Abstract
Persistent organic pollutants (POPs) are endocrine-disrupting chemicals associated with metabolic syndrome and type 2 diabetes. In humans, little is known about their potential role on obesity. Adiponectin augments the effects of insulin on glucose homeostasis. The expression of adiponectin is reduced in obesity, insulin resistance, and type 2 diabetes. The aim of this study is to reveal whether accumulation of the POPs, especially polychlorinated biphenyls (PCBs), is associated with serum levels of adiponectin in Koreans. This cross-sectional study includes 98 Koreans (49 men and 49 women). Serum levels of marker PCBs (PCB 28, 52, 101, 138, 153, and 180) were measured by Agilent 7890GC-micro-ECD (Gas chromatography-micro-electron capture detector). Total adiponectin levels were quantified by enzyme-linked immunosorbent assay. We defined high (≥Median) and low (<Median) body mass index (BMI) groups by using median value of BMI (24.6 kg/m2 for men; 23.0 kg/m2 for women). PCB28, PCB138, and PCB153 were significantly negatively associated with adiponectin levels (β-coefficients = −0.00741 for PCB28; −0.00438 for PCB138; −0.00406 for PCB153). When we divided subjects by sex, PCB28 and PCB153 were inversely associated with adiponectin in women. In the high BMI group (≥Median), PCB153 showed the significant negative associations with adiponectin levels (P < 0.05). However, these associations were not seen in the low BMI group. In conclusion, we found negative associations between PCBs and adiponectin. This cross-sectional study could provide support for the hypothesis that POPs exposure might contribute to type 2 diabetes as well as obesity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Persistent organic pollutants (POPs), widespread environmental contaminants, are currently considered as a global problem [1]. Persistent organic pollutants (POPs), such as organochlorine pesticides and polychlorinated biphenyls (PCBs), may be particularly interesting to many because the exposure of POPs are strongly linked to type 2 diabetes and insulin resistance, which are highly correlated to obesity is believed to play a critical role [1–3].
Despite the ban on the use of POPs in the United States and Europe, their stability, resistance to degradation, and lipophilicity have led to significant bioaccumulation in most compartments of the ecosystem and human tissues [4–7]. In adipose tissue, many POPs are known to have endocrine-disrupting potency [8].
Adiponectin, a hormone produced by adipocytes [9], augments the effects of insulin on glucose homeostasis. Adiponectin expression is reduced in obesity [10], insulin resistance [11], and type 2 diabetes [12]. Adiponectin was suggested as a strong predictor of diabetes [13].
Recent studies demonstrated that PCBs increased the risk of obesity [14, 15], but there have been only a few studies showing the relationship between adiponectin and PCBs, especially the non-dioxin-like PCB congeners. One study showed that PCB153 could downregulate adiponectin in Western obese subjects [8]. However, studies on Asian are very difficult to find.
Therefore, in this study, we investigated whether the accumulation of POPs, especially PCBs, is associated with the serum levels of adiponectin in Korean men and women.
Methods
Study subjects
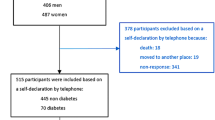
This study was designed as a cross-sectional study selecting 100 Koreans (men: 50, women: 50) from the Korean cancer prevention study-II (KCPS-II). KCPS-II included 185,331 individuals who visited eight health promotion centers nationwide from April 2004 to December 2011. A detailed description of the KCPS-II study design was published elsewhere [16, 17]. Of these, only 44,795 have adiponectin measurements. We only selected subjects aged 50–59 years who have adiponectin measurements because POPs concentrations depend on age [15]. We also excluded 1,721 participants who were missing anthropometric measurements (weight, height, body mass index (BMI), waist circumstance (WC), systolic blood pressure, diastolic blood pressure, total cholesterol, alanine aminotransferase, aspartate aminotransferase, high-density lipoprotein cholesterol (HDL-C), triglyceride, and carcinoembryonic antigen) and self-reported questionnaire information (age, smoking status, and alcohol consumption) [16].
