Abstract
Concerns raised by several animal studies, case reports, and pharmacovigilance warnings over incretin-based therapy potentially exposing type two diabetes patients to an elevated risk of pancreatitis have cast a shadow on the overall safety of this class of drugs. This systematic review evaluates the data from observational studies that compared treatment with or without incretins and the risk of pancreatitis. We searched PubMed for publications with the key terms incretins or GLP-1 receptor agonists or DPP-4 inhibitors or sitagliptin or vildagliptin or saxagliptin or linagliptin or alogliptin or exenatide or liraglutide AND pancreatitis in the title or abstract. Studies were evaluated against the following criteria: design (either cohort or case–control); outcome definition (incidence of pancreatitis); exposure definition (new or current or past incretins users); and comparison between patients receiving incretins or not for type 2 diabetes. Two authors independently selected the studies and extracted the data. Six studies meeting the inclusion criteria were reviewed. No difference was found in the overall risk of pancreatitis between incretin users and non-users (odds ratio 1.08; 95 % CI [0.84–1.40]). A risk increase lower than 35 % cannot be excluded according to the power calculation. This systematic review and meta-analysis suggests that type 2 diabetes patients receiving incretin-based therapy are not exposed to an elevated risk of pancreatitis. Limitations of this analysis are the low prevalence of incretin users and the lack of a clear distinction by the studies between therapy with DPP-4 inhibitors or with GLP-1 receptor agonists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Incretin-based therapies, because of their low hypoglycemic risk and apparently acceptable tolerability profile [1–3], are gaining wider use in current clinical practice for treating type 2 diabetes. However, incongruent data from experimental studies on rodents [4–6], recent case reports in humans [7–9], and pharmacovigilance analyses [10, 11] have been interpreted as providing evidence for the hypothesis that exposure to incretins may be associated with an increased risk of acute pancreatitis (AP), arousing concern about the overall safety of this drug class. Interpretations of the pharmacovigilance data in particular, based on self-reported, uncontrolled cases, supported the hypothesis for an almost 25-fold excess risk of developing AP [11, 12].
The relationship between diabetes and AP is a complex one in which many entangled factors, the disease itself, comorbidities, and medications, come into play. There is consensus that the incidence of AP is higher in patients with type 2 diabetes, especially among those receiving oral antidiabetic agents [13–16], and that there are known risk factors for AP, such as gallstones and obesity. Further complicating the issue is that several drugs commonly used in the treatment of type 2 diabetes have been reported to increase the risk of AP [17–19].
Involved in this association are both the glucagon-like peptide-1 (GLP-1) receptor agonist group, including injectable drugs that mimic the action of native GLP-1, such as the GLP-1 analogs exenatide and liraglutide, and the dipeptidyl peptidase 4 (DPP-4) inhibitor group, comprising oral agents that delay the catabolism of native GLP-1 mainly by inhibiting the endogenous enzyme DPP-4 [20].
Currently available randomized controlled trials (RCTs) do not suggest any increase in the risk of pancreatitis during treatment with incretins [21–23], but real-world data derived from post-marketing and observational studies leave open the possibility of an increased risk. Because AP is a relatively rare event with many possible confounders and because the current use of incretins is low, large-scale, rigorous observational studies are needed to detect this untoward effect and to define the underlying factors in the real world. In lieu of such studies, here we employed a meta-analytic approach to examine the possible association between incretin exposure and AP by gleaning salient information on available incretins from observational studies. This was done with a view to help practitioners evaluate the benefits versus risk when considering incretin-based therapy.
Methods
Search strategy and inclusion criteria
We searched the PubMed electronic database (last accessed November 15, 2013) using the key terms (“Pancreatitis”[MeSH Terms] OR “pancreatitis”[All Fields]) AND (incretin OR sitagliptin OR vildagliptin OR saxagliptin OR alogliptin OR linagliptin OR exenatide OR liraglutide OR glucagon-like peptide-1 agonist OR DPP-4 inhibitors) to identify articles published in English in the past 10 years (01/01/2003–31/10/2013) and referring to incretins and pancreatitis. The bibliographies of the retrieved publications, including reviews and meta-analyses, were also checked.
