Abstract
Despite the majority of papillary thyroid microcarcinoma (PTMC) patients has benign clinical courses, some PTMCs have a clinical presentation similar to conventional papillary thyroid carcinoma (PTC). The aim of this study was to identify risk factors for lymph node metastasis at presentation and prognostic parameters influencing nodal recurrence in PTMC. From January 1998 to October 2013, 556 consecutive patients had a diagnosis of differentiated thyroid carcinoma in our surgical department. A total of 219 (39.4 %) patients who had a pathological diagnosis of PTMC represented the cohort for the current study. We carried out a retrospective cohort study to compare 24 PTMC patients with lymph node metastasis at diagnosis (N1) and 195 PTMC patients without lymph node involvement (N0). The comparison between groups involved evaluation of patients and tumor characteristics. A diameter >8 mm, the presence of multifocality, and extrathyroid invasion (T3) were independent risk factors for nodal involvement at presentation. The presence of T3 was the only independent prognostic parameter influencing nodal recurrence. Prognostic factors for N1 at presentation and for recurrence are pathological parameters, thus it is not possible before surgery to detect PTMC patients who are at risk. However, we believe that a full treatment protocol should be also indicated in the case of PTMC according to risk stratification and cancer stage as for the conventional counterpart of PTC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The 2004 World Health Organization (WHO) classification of thyroid tumors has defined papillary thyroid microcarcinoma (PTMC) as a tumor of 1.0 cm or less in diameter [1]. The 2010 TNM staging system has included into stage T1 all tumors with a diameter ≤2.0 cm, where PTMCs are those T1a tumors ≤1.0 cm [2]. However, the updated TNM staging system has placed greater emphasis on the metastasis to lymph nodes than in the diameter alone in predicting prognosis of PTMC [2].
Despite the majority of PTMC patients has a benign clinical courses associated with good prognosis [3], some PTMCs have a clinical presentation similar to conventional papillary thyroid carcinoma (PTC) with the involvement of neck lymph node by the tumor [4, 5]. Detection of lymph node metastasis at diagnosis is fundamental also in the management of PTMC as up to 75.0 % of cancer recurrences will occur in the lymph nodes of the neck [6]. Indeed, several cases of PTMC with large neck nodal metastases affecting recurrence [7–10], and sometimes cancer-related death have been published [8, 11–15].
As evidence on the biological behavior and clinical courses of PTMC with nodal involvement at presentation are still controversial, we carried out a retrospective cohort study to identify risk factors for lymph node metastasis at presentation and prognostic parameters influencing late outcome such as nodal recurrence. The current research was based on a single-institution experience, with homogeneity in the clinical management of patients with PTMC.
Materials and methods
Over the period from January 1998 to October 2013, 556 consecutive patients had a diagnosis of differentiated thyroid carcinoma in our surgical department. Among them, 449 (80.8 %) were PTC, 71 (12.7 %) were follicular thyroid carcinoma, and 36 (6.5 %) were Hürthle cell thyroid carcinoma. A total of 219 patients (39.4 %) who had a pathological diagnosis of PTMC represented the cohort of patients for the current study. These patients accounted for 48.7 % of all PTCs.
PTMC was defined as a papillary thyroid tumor of 1.0 cm or less in diameter in accordance with the WHO classification [1]. In the current study, tumor size was the one reported by pathologists. Twenty-four patients (10.9 %) were included in the study as having a PTMC with lymph node metastasis at presentation (N1), while 195 patients (89.1 %) had a diagnosis of PTMC without macroscopic lymph node involvement (N0) (Fig. 1). Some patients of the N0 group could have had unknown microscopic lymph node metastasis being a Nx group rather than a N0 group. Nevertheless, patients without macroscopic lymph node involvement have been identified as N0PTMC group.