The final sampling frame consisted of 6,186 participants (men: 4,147, women: 2,040). Due to the high cost of PCB analysis, we could not include more than 100 subjects. To meet statistical representativeness, we randomly selected 100 participants (women: 50) when measuring the PCBs concentration. Two participants who had outlying values for PCB concentration (≥300 ng/g lipids) were not included in the analyses. Our final study subject consisted of 98 participants. A written consent form was signed by all participants, and the Institutional Review Board of Yonsei University approved the study protocol.
Chemistry and anthropometric measurements
Serum, separated from peripheral venous blood, was obtained from each participant after 12-h of fasting, and then was stored at −70 °C until it was further analyzed. We measured fasting blood glucose, total cholesterol, triglyceride, HDL-C, low-density lipoprotein cholesterol, and adiponectin. For anthropometric measurements, WC was measured by wrapping the measuring tape around the exposed waists midway between the lower rib and the iliac crest. Weight and height were measured while participants were wearing light clothing. BMI was calculated as weight (kg) divided by the square of their height in meters (m2).
Both systolic and diastolic blood pressures were measured after a 15-min rest. Each participant was interviewed using a structured questionnaire which was validated in previous studies [16–18]. We collected information on smoking and alcohol consumption as well as demographic characteristics such as age, sex, past history of clinical diseases, etc. Cigarette smoking was classified into never smokers, ex-smokers, and current smokers. Alcohol consumption was categorized by nondrinkers and current drinkers. The quality control of data was maintained in accordance with the procedures of the Korean Association of Laboratory Quality Control.
Adiponectin measurements
Adiponectin levels were measured via ELISA (Mesdia Co., Ltd., Seoul, Korea). Intra- and inter-assay variances for adiponectin ranged from 6.3 to 7.4 % and 4.5 to 8.6 %, respectively [17]. Quality control of data was in accordance with the procedure of the Korean Association of Laboratory Quality Control [18].
Determination of POPs
The six European Union marker PCBs (PCB 28, 52, 101, 138, 153, and 180) were taken into account in the estimation of the PCB concentration present in samples : PCB28 (2,4,4′-trichlorobiphenyl), PCB52 (2,2′,5,5′-tetrachlorobiphenyl), PCB101 (2,2′,4,5,5′-pentachlorobiphenyl), PCB138 (2,2′3,4,4′,5′-hexachlorobiphenyl), PCB153 (2,2′4,4′,5,5′-hexachlorobiphenyl), and PCB180 (2.2′,3,4,4′,5,5′-heptachlorobiphenyl) [19]. The analytical method was based on the method described by Lu–Lu Cao et al. [20] and was used with minor modifications for low serum volumes (≤1 mL). Serum samples were analyzed by Gas chromatography with micro-electron capture detection (Agilent 7890 Series, Palo Alto, CA, USA). The lower limit for the analyzed PCBs was detected as, 0.05 μg/L.
Statistical analysis
As POPs are predominantly carried in the lipid component of the blood, lipid-adjusted concentrations (ng/g lipid) have been used in epidemiological studies [21]. Lipid-adjusted concentrations (ng/g lipids) were calculated using the formula proposed by Bernert et al. [22]. In this study, we additionally conducted analyses using non-lipid-adjusted concentrations (POPs concentrations in μg/L are not divided by lipids). We used the median value of BMI (24.57 kg/m2 for men; 23.02 kg/m2 for women) to define high (≥Median) and low (<Median) BMI groups.
Due to POPs concentrations were not normally distributed, non-parametric tests were used: Wilcoxon’s test for comparison of high and low BMI groups and Spearman’s test for correlation of variables. Regression analyses were also conducted to display the association between adiponectin and PCB concentrations. Natural logarithms of the adiponectin data, having normal distribution, were used for the analyses. All analyses were performed using SAS statistical software, version 9.2 (SAS Institute Inc., Cary, NC) and STATA 11.0 (Stata Corporation, College Station, Texas, USA). Statistical power analyses were conducted using G*Power 3.1.7 [23, 24]. Null hypothesis of no difference was rejected if P values were less than 0.05.
Results
The basic characteristics of study subjects are depicted in Table 1. The average ln (adiponectin levels) was 1.51 μg/mL in men and 1.86 μg/mL in women. Men had a higher BMI than women.