Studies were screened for inclusion according to the following criteria:
-
Study design: cohort or case–control studies.
-
Outcome definition: incidence of and/or mortality and/or hospitalization due to pancreatitis.
-
Exposure definition: new or current or past incretins users: sitagliptin OR exenatide OR vildagliptin OR saxagliptin OR alogliptin OR linagliptin OR liraglutide.
-
Number of subjects, association measures, confidence intervals (CI): studies had to report the CI for the association measures at each exposure level.
Starting from the paper’s title and abstract, two investigators independently evaluated the study against the inclusion criteria.
Data collection
The review was performed in accordance with guidelines on meta-analyses. To diminish reporting bias and errors in data collection, two independent reviewers abstracted the data with a standardized form; disagreements were resolved through discussion and consensus.
Quality assessment
Two reviewers independently assessed the quality of each study against the Newcastle-Ottawa Scale (NOS). The NOS consists of three parameters of quality: selection, comparability, and exposure (case-control studies) or outcome (cohort studies). The NOS assigns a maximum of four points for selection, two for comparability, and three for exposure/outcome, wherein a score of nine designates the highest quality. Any scoring discrepancies were addressed by a joint re-evaluation of the original article with a third reviewer. Five was the minimum NOS score for including a study in the analysis.
Statistical analysis
The estimates of relative risks (odds ratios for case–control studies and risk ratios or hazard ratios for cohort studies) were transformed to their natural logarithm before pooling. Variances of the adjusted estimates on the log-scale are derived from the 95 % confidence intervals (95 % CI) reported in the original articles as ((log (95 % CI upper limit)−log (95 % CI lower limit))/3.92)2.
The analysis was stratified by study design (case–control and cohort studies) using the Der Simonian and Laird random effect model, with the estimate of heterogeneity being taken from the inverse-variance fixed-effect model. The I2 statistic for heterogeneity was used. Although different summary measures have different interpretations, we pooled hazard ratios (HRs) and risk ratios (RRs) since they originate from time data based on the same study design.
Because pancreatitis is a rare event and because case–control studies are nested in cohorts, we assumed that the ORs would be unbiased estimates of the RRs; hence, we report an overall estimate resulting from pooling all studies.
To assess the statistical power of the meta-analysis, we computed the power values for the two-sided Z test of no population effect (pooled RR = 1) against several alternative hypotheses (RR ranging from 1 to 1.5) [24]. As an estimate of the variance of population effect, we considered the one coming from pooling all studies and the other only from cohort studies, both under fixed effect models.
Statistical analyses were performed using R version 3.0.2 (R Core Team (2013). (A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org).
Results
Search results
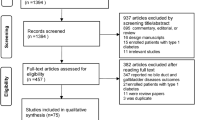
In all, 15 articles were fully reviewed from among the 136 papers retrieved. Figure 1 illustrates the search flow. Among the papers excluded because they did not meet the criteria for study design, Dore et al. 2013 [25] was omitted because it is a pooled analysis of two studies already included in this meta-analysis (Dore et al. 2011 and Wenten et al. 2012) [26, 27]. Tables 1 and 2 report the characteristics of the eight studies included in the final evaluation. The quality of included studies was poor, with NOS ranging from 1 to 6. The study by Elashoff et al. [10] was excluded because it did not fulfill the quality conditions, and a second study by Dore published in 2011 [26] was also excluded because it did not report the risk for the exposure category “any use” or “initiators”.
At the end of selection, after excluding the two papers mentioned above, six articles [27–32] met the inclusion criteria for quality assessment and for reporting the risk estimation for the exposure category “any use” or “initiators”. Only one European study reported on exposure to all the available compounds [32], two studies analyzed exposure to exenatide, and three studies to either exenatide or sitagliptin.