Preoperative diagnosis of PTMC was made by means of fine-needle aspiration cytology (FNAC), while in another subset of patients, the diagnosis of PTMC was incidentally found at final histology. All patients underwent ultrasonography of both thyroid and neck before operation. Those patients with suspect of lymph node involvement by the tumor at ultrasonography underwent magnetic resonance imaging (MRI) of the neck and mediastinum. In a subgroup of patients, lateral lymph node metastasis was assessed by means of both FNAC and cytological detection of thyroglobulin in cervical lymph nodes.
Very thin anatomical slices were made to carefully analyze thyroid specimen. Multifocality was considered, when two or more tumor foci were found in one lobe or in two thyroid lobes. Metastatic lymph nodes were routinely examined with four histological sections.
We carried out a retrospective cohort study in order to compare 24 N1PTMC patients and 195 N0PTMC patients. The allocation of patients to the group N1 or N0 was based on the pathological epicrisis.
This study was conducted in accordance with the recommendations from the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational studies [16]. All the medical records were retrospectively reviewed. The comparison between the two groups involved evaluation of patients characteristics (age and gender), body mass index (BMI), familiarity for thyroid cancer, tumor characteristics such as tumor size, histology, the incidental or nonincidental diagnosis, multifocality, and extrathyroid invasion (T3 of the pathologic tumor node metastasis, (pTNM) classification, such as extension to sternothyroid muscle or perithyroid soft tissues). Pathologic cancer staging and risk assessment were in accordance with the American Joint Committee on Cancer (AJCC) pTNM staging, following the 2010 pTNM-AJCC system [2].
Indications for surgical treatment, management characteristics, surgical complications such as inferior laryngeal nerve palsy and post-operative hypocalcemia, and follow-up results were also evaluated. Post-operative hypocalcemia was defined as a serum calcium level less than 8.0 mg/dl; patients were considered as having permanent hypoparathyroidism and permanent inferior laryngeal nerve palsy, if the impaired function persisted after six months from the operation [17].
Ipsilateral, bilateral, and central neck lymphadenectomy was always performed as scheduled procedure with curative intent, on the basis of preoperative or intraoperative detection of lymph node involvement by the tumor. Prophylactic lymphadenectomy was not performed in any case.
After discharge, all patients entered a scheduled clinical and instrumental follow-up program in the endocrinological department on an average duration of 90.0 months (range 15–249 months, median 95 months).
All 24 N1PTMC patients and 135 N0PTMC patients (69.2 %) underwent iodine-131 whole-body scan (WBS) 4–6 weeks after surgery, without l-thyroxin treatment and when the TSH level was higher than 30 μU/ml. A radioactive-iodine (RAI) ablating dose of 100 mCi (3700 MBq) was used to ablate microscopic thyroid remnants and micrometastases. A post-ablating WBS was done to search for missed metastases on the previous WBS. l-thyroxin suppressive treatment was started to maintain a serum TSH concentration of 0.1 μU/ml or less. Patients were followed-up at 6 and 12 months after treatment and then on a yearly basis. Only patients with tumor recurrence were resubmitted to RAI ablation therapy. Patients with undetectable TSH-stimulated serum thyroglobulin (Tg) levels, without ultrasonographic evidence of neck lymph node metastases and negative WBS, were defined as cancer-free, as described elsewhere [17].
Patients with incidental N0PTMC <3 mm in diameter, those patients with a N0PTMC not showing multifocality, and extrathyroid invasion did not undergo RAI ablation therapy. According to the treatment protocol of other authors [18], the diameter cut-off of 3 mm was chosen because none of the PTMCs <3 mm showed lymph node involvement or extrathyroid invasion.
Statistical analysis and synthesis of the results
Data were collected in a planned relational computer database (Microsoft Access®) including patients and tumors characteristics. All statistical analyses were carried out using the MedCalc® statistical software version 12.7.5 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2013).
