presence of or of not of hypertension, type 2 diabetes, and fractures in 206 postmenopausal females without adrenal tumors. They found that the presence of serum post-DST cortisol concentrations >0.9 μg/dL plus a ratio between 24-h urinary free cortisol and cortisone >0.17 showed 82.1% specificity for predicting the presence of ≥1 comorbidities. In the same line, several studies have reported a higher cardiometabolic risk, including a greater rate of mortality, in patients with serum post-DST cortisol concentrations above 1.8 µg/dL compared to patients with levels below this threshold [5, 6, 32].
In contrast with our present study, where DST was a fair test for the prediction of ACS-related comorbidities, we recently found in a different study that the AUC of the serum post-DST cortisol concentrations for the prediction of ACS-related comorbidities was actually poor, with an AUC of 0.58 [8]. These discrepancies were probably related to the different design of the study: the earlier report was a multicenter retrospective study, and although treatment with drugs that affect dexamethasone metabolism was also considered an exclusion criterion, we cannot ensure that there were no other conditions that could affect dexamethasone levels such as patient compliance with the ingestion of the drug, or individual differences in the intestinal absorption and hepatic metabolism of dexamethasone [33].
However, one of the most important findings of our present research is that the addition of USP results to serum post-DST cortisol concentrations significantly improved the diagnostic accuracy of the latter to detect ACS-related comorbidities. Thus, these results suggest that USP is not only useful for the differential diagnosis of benign and malignant adrenal tumors [34, 35] and as an earlier marker of ACS [10, 20], but also for subtyping those AIs with higher cardiometabolic risk.
Despite the novelty of our study, we are aware of several limitations. First, we did not measure serum dexamethasone levels at the time of the DST. Thus, it is possible that serum post-DST cortisol concentrations were falsely elevated in some patients. Moreover, with the design of this study, we could not demonstrate the directionality of the association between the observed changes in the USP and comorbidities. Therefore, it would be desirable to perform prospective studies analyzing if the changes in the USP are also associated with a higher risk of developing ACS-related comorbidities during follow-up.
Conclusion
The DST shows positive, but modest, correlations with urinary glucocorticoid excretion. On the other hand, the diagnostic accuracy of the DST for the prediction of ACS-related comorbidities is fair but may be improved when combined with the results of the USP and DHEAS.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Abbreviations
- AIs:
-
adrenal incidentalomas
- ACS:
-
autonomous cortisol secretion
- BMI:
-
body mass index
- CV:
-
coefficient of variation
- DST:
-
dexamethasone suppression test
- 20α-DHF:
-
20α-dihydrocortisol
- 20β-DHF:
-
20β-dihydrocortisol
- DHEA:
-
Dehydroepiandrosterona
- FPG:
-
fasting plasma glucose
- GFR:
-
glomerular filtration rate
- NFAI:
-
non-functioning adrenal incidentalomas
- 17HP:
-
17-OH-Pregnanolone
- 5PD:
-
5-Pregnenediol
- 5PT:
-
5-Pregnenetriol
- PD:
-
Pregnanediol
- PT:
-
Pregnanetriol
- PTONE:
-
Pregnanetriolone
- UFC:
-
urinary free cortisol
- THF:
-
Tetrahydrocortisol
- THE:
-
Tetrahydrocortisone
- THS:
-
Tetrahydro-11-deoxycortisol
- THA:
-
Tetrahydro-11-dehydrocorticosterone
- 5 alfa-THA:
-
5α-Tetrahydro-11-dehydrocorticosterone
- THB:
-
Tetrahydrocortisone
- 5α-THB:
-
5α-Tetrahydrocorticosterone
- THAldo:
-
Tetrahydroaldosterone
- THDOC:
-
Tetrahydrodeoxycorticosterone
- 5 alfa-THDOC:
-
5α-Tetrahydrodesoxycorticosterone
- USP:
-
urinary steroid profile
References
I. Bancos, Adrenal incidentalomas: insights into prevalence. Ann. Intern. Med. 2022. https://doi.org/10.7326/m22-2600