Contrasting estimates from the case–control studies (I 2 = 86 %) resulted in a pooled OR = 1.41 (95 % CI 0.68–2.94). For the sensitivity analysis, we included the nested case–control reported in the 2011 paper by Dore et al. [26], but the result was basically unchanged (OR 1.42; 95 % CI 0.75–2.69), overall OR 1.09 (95 % CI 0.86–1.38).
In contrast, individual estimates from the cohort studies were largely homogeneous—as demonstrated by the random effect models which coincided with those of the fixed effect model—and were not suggestive of an increased risk (RR = 0.94, 95 % CI 0.76–1.17) (Fig. 2). As shown by the power functions (Fig. 3), this analysis lacked sufficient statistical power to rule out positive associations measured by RRs < 1.35. As expected, the global analysis suffers from moderate heterogeneity, yielding an intermediate weighted estimate of 1.08, which was not statistically significant irrespective of the model assumptions (fixed or random effect).
Discussion
This systematic review and meta-analysis of data from observational studies suggests no alarming increase in the risk of pancreatitis during treatment with incretins. This finding is shared by those of two meta-analyses of registration trials of the DPP-4 inhibitor [21, 33], which excluded a higher risk of AP in treated patients. But because the sample size and duration of the trials were limited, the number of observed cases of incident pancreatitis was small and the final confidence intervals were too wide.
Five out of the six retrieved observational studies failed to detect a significant association between incretin exposure and an elevated risk of AP. Interpreting observational studies on the effects of drugs is always problematic owing to the possible effect of bias and confounders. The lack of randomization makes observational effect estimates vulnerable to bias by indication, due to the different prognosis of individuals between treatment groups. Despite attempts to adjust analyses for potentially relevant covariates, some parameters are difficult to measure reliably in large patient populations. For example, defining obesity or excluding cases of pre-existing milder forms of chronic pancreatitis is complicated in large datasets. Nonetheless, observational studies have the advantage that they collect vast amounts of information in a routine clinical setting, whereas RCTs involve only a limited number of patients who are not necessarily representative of those receiving prescriptions in the real world.
There are many reasons to remain cautious when making causal attributions between observations and interventions. Such data require circumspect interpretation. This holds particularly true for the management of information from self-reported, uncontrolled cases contained in pharmacovigilance databases, like the U.S. Food and Drug Administration (FDA) database and the very recent French one [10, 11], that may amplify public responses to risk or a risk event. The FDA Adverse Event Reporting System (FAERS, formerly AERS) has come under criticism for being based on self-reported, non-standardized adverse events, which has manifold implications for how FDA notifications and safety alerts are reported and interpreted. Consistent with the concept of a Weber effect (the so-called notoriety bias), a recent retrospective investigation applying a case/non-case method to the FDA FAERS database [34] found a significant disproportionality in the first quarter of 2008 (ROR, 1.24; 95 % CI 1.10–1.40) soon after an FDA alert was issued for exenatide and in the second quarter of 2008 (95 % CI 1.41, 1.05–1.90) for sitagliptin. The time-trend revealed a striking influence of FDA warnings on the reporting of pancreatitis, leading the authors to suspect that a pharmacovigilance alert signal had been automatically transformed into an alarm.
Two limitations of the studies included in our systematic review and meta-analysis were the low rate of incretin use (predominantly the GLP-1 receptor agonists exenatide and, to a lower extent, sitagliptin) and the relatively short study duration, which precluded ruling out less powerful associations and somehow reduced the overall accurateness of the investigation. A further weak point of our results, shared by all the observational studies on incretins, is that, owing to the low percentage of patients receiving incretin-based therapy, the two drug classes are not factored in. And because of the large differences in the mode of action of these two drug classes, future analyses need to be done separately for GLP-1 receptor agonists and DPP-4 inhibitors if the accuracy is to be improved [35, 36].