Data for age, tumor size, BMI, and post-operative hospital stay were presented as the mean ± standard deviation (SD). Data were compared for statistical analysis using the χ 2 test to evaluate differences between qualitative variables, and using the Student’s t test to compare quantitative variables. The objective of statistical analysis was mainly to identify independent risk factors significantly related to the presence of lymph node metastasis at presentation (N1) and lymph node recurrences at follow-up by means of stepwise logistic regression analysis. Differences were considered significant, when p < 0.05. p values of the study have been reported as calculated by the statistical software, which were both bilateral, i.e., p= and unilateral i.e., p<.
Results
N1PTMC group included 19 women and 5 men (at a ratio of 3.8:1.0) with a mean age of 48.9 years (median 50, range 27–75 years). N0PTMC group consisted of 168 women and 27 men (at a ratio of 6.2:1.0) with a mean age of 51.1 years (median 50, range 18–78 years). Age and gender were not significantly different between groups. In the N1PTMC group, there were 17 patients (70.8 %) ≥45 year-old, while in the N0PTMC group, there were 116 patients (53.0 %) ≥45 year-old, and this difference was not statistically significant (p = 0.146).
BMI was similar in both groups of study (mean ± SD: 25.8 ± 4.8 vs. 24.7 ± 4.5 kg/m2, p = 0.241, in N1PTMC and N0PTMC group, respectively).
In the N1PTMC group, familiarity for thyroid cancer was found in two patients (8.3 %): one 43-year-old male patient with a multifocal tumor and bilateral and central neck nodal metastasis; and one 75-year-old female patient with a 10 mm tumor and ipsilateral and central neck nodal metastasis. In the N0PTMC group, familiarity for thyroid cancer was found in three female patients (1.5 %) aged 39, 43, and 57 with multifocal tumor in all cases.
The comparative cohort study revealed that preoperative cytological diagnosis of primary thyroid tumor was significantly more frequent in N1 group (p = 0.002) (Table 1).
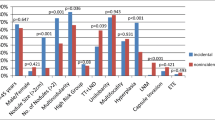
Mean tumor size was significantly greater in the N1PTMC than in the N0PTMC (7.5 vs. 5.7 mm, p = 0.011). The prevalence of lymph node involvement by the tumor in the N1PTMC group was also stratified per millimeter cut-offs from 1 to 10 mm. No patient with a tumor diameter <3 mm showed nodal metastasis at presentation, and 11 patients (45.8 %) had a tumor diameter >8 mm (Fig. 2). Moreover, after comparing the two groups per different millimeter cut-offs from 1 to 10 mm, a diameter >8 mm was more frequently found in the N1PTNM group, and this difference was statistically significant (45.8 vs. 23.0 %, respectively, p = 0.031) (Table 1). The presence of a papillary classical variant of carcinoma at histology was significantly related to the presence of nodal metastasis [23 patients (95.8 %) of N1PTMC group vs. 134 patients (68.7 %) of N0PTMC group, respectively, p < 0.0001] (data not shown in tables). The only patient with encapsulated variant of PTMC and lymph node involvement by the tumor showed papillae and follicles as in the classical variant of PTC. Tumor multifocality, T3, and a nonincidental diagnosis were significantly associated with the presence of nodal metastasis (p < 0.0001 and p = 0.002, respectively) (Table 1).
Eleven out of 24 N1PTMC patients (45.9 %) had a preoperative diagnosis of nodal involvement: five patients had a preoperative cytological diagnosis of both primary tumor and lateral neck lymph nodes; three patients had cytological detection of lymph nodes involvement only (occult primary); and three patients had cytological thyroglobulin detection in lymph nodes (FNAC–TG); eight patients (33.3 %) had a suspicion of nodal involvement following neck ultrasonography and MRI; and five patients (20.8 %) with nodal metastasis in the central compartment only were diagnosed intraoperatively.