Table 2 shows the concentrations of POPs by BMI group. There was a significant difference in PCB28 (μg/L) between two BMI groups (P = 0.0057). After adjusting for age, sex, and BMI, significant inverse correlations of PCB153 (ng/g lipid) with adiponectin levels were found in high BMI group (P = 0.0310, Table 3). PCB138 (ng/g lipid) and PCB180 (ng/g lipid) also showed a negative correlation with borderline P value (P < 0.1, Table 3). However, those associations disappeared in the low BMI group (Table 3).
In regression analyses of lipid-adjusted PCBs concentrations and adiponectin levels, both PCB28, PCB138 and PCB153 were significantly negatively associated with adiponectin levels. When we divided subjects by sex, PCB28 and PCB153 were significantly inversely associated with adiponectin levels in women only. The sum of six PCBs concentrations showed negative association with adiponectin with borderline P value (P = 0.0598) (Table 4).
In high BMI group, the significant, negative correlations between adiponectin levels and lipid- adjusted PCBs (PCB138, PCB153) were confirmed by regression analysis (PCB138: P = 0.0149, R 2 = 0.1220; PCB153: P = 0.0059, R 2 = 0.1535, Fig. 1). However, negative correlations between adiponectin and PCBs were not seen in the low BMI group (PCB138: P = 0.3425, R 2 = 0.0188; PCB153: P = 0.4004, R 2 = 0.0148, Fig. 1). These results were similar in additional analyses which used non-lipid-adjusted concentrations (data not shown).
Negative correlations between adiponectin and lipid-adjusted polychlorinated biphenyls determined by body mass index (BMI) level. Low BMI group is composed of subjects with BMI lower than median values; high BMI group is composed of subjects with BMI higher than median values. The median of BMI was 24.57 kg/m2 for men and 23.02 kg/m2 for women. Each graph in the figure illustrates one of the two independent variables: a lipid-adjusted PCB138, b lipid-adjusted PCB153
Discussion
In this study, we found negative correlations between adiponectin and concentrations of PCBs in Koreans. These correlations were not apparent in lean subjects. To the best of our knowledge, this is the first study that explores the association between the concentrations of non-dioxin-like PCBs and adiponectin level in human.
In previous studies, inverse association between dioxin-like PCBs, especially 2,3,7,8-tetrachlorodibenzo-p-dioxin [25] and PCB 77 [26], and adiponectin was found. In vitro study suggested that exposure to the organochlorine compounds, especially p,p′-dichlorodiphenyldichloroethylene (DDE), increased the release of adiponectin from mature adipocytes [27]. However, there were a few prior studies on the association between adiponectin and non-dioxin-like PCBs. A study of Czech Republic showed a negative correlation between total adiponectin levels and PCB 153 serum levels in obese patients (BMI > 30 kg/m2). The parameters of the described regression model equal r = −2.567, a = 9.2 (P < 0.003, R 2 = 0.332), which are consistent with our study (r = −0.006, a = 1.8 (P < 0.006, R 2 = 0.154)).
As non-dioxin-like PCBs, both PCB 138 and PCB 153 display little or no binding affinity for aryl hydrocarbon receptor, and those are considered to be a partial androgen antagonist [28]. Because of their androgen antagonistic property, they could give different results by sex. According to our result, PCB28 and PCB153 were not significantly and inversely associated with adiponectin in men (β-coefficients = −0.00151, P = 0.5700) but in women (β-coefficients = −0.00565, P = 0.0240) (Table 4).
Our results may suggest suppression of adiponectin by PCB28, PCB138, and PCB 153 in obese patients. This may explain the association of POPs with type 2 diabetes [29–31]. It has been suggested that diabetes may be promoted by an immunotoxic effect of POPs via their binding with estrogenic receptors [32]. This mechanism would induce a chronic low-grade inflammation process, a decreased mitochondrial function, a fatty acid oxidation, and an increase lipolysis, which are all related to the insulin resistance syndrome [33, 34]. The changes in insulin sensitivity achieved in insulin resistant populations may be strongly associated with the changes in adiponectin [35].