In detail, despite the inhibition of GLP-1 degradation by DPP-4 inhibitors, the pancreas might not be exposed to harmfully high GLP-1 concentrations when DPP-4 inhibitors are used. In such treatment, high concentrations of active GLP-1 in portal blood are substantially diluted before reaching the pancreatic arteries via the general circulation [35]. Furthermore, the pancreatic effects of other DPP-4 substrates (e.g., [GIP] gastric inhibitory peptide or [VIP] vasoactive, intestinal peptide) cannot be excluded because their concentrations and biological activities might also be increased by DPP-4 inhibition. By contrast, since GLP-1 receptor agonists significantly augment circulating concentrations of the incretin mimetic throughout the body, this could potentially exert a more aggressive action on pancreatic cells.
Finally, genetically predisposed persons may be at increased risk of developing AP, a condition similar to the recent demonstration that certain genotypes and epitopes predispose to autoimmune AP [37], which might explain the severe pancreatitis some case reports have described [7–9]. Likewise, our findings cannot rule out the silent destruction of pancreatic cells, as revealed by increased serum amylase and lipase levels [38]. This issue will need to be addressed with a prospective analysis of the outcome of patients found to have abnormal levels of pancreatic enzymes.
In conclusion, the major strength of this study is that the systematic review and meta-analysis of available studies is robust enough to reassure the practitioner that the claims for a 25-fold excess of risk are unfounded, even though a risk increase lower than 35 % cannot be completely excluded. The safety profile of type 2 diabetes drugs has come under increased scrutiny as the prevalence of diabetes continues to rise and ever more patients receive treatment for the condition. More research is needed, especially large cohort studies that could account for covariates in drug- and patient-related characteristics. A further area of focus is research into potential harmful effects on the exocrine pancreas beyond AP.
References
C.F. Deacon, E. Mannucci, B. Ahre´n, Glycaemic efficacy of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors as add-on therapy to metformin in subjects with type 2 diabetes-a review and meta analysis. Diabetes. Obes. Metab. 14, 762–767 (2012)
B. Gallwitz, Extra-pancreatic effects of incretin-based therapies. Endocrine (2014). doi:10.1007/s12020-014-0223-0
N. Mikhail, Effects of incretin-based therapy in patients with heart failure and myocardial infarction. Endocrine 47(1), 21–28 (2014). doi:10.1007/s12020-014-0175-4
J.A. Koehler, L.L. Baggio, B.J. Lamont, S.D. Ali, D.J. Drucker, Glucagon-like peptide-1 receptor activation modulates pancreatitis-associated gene expression but does not modify the susceptibility to experimental pancreatitis in mice. Diabetes. 58, 2148–2161 (2009)
J.S. Nachnani, D.G. Bulchandani, A. Nookala, B. Herndon, A. Molteni, P. Pandya, R. Taylor, T. Quinn, L. Weide, L.M. Alba, Biochemical and histological effects of exendin-4 (exenatide) on the rat pancreas. Diabetologia. 53, 153–159 (2010)
K. Tatarkiewicz, P.A. Smith, E.J. Sablan, C.J. Polizzi, D.E. Aumann, C. Villescaz, D.M. Hargrove, B.R. Gedulin, M.G.W. Lu, L. Adams, T. Whisenant, D. Roy, D.G. Parkes, Exenatide does not evoke pancreatitis and attenuates chemically induced pancreatitis in normal and diabetic rodents. Am. J. Physiol. Endocrinol. Metab. 299, E1076–E1086 (2010)