Among those 24 patients who underwent lymph node dissection, four had a total thyroidectomy associated with central neck dissection; 14 had a total thyroidectomy and central and ipsilateral neck dissection; and six had a total thyroidectomy associated with central and bilateral neck dissection. In the latter cases, four patients were submitted to bilateral neck dissection after cytological detection of lymph node involvement in both sides, while lymph node frozen section modulated the extent of lymphadenectomy to the other side in two patients. Tumor lymph node recurrence was shown in four patients after at least six months from the operation. In all cases, tumor recurrence in neck lymph nodes was ipsilateral to the previous functional neck dissection. The rate of nodal recurrence was 16.6 % of a total of 24 patients with lymph node metastasis at presentation. However, the overall rate of recurrence was 1.8 %, when considering the whole cohort of 219 patients.
Operative results, post-operative morbidity, and data about post-operative ablation therapy with I-131 have been reported in details in Table 2. Two patients of the N0PTMC group had completion thyroidectomy because permanent histology demonstrated an unexpected PTMC of 8 and 10 mm.
Post-operative hypocalcemia was mainly found in those patients who underwent central neck dissection. However, none of the patients of this cohort had permanent inferior laryngeal nerve palsy or permanent hypoparathyroidism.
At a mean follow-up of 90.0 months, four patients (16.6 %) of the N1PTMC group showed nodal recurrence: three patients in the ipsilateral neck compartment 12, 15, and 20 months after surgery, and one patient in the central compartment 16 months after thyroidectomy (Table 2). In all cases, patients with recurrence showed levels of Tg >10 ng/ml, and nodal recurrence was detected by means of FNAC. After lymph node removal, these four patients were once more submitted to RAI ablation therapy. One 53-year-old female patient of the N0PTMC group was submitted to ipsilateral modified lateral neck dissection five years after total thyroidectomy for a multifocal N0PTMC (with a greatest diameter of 8 mm). In this case, preoperative diagnosis of lymph node metastasis was made by FNAC, while Tg serum level was <0.5 ng/ml and WBS was negative. Pathological result showed three lymph nodes involved by the tumor. Thereafter, she was submitted to a second dose of RAI ablation therapy. None of the patients of both groups died because of PTMC.
Multivariate analysis showed that a diameter >8 mm, the presence of multifocality, and T3 were independent risk factors for nodal involvement at presentation. The presence of T3 was the only independent prognostic parameter influencing nodal recurrence (Table 3).
Discussion
The extensive uses of thyroid ultrasonography and FNAC, together with a more accurate thyroid specimen examination, are the most important reasons for the reported increasing incidence of PTMC [19, 20]. According to the WHO classification, microcarcinoma should indicate a papillary carcinoma ≤1.0 cm in diameter, which is incidentally found at histology and reported to have indolent clinical courses [1]. The same guidelines report that PTMC can behave more aggressively and it may present with bulky cervical node metastasis [1]. Thus, a distinction between clinically relevant and irrelevant PTMCs is absolutely needed by physicians.
The main results of the current investigation show that there are two different varieties of PTMC: tumors with benign biological and clinical courses and those aggressive tumors with a diameter >8 mm, multifocality, and extrathyroid invasion, which are at high risk for lymph node involvement at presentation. In these latter tumors, the risk for nodal recurrence is related to the extrathyroid invasion in PTMC patients with lymph node metastasis at diagnosis aged more than 45 (pTNM IVA).
Our data confirm other studies in which PTMCs 8 mm or larger in diameter are more aggressive in terms of lymph node and distant metastasis [5, 12]. Other reports do not agree showing no statistically significant difference in maximum diameter cutoff of PTMCs affecting cancer recurrence and death [8, 21, 22]. Different tumor size, however, had no impact on late outcome such as nodal recurrence, also in our patients. As shown in fig. 2, all PTMCs <3 mm in diameter showed neither lymph node metastasis nor extrathyroid invasion. This subgroup of patients was not submitted to a full treatment protocol (surgery, RAI ablation, and l-thyroxin TSH-suppressive therapy), and thyroidectomy alone was considered as a definitive treatment.