G. Reimondo, E. Castellano, M. Grosso, R. Priotto, S. Puglisi, A. Pia et al. Adrenal incidentalomas are tied to increased risk of diabetes: findings from a prospective study. J. Clin. Endocrinol. Metab. 105, E973–E981 (2020). https://doi.org/10.1210/clinem/dgz284
M. Fassnacht, W. Arlt, I. Bancos, H. Dralle, J. Newell-Price, A. Sahdev et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 175, G1–G34 (2016). https://doi.org/10.1530/EJE-16-0467
M. Araujo-Castro, Cardiometabolic profile and urinary metabolomic alterations in non-functioning adrenal incidentalomas: a review. Clin. Endocrinol. 2022. https://doi.org/10.1111/cen.14745
A. Adamska, V. Ulychnyi, K. Siewko, A. Popławska-Kita, M. Szelachowska, M. Adamski, et al. Cardiovascular risk factors in mild adrenal autonomous cortisol secretion in a Caucasian population. Endocr. Connect. 2022;11. https://doi.org/10.1530/EC-22-0074
A. Kjellbom, O. Lindgren, S. Puvaneswaralingam, M. Löndahl, H. Olsen, Association between mortality and levels of autonomous cortisol secretion by adrenal incidentalomas. Ann. Intern. Med. 174, 1041–1049 (2021). https://doi.org/10.7326/M20-7946
M. Araujo-Castro, A. García Cano, L. Jiménez Mendiguchía, H.F. Escobar-Morreale, P. Valderrábano, Diagnostic accuracy of the different hormonal tests used for the diagnosis of autonomous cortisol secretion. Sci. Rep. 2021;11. https://doi.org/10.1038/s41598-021-00011-4
M. Araujo-Castro, P.P. Ramírez, C.R. Lázaro, R.G. Centeno, P.G. Gimeno, M.T. Fernández-Ladreda et al. Accuracy of the dexamethasone suppression test for the prediction of autonomous cortisol secretion-related comorbidities in adrenal incidentalomas. Horm 2021 20, 1–10 (2021). https://doi.org/10.1007/S42000-021-00308-Z
M. Araujo-Castro, P. Valderrábano, H.F. Escobar-Morreale, F.A. Hanzu, G. Casals, Urine steroid profile as a new promising tool for the evaluation of adrenal tumors. Lit. Rev. Endocr. 72, 40–48 (2020). https://doi.org/10.1007/s12020-020-02544-6
M. Araujo-Castro, G. Casals G., F.A. Hanzu, E. Pascual-Corrales, A.M. García Cano, V.F. Lanza, et al. Characterisation of the urinary steroid profile of patients with nonfunctioning adrenal incidentalomas: A matched controlled cross-sectional study. Clin. Endocrinol. 2022. https://doi.org/10.1111/cen.14811
M. Araujo-Castro, M.A. Sampedro Núñez, M. Marazuela, Autonomous cortisol secretion in adrenal incidentalomas. Endocrine 2019;64. https://doi.org/10.1007/s12020-019-01888-y
C.H.L Shackleton. Steroid profiling: diagnosis of disorders affecting steroid synthesis and metabolism. In: M.L Gross, W Niessen, R.M Caprioli, (eds.) The Encyclopedia of Mass Spectrometry. 8 Elsevier: Amsterdam, 2006; 789–813
C.H.L. Shackleton, Mass spectrometry in the diagnosis of steroid-related disorders and in hypertension research. J. Steroid Biochem. Mol. Biol. 45, 127–140 (1993). https://doi.org/10.1016/0960-0760(93)90132-G
G. Casals, J. Marcos, O.J. Pozo, J. Alcaraz, M.J. Martínez de Osaba, W. Jiménez, Microwave-assisted derivatization: application to steroid profiling by gas chromatography/mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 960, 8–13 (2014). https://doi.org/10.1016/j.jchromb.2014.04.015
G. Casals, J. Marcos, Ó.J. Pozo, P. Aguilera, C. Herrero, J. To-Figueras, Gas chromatography-mass spectrometry profiling of steroids in urine of patients with acute intermittent porphyria. Clin. Biochem. 46, 819–824 (2013). https://doi.org/10.1016/j.clinbiochem.2013.03.001
A. Vega-Beyhart, J. Laguna-Moreno, D. Díaz-Catalán, L. Boswell, M. Mora, I. Halperin, et al. Ketoconazole- and metyrapone-induced reductions on urinary steroid metabolites alter the urinary free cortisol immunoassay reliability in cushing syndrome. Front. Endocrinol. 2022;13. https://doi.org/10.3389/FENDO.2022.833644
P.A. Harris, R. Taylor, R. Thielke, J. Payne, N. Gonzalez, J.G. Conde, Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42, 377–381 (2009). https://doi.org/10.1016/j.jbi.2008.08.010
P.A. Harris, R. Taylor, B.L. Minor, V. Elliott, M. Fernandez, L. O’Neal, et al. The REDCap consortium: building an international community of software platform partners. J. Biomed. Inform. 2019;95. https://doi.org/10.1016/j.jbi.2019.103208.