S.N. Iyer, A.J. Drake, R.I. West, C.E. Mendez, R.J. Tanenberg, Case report of acute necrotizing pancreatitis associated with combination treatment of sitagliptin and exenatide. Endocr. Pract. 18, 10–13 (2012)
A.S. Franks, P.H. Lee, C.M. George, Pancreatitis: a potential complication of liraglutide? Ann. Pharmacother. 46, 1547–1553 (2012)
M. Sue, A. Yoshihara, K. Kuboki, N. Hiroi, G. Yoshino, A case of severe acute necrotizing pancreatitis after administration of sitagliptin. Clin. Med. Insights. Case. Rep. 6, 23 (2013)
M. Elashoff, A.V. Matveyenko, B. Gier, R. Elashoff, P.C. Butler, Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 141, 150–156 (2011)
J.L. Faillie, S. Babai, S. Crépin, V. Bres, M.L. Laroche, H. Le Louet, P. Petit, J.L. Montastruc, D. Hillaire-Buys, The French Pharmacovigilance Centers Network. Pancreatitis associated with the use of GLP-1 analogs and DPP-4 inhibitors: a case/non-case study from the French Pharmacovigilance Database. Acta Diabetol. 51(3), 491–497 (2014)
E.A. Gale, GLP-1 based agents and acute pancreatitis: drug safety falls victim to the three monkey paradigm. BMJ. 346, f1263 (2013)
C.J. Girman, T.D. Kou, B. Cai, C.M. Alexander, E.A. O’Neill, D.E. Williams-Herman, L. Katz, Patients with type 2 diabetes mellitus have higher risk for acute pancreatitis compared with those without diabetes. Diabetes. Obes. Metab. 12, 766–771 (2010)
R.A. Noel, D.K. Braun, R.E. Patterson, G.L. Bloomgren, Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes. Care. 32, 834–838 (2009)
A. Gonzalez-Perez, R.G. Schlienger, L.A. Rodríguez, Acute pancreatitis in association with type 2 diabetes and antidiabetic drugs: a population-based cohort study. Diabetes. Care. 33(12), 2580–2585 (2010)
S.-W. Lai, C.-H. Muo, K.-F. Liao, F.-C. Sung, P.-C. Chen, Risk of acute pancreatitis in type 2 diabetes and risk reduction on anti-diabetic drugs: A population-based cohort study in Taiwan. Am. J. Gastroenterol. 106, 1697–1704 (2011)
K.B. Blomgren, A. Sundstrom, G. Steineck, B.E. Wiholm, Obesity and treatment of diabetes with glyburide may both be risk factors for acute pancreatitis. Diabetes. Care. 25, 298–302 (2002)
F.L. Fimognari, A. Corsonello, R. Pastorell, R. Antonelli-Incalzi, Metformin-induced pancreatitis: a possible adverse drug effect during acute renal failure. Diabetes. Care. 29, 1183 (2006)
S. Mallick, Metformin-induced acute pancreatitis precipitated by renal failure. Postgrad. Med. J. 80, 239–240 (2004)
C.B. Giorda, E. Nada, B. Tartaglino, Pharmacokinetics, safety, and efficacy of DPP-4 inhibitors and GLP-1 receptor agonists in patients with type 2 diabetes mellitus and renal or hepatic impairment. A systematic review of the literature. Endocrine 46(3), 406–419 (2014)
M. Monami, I. Dicembrini, E. Mannucci, Dipeptidyl peptidase-4 inhibitors and pancreatitis risk: a meta-analysis of randomized clinical trials. Diabetes. Obes. Metab. 16(1), 48–56 (2014)
B.M. Scirica, D.L. Bhatt, E. Braunwald, P.G. Steg, J. Davidson, P. Hirshberg, R. Frederich, S.D. Wiviott, E.B. Hoffman, M.A. Cavender, J.A. Udell, N.R. Desai, O. Mosenzon, D.K. McGuire, K.K. Ray, L.A. Leiter, I. Raz, the SAVOR-TIMI 53 Steering Committee and Investigators, Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 369(14), 1317–1326 (2013)
W.B. White, C.P. Cannon, S.R. Heller, S.E. Nissen, R.M. Bergenstal, G.L. Bakris, A.T. Perez, P.R. Fleck, C.R. Mehta, S. Kupfer, C. Wilson, W.C. Cushman, F. Zannad, the EXAMINE Investigators, Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 369(14), 1327–1335 (2013)
L.V. Hedges, T.D. Pigott, The power of statistical test in meta-analysis. Psychol. Methods. 6, 203–217 (2001)