Multivariate analysis indicated multifocality as an independent risk factor for nodal involvement by the tumor. Multifocal PTMC is deemed to have similar risk of lymph node metastasis as conventional PTC, especially when the foci of summed carcinoma are >1.0 cm in size [8, 23]. In the case of multifocal PTMC, we suggest the same treatment as for the conventional PTC, since 75.0 % of our PTMCs with nodal metastasis were multicentric. In our experience, multifocality was also found in the only patient of the N0PTNM group who had metachronous lymph node metastasis five years after total thyroidectomy. Moreover, four out of our five patients with familiarity for thyroid cancer showed multifocality at final histology. Indeed, in about 6 % of cases of PTMC, there is a familial occurrence, and most of these tumors seem to have an unfavorable behavior needing a full treatment protocol [24].
Extrathyroid invasion was found to be a relevant risk factor for both lymph node involvement and nodal recurrence. These results were an evident confirmation of the correct inclusion of extrathyroid tumor extension in the pTNM staging system [25]. Recently, higher BMI has been reported as a prognostic factor for predicting the presence of extrathyroid extension in PTMC patients being helpful in deciding the appropriate surgical extent [26]. The current study, however, does not confirm these data about BMI, and PTMC maybe because our cohort of patients was quite small.
The 2010 pTNM system appears to be the most appropriate staging system classification showing that lymph node metastases are more important than size alone in predicting prognosis [2]. Unfortunately, the TNM staging is available as a post-operative outcome. In our comparative cohort study, preoperative staging was allowed by cytological diagnosis and both ultrasonographic and MRI findings. Other authors reported that the risk of lateral lymph node metastasis at presentation in PTMC is related to some preoperative findings at ultrasonography such as upper pole location, the presence of calcifications, and contact of >25 % with the adjacent capsule [6]. Conversely, the preoperative vascular index of PTMC on Doppler ultrasonography has been reported as not significantly associated with both central and lateral lymph node metastasis [27].
None of our patients died of the disease and all patients with nodal recurrence recovered, showing an excellent prognosis. However, a long-term follow-up is needed for definitive recommendations and to confirm the clinical relevance of the prognostic factor for lymph node involvement and recurrence [28]. In this way, the use of molecular tests will help clinicians in distinguish among more aggressive variants of PTMC [29]. BRAF (V600E) mutational status has been reported as associated with PTMC tumor size, multifocality, extrathyroid invasion, and lymph node metastasis [30].
Conclusions
The results of our study show that it is not possible before surgery to detect PTMC patients who are at risk, since risk factors for N1 at presentation and for nodal recurrence are pathological parameters. These results are useful after the operation to identify those PTMC patients who need RAI therapy, a l-thyroxin suppressive treatment to maintain a very low serum TSH concentration, and a close follow-up. In the case of more aggressive PTMCs, we believe that a full treatment protocol should be indicated as for the conventional counterpart of PTC according to risk stratification and cancer stage.