J. Brossaud, D. Ducint, J.B. Corcuff, Urinary glucocorticoid metabolites: biomarkers to classify adrenal incidentalomas. Clin. Endocrinol. 84, 236–243 (2016). https://doi.org/10.1111/cen.12717
A. Kotłowska, E. Maliński, K. Sworczak, J. Kumirska, P. Stepnowski, The urinary steroid profile in patients diagnosed with adrenal incidentaloma. Clin. Biochem. 42, 448–454 (2009). https://doi.org/10.1016/j.clinbiochem.2008.12.027
M. Araujo-Castro, H.F. Escobar-Morreale, P. Valderrábano, P. Valderrabano, A proposal for nomenclature revision of non-functioning adrenal incidentalomas as adrenal lesions of Undetermined Secretion of Adrenal Steroids (ALUSAS). Endocr. Pract. 28, 918–920 (2022). https://doi.org/10.1016/J.EPRAC.2022.06.007
T.M.A. Kerkhofs, M.N. Kerstens, I.P. Kema, T.P. Willems, H.R. Haak, Diagnostic value of urinary steroid profiling in the evaluation of adrenal tumors. Horm. Cancer 6, 168–175 (2015). https://doi.org/10.1007/s12672-015-0224-3
A. Kotłowska, K. Sworczak, P. Stepnowski, Urine metabolomics analysis for adrenal incidentaloma activity detection and biomarker discovery. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 879, 359–363 (2011). https://doi.org/10.1016/j.jchromb.2010.12.021
A. Kotłowska, T. Puzyn, K. Sworczak, P. Stepnowski, P. Szefer, Metabolomic biomarkers in urine of cushing’s syndrome patients. Int J. Mol. Sci. 18, 1–15 (2017). https://doi.org/10.3390/ijms18020294
O. Chabre, The difficulties of pseudo-Cushing’s syndrome (or “non-neoplastic hypercortisolism”). Ann. Endocrinol. 79, 138–145 (2018). https://doi.org/10.1016/J.ANDO.2018.04.017
Y.J. Bae, J. Kratzsch, Corticosteroid-binding globulin: modulating mechanisms of bioavailability of cortisol and its clinical implications. Best. Pract. Res Clin. Endocrinol. Metab. 29, 761–772 (2015). https://doi.org/10.1016/J.BEEM.2015.09.001
A.W. MEIKLE, Dexamethasone suppression tests: usefulness of simultaneous measurement of plasma cortisol and dexamethasone. Clin. Endocrinol. 16, 401–408 (1982). https://doi.org/10.1111/j.1365-2265.1982.tb00733.x
W. Liu, D. Yuan, M. Han, J. Huang, Y. Xie, Development and validation of a sensitive LC-MS/MS method for simultaneous quantification of thirteen steroid hormones in human serum and its application to the study of type 2 diabetes mellitus. J. Pharm. Biomed. Anal. 2021;199. https://doi.org/10.1016/J.JPBA.2021.114059
V.L. Wester, J.W. Koper, E.L.T. Van Den Akker, O.H. Franco, R. P. Stolk, E.F.C. Van Rossum, Glucocorticoid receptor haplotype and metabolic syndrome: the Lifelines cohort study. Eur. J. Endocrinol. 175, 645–651 (2016). https://doi.org/10.1530/EJE-16-0534
M.S. Cooper, E.H. Rabbitt, P.E. Goddard, W.A. Bartlett, M. Hewison, P.M. Stewart, Osteoblastic 11beta-hydroxysteroid dehydrogenase type 1 activity increases with age and glucocorticoid exposure. J. Bone Min. Res 17, 979–986 (2002). https://doi.org/10.1359/JBMR.2002.17.6.979
V. Morelli, C. Aresta, A. Gaudio, C. Eller-Vainicher, V.V. Zhukouskaya, D. Merlotti et al. Prediction of hypertension, diabetes and fractures in eucortisolemic women by measuring parameters of cortisol milieu. Endocrine 68, 411–419 (2020). https://doi.org/10.1007/S12020-020-02212-9
T. Deutschbein, G. Reimondo, G. Di Dalmazi, I. Bancos, J. Patrova, D.A. Vassiliadi, et al. Age-dependent and sex-dependent disparity in mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: an international, retrospective, cohort study. Lancet Diabetes Endocrinol. 2022. https://doi.org/10.1016/S2213-8587(22)00100-0
V. Bansal, N. Asmar, W.W.R. Selman, B.B.M. Arafah, N. El Asmar, W.W.R. Selman et al. Pitfalls in the diagnosis and management of Cushing’s syndrome. Neurosurg. Focus 38, 1–11 (2015). https://doi.org/10.3171/2014.11.FOCUS14704.Disclosure
V. Chortis, I. Bancos, T. Nijman, L.C. Gilligan, A.E. Taylor, C.L. Ronchi, et al. Urine steroid metabolomics as a novel tool for detection of recurrent adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2020;105. https://doi.org/10.1210/clinem/dgz141
I. Bancos, W. Arlt, Diagnosis of a malignant adrenal mass: the role of urinary steroid metabolite profiling. Curr. Opin. Endocrinol. Diabetes Obes. 24, 200–207 (2017). https://doi.org/10.1097/MED.0000000000000333
Funding
SENDIMAD: BECA SENDIMAD de Ayuda a la Investigación en Endocrinología, Nutrición y Diabetes 2019. IRYCIS: Convocatoria intramural de ayudas a proyectos de investigación de investigadores noveles, investigadores clínicos asociados y/o grupos emergentes del Hospital Universitario Ramón y Cajal 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All procedures performed in the participants of the study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All patients signed an informed consent to participate in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Araujo-Castro, M., Hanzu, F.A., Pascual-Corrales, E. et al. Is the 1mg-dexamethasone suppression test a precise marker of glucocorticoid excess and cardiometabolic risk in patients with adrenal incidentalomas?. Endocrine 82, 161–170 (2023). https://doi.org/10.1007/s12020-023-03429-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03429-0