Unlike the previous studies, we provided information about the association between various non-dioxin-like PCBs and adiponectin. Moreover, we also confirmed the findings from the Western studies in an Asian group.
This study has the following limitations: (i) This is a cross-sectional study; (ii) We could not offer information about dioxin-like PCBs because we only measured the concentration of 6 marker PCBs which belong to non-dioxin-like PCBs; and (iii) We used small number of participants to analyze PCBs concentration. However, our data included both adiponectin and POPs measurements, which are not commonly available in many studies. Moreover, the power of the study was not limited as statistical power (1 − type II error) equaled 0.87 even after stratified by gender.
In conclusion, we found negative associations of PCBs with adiponectin. These associations were different between men and women. Even, if we cannot exclude the possibility of a reversed causality, our preliminary results support for the hypothesis that POPs, endocrine-disrupting chemicals, might contribute to type 2 diabetes. Certainly, the results may be the good basis for further research on this topic. Although non-dioxin-like PCBs act through a series of toxicity pathways and elicit a wide range of effects on cancer, neurotoxicity, and endocrine disruption, there were not many studies assessing the toxicity of non-dioxin-like PCBs. Further studies are necessary to confirm the association of non-dioxin-like PCBs with obesity-related diseases to describe their harmful effects. Biological studies are also necessary to clarify the mechanism that describes how POPs down-regulate adiponectin level in human.
References
D.H. Lee, I.K. Lee, K. Song, M. Steffes, W. Toscano, B.A. Baker, D.R. Jacobs, A strong dose–response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999–2002. Diabetes Care 29, 1638–1644 (2006)
D.H. Lee, I.K. Lee, S.H. Jin, M. Steffes, D.R. Jacobs, Association between serum concentrations of persistent organic pollutants and insulin resistance among nondiabetic adults: results from the National Health and Nutrition Examination Survey 1999–2002. Diabetes Care 30, 622–628 (2007)
D.H. Lee, I.K. Lee, M. Porta, M. Steffes, D.R. Jacobs, Relationship between serum concentrations of persistent organic pollutants and the prevalence of metabolic syndrome among non-diabetic adults: results from the National Health and Nutrition Examination Survey 1999-2002. Diabetologia 50, 1841–1851 (2007)
D.G. Patterson, W.E. Turner, S.P. Caudill, L.L. Needham, Total TEQ reference range (PCDDs, PCDFs, cPCBs, mono-PCBs) for the US population 2001–2002. Chemosphere 73, S261–S277 (2008)
A. Agudo, F. Goni, A. Etxeandia, A. Vives, E. Millan, R. Lopez, P. Amiano, E. Ardanaz, A. Barricarte, M.D. Chirlaque, M. Dorronsoro, P. Jakszyn, N. Larrañaga, C. Martínez, C. Navarro, L. Rodríguez, M.J. Sánchez, M.J. Tormo, C.A. González, Polychlorinated biphenyls in Spanish adults: determinants of serum concentrations. Environ. Res. 109, 620–628 (2009)
E. Den Hond, E. Govarts, L. Bruckers, G. Schoeters, Determinants of polychlorinated aromatic hydrocarbons in serum in three age classes—methodological implications for human biomonitoring. Environ. Res. 109, 495–502 (2009)
C. La Rocca, S. Alivernini, M. Badiali, A. Cornoldi, N. Iacovella, L. Silvestroni, G. Spera, L. Turrio-Baldassarri, TEQ(S) and body burden for PCDDs, PCDFs, and dioxin-like PCBs in human adipose tissue. Chemosphere 73, 92–96 (2008)