D.D. Dore, M. Hussein, C. Hoffman, E.M. Pelletier, D.B. Smith, J.D. Seeger, A pooled analysis of exenatide use and risk of acute pancreatitis. Curr. Med. Res. Opin. 29(12), 1577–1586 (2013)
D.D. Dore, G.L. Blomgren, M. Wenten et al., A cohort study of acute pancreatitis in relation to exenatide use. Diabetes. Obes. Metab. 13(6), 559–566 (2011)
M. Wenten, J.A. Gaebler, M. Hussein, E.M. Pelletier, D.B. Smith, P. Girase, R.A. Noel, D.K. Braun, G.L. Bloomgren, Relative risk of acute pancreatitis in initiators of exenatide twice daily compared with other anti-diabetic medication: a follow-up study. Diabet. Med. 29(11), 1412–1418 (2012)
D.D. Dore, D. John, J.D. Seeger, Arnold Chan K. Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr. Med. Res. Opin. 25(4), 1019–1027 (2009)
R. Garg, W. Chen, M. Pendergrass, Acute pancreatitis in type 2 diabetes treated with exenatide or sitagliptin: a retrospective observational pharmacy claims analysis. Diabetes. Care. 33(11), 2349–2354 (2010)
J.A. Romley, D.P. Goldman, M. Solomon, D. McFadden, A.L. Peters, Exenatide therapy and the risk of pancreatitis and pancreatic cancer in a privately insured population. Diabetes. Technol. Ther. 14(10), 904–911 (2012)
S. Singh, H.Y. Chang, T.M. Richards, J.P. Weiner, J.M. Clark, J.B. Segal, Glucagon-like peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMA. Intern. Med. 173(7), 534–539 (2013)
C.B. Giorda, R. Picariello, E. Nada, B. Tartaglino, L. Marafetti, G. Costa, R. Gnavi, Incretin therapies and risk of hospital admission for acute pancreatitis in an unselected population of European patients with type 2 diabetes: a case-control study. www.thelancet.com/diabetes-endocrinology. Accessed 12 Nov 2013. doi:10.1016/S2213-8587(13)70147-5
M. Monami, I. Iacomelli, N. Marchionni, E. Mannucci, Dipeptidyl peptidase-4 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Nutr. Metab. Cardiovasc. Dis. 20, 224–235 (2010)
E. Raschi, C. Piccinni, E. Poluzzi, G. Marchesini, F. De Ponti, The association of pancreatitis with antidiabetic drug use: gaining insight through the FDA. Acta Diabetol. 50(4), 569–577 (2013)
M.A. Nauck, J.J. Meier, A critical analysis of the clinical use of incretin-based therapies: the benefits by far outweigh the potential risks. Diabetes. Care. 36, 2126–2132 (2013)
M.A. Nauck, J.J. Meier, Pancreatitis and incretin-based drugs: clarity or confusion? Comment. www.thelancet.com/diabetes-endocrinology. Accessed 12 Nov 2013. doi:10.1016/S2213-8587(13)70186-4
L. Frulloni, C. Lunardi, R. Simone et al., Identification of a novel antibody associated with autoimmune pancreatitis. N. Engl. J. Med. 361, 2135–2142 (2009)
H.M. Lando, M. Alattar, A.P. Dua, Elevated amylase and lipase levels in patients using glucagon-like peptide-1 receptor agonists or dipeptidyful-peptidase-4 inhibitors in the outpatient setting. Endocr. Pract. 18, 472–477 (2012)
Acknowledgments
This study was supported by Chaira Medica Association (non-profit organization for the study of endocrine and metabolic disorders), Chieri, Italy; EN is employed with the Association.
Conflict of interest
CBG discloses teaching and speaking fees from Merck, Novo Nordisk, BMS/AZ Alliance, and BI/Lilly Alliance. All the other authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giorda, C.B., Sacerdote, C., Nada, E. et al. Incretin-based therapies and acute pancreatitis risk: a systematic review and meta-analysis of observational studies. Endocrine 48, 461–471 (2015). https://doi.org/10.1007/s12020-014-0386-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-014-0386-8