References
R.A. DeLellis, R.V. Lloyd, P.U. Heitz, Pathology and genetics of tumors of endocrine organs, in World Health Organization classification of tumors, ed. by R.A. DeLellis, R.V. Lloyd, et al. (IARC Press, Lyon, 2004), pp. 64–66
S.B. Edge, C.C. Compton, The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 17, 1471–1474 (2010)
Y. Ito, T. Uruno, K. Nakano, Y. Takamura, A. Miya, K. Kobayashi, T. Yokozawa, F. Matsuzuka, S. Kuma, K. Kuma, A. Miyauchi, An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid 13, 381–387 (2003)
E. Baudin, J.P. Travagli, J. Ropers, F. Mancusi, G. Bruno-Bossio, B. Caillou, A.F. Cailleux, J.D. Lumbroso, C. Parmentier, M. Schlumberger, Microcarcinoma of the thyroid gland: the Gustave-Roussy Institute experience. Cancer 83, 553–559 (1998)
E. Roti, R. Rossi, G. Trasforini, F. Bertelli, M.R. Ambrosio, L. Busutti, E.N. Pearce, L.E. Braverman, E.C. Degli Uberti, Clinical and histological characteristics of papillary thyroid microcarcinoma: results of a retrospective study in 243 patients. J. Clin. Endocrinol. Metab. 91, 2171–2178 (2006)
J.Y. Kwak, E.-K. Kim, M.J. Kim, E.J. Son, W.Y. Chung, C.S. Park, K.-H. Nam, Papillary microcarcinoma of the thyroid: predicting factors of lateral neck node metastasis. Ann. Surg. Oncol. 16, 1348–1355 (2009)
I.D. Hay, E.J. Bergstralh, J.R. Goelner, J.R. Ebersold, C.S. Grant, Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 114, 1050–1058 (1993)
S.M. Chow, S.C.K. Law, J.K. Chan, S.K. Au, S. Yau, W.H. Lau, Papillary microcarcinoma of the thyroid. Prognostic significance of lymph node metastasis and multifocality. Cancer 98, 31–40 (2003)
S. Noguchi, H. Yamashita, S. Uchino, S. Watanabe, Papillary microcarcinoma. World. J. Surg. 32, 747–753 (2008)
I.D. Hay, C.S. Grant, J.A. van Heerden, J.R. Goellner, J.R. Ebersold, E.J. Bergstralh, Papillary thyroid microcarcinoma: a study of 535 cases observed in a 50-year period. Surgery 112, 1139–1146 (1992)
N. Wada, Q.Y. Duh, K. Sugino, H. Iwasaki, K. Kameyama, T. Mimura, K. Ito, H. Takami, Y. Takanashi, Lymph node metastasis from 259 papillary thyroid microcarcinomas. Frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann. Surg. 237, 399–407 (2003)
K.D. Lin, J.D. Lin, M.J. Huang, H.S. Huang, L.B. Jeng, T.C. Chao, Y.S. Ho, Clinical presentations and predictive variables of thyroid microcarcinoma with distant metastasis. Int. Surg. 82, 378–381 (1997)
Y. Ito, T. Jikuzono, T. Higashiyama, S. Asahi, C. Tomoda, Y. Takamura, A. Miya, K. Kobayashi, F. Matsuzuka, K. Kuma, A. Miyauchi, Clinical significance of lymph node metastasis of thyroid papillary carcinoma located in one lobe. World J. Surg. 30, 1821–1828 (2006)
C. Cappelli, M. Castellano, M. Braga, E. Gandossi, I. Pirola, E. De Martino, B. Agosti, E.A. Rosei, Aggressiveness and outcome of papillary thyroid carcinoma (PTC) versus microcarcinoma (PMC): a mono-institutional experience. J. Surg. Oncol. 95, 555–560 (2007)
N. Arora, H.K. Turbedian, M.A. Kato, T.A. Moo, R. Zarnegar, T.J. Fahey 3rd, Papillary thyroid carcinoma and microcarcinoma: is there a need to distinguish the two? Thyroid 5, 473–477 (2009)
J.P. Vandenbroucke, E. von Elm, D.G. Altman, P.C. Gøtzsche, C.D. Mulrow, S.J. Pocock, C. Poole, J.J. Schlesselman, M. Egger, Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS. Med. 4, e297 (2007). doi:10.1371/journal.pmed
A. Pisanu, I. Reccia, O. Nardello, A. Uccheddu, Risk factors for nodal metastasis and recurrence among patients with papillary thyroid microcarcinoma: differences in clinical relevance between nonincidental and incidental tumors. World J. Surg. 33, 460–468 (2009)