D. Mullerova, J. Kopecky, D. Matejkova, L. Muller, J. Rosmus, J. Racek, F. Sefrna, S. Opatrna, O. Kuda, M. Matejovic, Negative association between plasma levels of adiponectin and polychlorinated biphenyl 153 in obese women under non-energy-restrictive regime. Int. J. Obes. 32, 1875–1878 (2008)
P.E. Scherer, S. Williams, M. Fogliano, G. Baldini, H.F. Lodish, A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270, 26746–26749 (1995)
Y. Arita, S. Kihara, N. Ouchi, M. Takahashi, K. Maeda, J. Miyagawa, K. Hotta, I. Shimomura, T. Nakamura, K. Miyaoka, H. Kuriyama, M. Nishida, S. Yamashita, K. Okubo, K. Matsubara, M. Muraguchi, Y. Ohmoto, T. Funahashi, Y. Matsuzawa, Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 257, 79–83 (1999)
F. Abbasi, J.W. Chu, C. Lamendola, T. McLaughlin, J. Hayden, G.M. Reaven, P.D. Reaven, Discrimination between obesity and insulin resistance in the relationship with adiponectin. Diabetes 53, 585–590 (2004)
K. Hotta, T. Funahashi, Y. Arita, M. Takahashi, M. Matsuda, Y. Okamoto, H. Iwahashi, H. Kuriyama, N. Ouchi, K. Maeda, M. Nishida, S. Kihara, N. Sakai, T. Nakajima, K. Hasegawa, M. Muraguchi, Y. Ohmoto, T. Nakamura, S. Yamashita, T. Hanafusa, Y. Matsuzawa, Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler. Thrombo. Vasc. Biol. 20, 1595–1599 (2000)
D.H. Lee, L. Lind, D.R. Jacobs, S. Salihovic, B. van Bavel, P.M. Lind, Associations of persistent organic pollutants with abdominal obesity in the elderly: the prospective investigation of the vasculature in uppsala seniors (PIVUS) study. Environ. Int. 40, 170–178 (2012)
M.A. Elobeid, M.A. Padilla, D.W. Brock, D.M. Ruden, D.B. Allison, Endocrine disruptors and obesity: an examination of selected persistent organic pollutants in the NHANES 1999-2002 data. Int. J. Environ. Res. Public Health 7, 2988–3005 (2010)
E. Hardell, M. Carlberg, M. Nordstrom, B. van Bavel, Time trends of persistent organic pollutants in Sweden during 1993–2007 and relation to age, gender, body mass index, breast-feeding and parity. Sci. Total Environ. 408, 4412–4419 (2010)
J. Jo, C.M. Nam, J.W. Sull, J.E. Yun, S.Y. Kim, S.J. Lee, Y.N. Kim, E.J. Park, H. Kimm, S.H. Jee, Prediction of colorectal cancer risk using a genetic risk score: the Korean cancer prevention study-II (KCPS-II). Genomics Inform. 10, 175–183 (2012)
S.H. Jee, S. Lee, S. Min, J. Park, H.S. Kim, S.Y. Kim, J.E. Yun, S.J. Lee, E.J. Jee, H.Y. Lee, H.Y. Song, Development of ELISA-kit of quantitative analysis for adiponectin and their correlation with cardiovascular risk factors. Korean J. Epidemiol. 29, 165–175 (2007)
S.H. Jee, J.W. Sull, J.E. Lee, C. Shin, J. Park, H. Kimm, E.Y. Cho, E.S. Shin, J.E. Yun, J.W. Park, S.Y. Kim, S.J. Lee, E.J. Jee, I. Baik, L. Kao, S.K. Yoon, Y. Jang, T.H. Beaty, Adiponectin concentrations: a genome-wide association study. Am. J. Hum. Genet. 87, 545–552 (2010)
C. Pirard, J.F. Focant, P.E. De, An improved clean-up strategy for simultaneous analysis of polychlorinated dibenzo-p-dioxins (PCDD), polychlorinated dibenzofurans (PCDF), and polychlorinated biphenyls (PCB) in fatty food samples. Anal. Bioanal. Chem. 372, 373–381 (2002)
L.L. Cao, C.H. Yan, X.D. Yu, Y. Tian, X.Y. Zou, D.S. Lu, X.M. Shen, Determination of polychlorinated biphenyls and organochlorine pesticides in human serum by gas chromatography with micro-electron capture detector. J. Chromatogr. Sci. 50, 145–150 (2012)
D.H. Lee, P.M. Lind, D.R. Jacobs, S. Salihovic, B. van Bavel, L. Lind, Polychlorinated biphenyls and organochlorine pesticides in plasma predict development of type 2 diabetes in the elderly: the prospective investigation of the vasculature in Uppsala Seniors (PIVUS) study. Diabetes Care 34, 1778–1784 (2011)
J.T. Bernert, W.E. Turner, D.G. Patterson, L.L. Needham, Calculation of serum “total lipid” concentrations for the adjustment of persistent organohalogen toxicant measurements in human samples. Chemosphere 68, 824–831 (2007)
F. Faul, E. Erdfelder, A.G. Lang, A. Buchner, G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 (2007)
F. Faul, E. Erdfelder, A. Buchner, A.G. Lang, Statistical power analyses using G*Power 3.1 tests for correlation and regression analyses. Behav. Res. Methods 41, 1149–1160 (2009)
P.A. Kern, S. Said, W.G. Jackson, J.E. Michalek, Insulin sensitivity following agent orange exposure in Vietnam veterans with high blood levels of 2,3,7,8-tetrachlorodibenzo-p-dioxin. J. Clin. Endocrinol. Metab. 89, 4665–4672 (2004)
V. Arsenescu, R.I. Arsenescu, V. King, H. Swanson, L.A. Cassis, Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ. Health Perspect. 116, 761–768 (2008)
G. Howell 3rd, L. Mangum, Exposure to bioaccumulative organochlorine compounds alters adipogenesis, fatty acid uptake, and adipokine production in NIH3T3-L1 cells. Toxicol. In Vitro 25, 394–402 (2011)
T.J. Schrader, G.M. Cooke, Effects of Aroclors and individual PCB congeners on activation of the human androgen receptor in vitro. Reprod. Toxicol. 17, 15–23 (2003)
A. Rignell-Hydbom, L. Rylander, L. Hagmar, Exposure to persistent organochlorine pollutants and type 2 diabetes mellitus. Hum. Exp. Toxicol. 26, 447–452 (2007)
L. Rylander, A. Rignell-Hydbom, L. Hagmar, A cross-sectional study of the association between persistent organochlorine pollutants and diabetes. Environ. Health 4, 28 (2005)
H.S. Kim, J. Jo, J.E. Lim, Y.D. Yun, S.J. Baek, T.Y. Lee, K.B. Huh, S.H. Jee, Adiponectin as predictor for diabetes among pre-diabetic groups. Endocrine 44, 411–418 (2013)
F. Wang, S.M. Roberts, E.J. Butfiloski, L. Morel, E.S. Sobel, Acceleration of autoimmunity by organochlorine pesticides: a comparison of splenic B-cell effects of chlordecone and estradiol in (NZBxNZW)F1 mice. Toxicol. Sci. 99, 141–152 (2007)
A. Guilherme, J.V. Virbasius, V. Puri, M.P. Czech, Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9, 367–377 (2008)
J.P. Arrebola, J. Pumarega, M. Gasull, M.F. Fernandez, P. Martin-Olmedo, J.M. Molina-Molina, M. Fernandez-Rodriguez, M. Porta, N. Olea, Adipose tissue concentrations of persistent organic pollutants and prevalence of type 2 diabetes in adults from Southern Spain. Environ. Res. 122, 31–37 (2013)
J. Polak, Z. Kovacova, C. Holst, C. Verdich, A. Astrup, E. Blaak, K. Patel, J.M. Oppert, D. Langin, J.A. Martinez, T.I. Sørensen, V. Stich, Total adiponectin and adiponectin multimeric complexes in relation to weight loss-induced improvements in insulin sensitivity in obese women: the NUGENOB study. Eur. J. Endocrinol. 158, 533–541 (2008)
Acknowledgments
This research was supported by a Grant (13162KFDA891) from Korea Food & Drug Administration in 2013 and the National Research Foundation of Korea (NRF) Grant funded by the Korea government (MEST) (2011-0029348).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, Je., Jee, S.H. Association between serum levels of adiponectin and polychlorinated biphenyls in Korean men and women. Endocrine 48, 211–217 (2015). https://doi.org/10.1007/s12020-014-0231-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-014-0231-0