J. Schonberger, J. Marienhagen, A. Agha, S. Rozeboom, E. Bachmeier, H. Schlitt, C. Eilles, Papillary microcarcinoma and papillary cancer of the thyroid B1 cm. Modified definition of the WHO and the therapeutic dilemma. Nuklearmedizin 46, 115–120 (2007)
S. Grodski, T. Brown, S. Sidhu, A. Gill, B. Robinson, D. Learoyd, M. Sywak, T. Reeve, L. Delbridge, Increasing incidence of thyroid cancer is due to increased pathologic detection. Surgery 144, 1038–1043 (2008)
E.L. Mazzaferri, J. Sipos, Should all patients with subcentimeter thyroid nodules undergo fine-needle aspiration biopsy and pre-operative neck ultrasonography to define the extent of tumor invasion? Thyroid 18, 597–602 (2008)
M.R. Pelizzo, I.M. Boschin, A. Toniato, C. Pagetta, A. Piotto, P. Bernante, D. Casara, G. Pennelli, D. Rubello, Natural history, diagnosis, treatment and outcome of papillary thyroid microcarcinoma (PTMC): a mono-institutional 12-year experience. Nucl. Med. Commun. 25, 547–552 (2004)
I. Sugitani, Y. Fujimoto, Symptomatic versus asymptomatic papillary thyroid microcarcinoma: a retrospective analysis of surgical outcome and prognostic factors. Endocr. J. 46, 209–216 (1999)
Q. Zhao, J. Ming, C. Liu, L. Shi, X. Xu, X. Nie, T. Huang, Multifocality and total tumor diameter predict central neck lymph node metastases in papillary thyroid microcarcinoma. Ann. Surg. Oncol. 20, 746–752 (2013)
G. Lupoli, G. Vitale, M. Caraglia, M.R. Fittipaldi, A. Abbruzzese, P. Tagliaferri, A.R. Bianco, Familial papillary thyroid microcarcinoma: a new clinical entity. Lancet 353, 637–639 (1999)
G. Mercante, A. Frasoldati, C. Pedroni, D. Formisano, L. Renna, S. Piana, G. Gardini, R. Valcavi, V. Barbieri, Prognostic factors affecting neck lymph node recurrence and distant metastasis in papillary microcarcinoma of the thyroid: results of a study in 445 patients. Thyroid 19, 707–716 (2009)
J.S. Choi, E.-K. Kim, H.J. Moon, J.Y. Kwak, Higher body mass index may be a predictor of extrathyroidal extension in patients with papillary thyroid microcarcinoma. Endocrine (2014). doi:10.1007/s120120-014-0293-z
H.J. Shin, E.-K. Kim, H.J. Moon, J.H. Yoon, K.-H. Han, J.Y. Kwak, Can increased tumoral vascularity be a quantitative predicting factor of lymph node metastasis in papillary thyroid microcarcinoma? Endocrine (2013). doi:10.1007/s12020-013-0131-8
T.Y. Kim, S.J. Hong, M. Kim, W.G. Kim, G. Gong, J.S. Ryu, W.B. Kim, S.-C. Yun, Y.K. Shong, Prognostic parameters for recurrence of papillary thyroid microcarcinoma. B.M.C. Cancer 8, 296 (2008)
E.J. Kuo, P. Goffredo, J.A. Sosa, S.A. Roman, Aggressive variants of papillary thyroid microcarcinoma are associated with extrathyroidal spread and lymph node metastases: a population-level analysis. Thyroid 23, 1305–1311 (2013)
K.L. Lin, O.C. Wang, X.H. Zhang, X.X. Dai, X.Q. Hu, J.M. Qu, The BRAF mutation is predictive of aggressive clinicopathological characteristics in papillary thyroid microcarcinoma. Ann. Surg. Oncol. 17, 3294–3300 (2010)
Acknowledgments
This study was supported by a Grant from the University of Cagliari, Italy (CAR 2012).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pisanu, A., Saba, A., Podda, M. et al. Nodal metastasis and recurrence in papillary thyroid microcarcinoma. Endocrine 48, 575–581 (2015). https://doi.org/10.1007/s12020-014-0350-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-014-0350-7