Abstract
The serum levels of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) were measured in a middle-aged Korean population and investigated associations with age, gender, body mass index (BMI), metabolic syndrome (MS), type 2 diabetes mellitus (T2DM), and dietary habits. The median concentrations of 22 OCPs and 34 PCBs in the serum samples were 483 and 216 ng g−1 lipid, respectively. The most abundant compound was p,p’-DDE, followed by PCB 153, β-HCH, PCB 118, and PCB 180. The results of multiple linear regression and other statistical analyses revealed that serum OCP and PCB levels were higher in women and were positively correlated with age. BMI was positively associated with serum OCP and PCB levels, reflecting the influence of food intake and the preserving effect of body fat. MS and T2DM were significantly associated with serum OCP and PCB levels. The intake of animal foods had positive associations with serum OCP and PCB levels, whereas the intake of phytogenic foods showed negative associations, presumably because of contamination levels in food items and food matrices that governs absorption and excretion of OCPs and PCBs in the body. The relationship between dietary habits and serum OCP and PCB levels were different in participants with MS compared to healthy participants, suggesting MS may alter the influence of food intake on serum OCP and PCB levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) are groups of persistent organic pollutants (POPs) that are frequently detected at high levels in the environment (El-Shahawi et al. 2010). Although the Stockholm Convention on POPs agreed to cease production and usage of the chemicals, OCPs and PCBs persist in nature because of their physicochemical and biological stability. Resistant to metabolism and excretion, the chemicals accumulate in organisms and increase through the trophic levels (Metcalf et al. 1973). Humans, as a top predator, are also exposed to OCPs and PCBs via dietary intake, respiration, dermal contact, and intrauterine exposure. OCPs and PCBs in the human body have been reported to be associated with various adverse health effects, including immunological, neurological, and reproductive effects, in addition to the development of cancer (ATSDR 2000, 2002, 2005).
Recently, a growing body of evidence has suggested that exposure to low levels of OCPs and PCBs is associated with environmental diseases, such as metabolic syndrome (MS), including obesity, dyslipidemia, and insulin resistance (Lee et al. 2011a), and type 2 diabetes mellitus (T2DM) (Lee et al. 2010). Furthermore, some toxic effects of POPs showed higher responses at lower doses, suggesting non-monotonic dose-response relationships (Vandenberg et al. 2012). Therefore, the importance of risk assessment for low-dose and chronic exposure in daily life, even without occupational and accidental exposure, has been increasing.
As the primary route of human exposure to legacy POPs is dietary intake (Lohmann et al. 2007), previous studies have attempted to evaluate dietary POP exposure by analyzing POP levels in food items and total daily intake (Storelli et al. 2011; Trudel et al. 2011). These approaches have helped provide estimations of total external dietary exposure and major food sources of POPs. The foodstuff analysis and total daily evaluations, however, could not determine the influence of dietary intake on POP levels in the body, because the absorption, distribution, and excretion of POPs vary with the physicochemical properties of individual compounds, the physiological characteristics of subjects, and the matrices of foods (Escher and Hermens 2004; Jandacek and Tso 2001).
A biomonitoring study combined with lifestyle surveys could be appropriate to investigate the actual influence of dietary intake on POP levels in the body as these approaches can identify direct associations between dietary habits and serum POP levels. Previous studies reported associations between dietary habits and serum POP levels (Gasull et al. 2011; Lee et al. 2007; Tsukino et al. 2006), but discussions regarding the causal relationship were limited. While several studies reported OCP and PCB levels in food items (Moon and Choi 2009; Son et al. 2012) and human samples (Kang et al. 2008; Park et al. 2014, 2007) in Korea, there have been no studies that investigated the association between dietary habits and body conditions with internal OCP and PCB exposure in Korea.
The aims of this study were to determine serum OCP and PCB levels and investigate their associated factors in a Korean population; we investigated associations of OCP and PCB levels with age, gender, body mass index (BMI), MS, T2DM, and dietary habits.
Material and methods
Study subjects
A community-based health survey was conducted between June and December 2006 in Uljin County, South Korea. This county is located on the shore of the East Sea, and the majority of the population is involved in fishery and agriculture. Residents over 40 years of age volunteered, and a total of 180 subjects who had not been occupationally or accidentally exposed to OCPs or PCBs were selected. As all participants were life-long residents of this county, there were no geographical differences among the participants. Demographic information and dietary intake were recorded for all participants by trained interviewers using a standardized questionnaire during face-to-face interviews. This study was approved by the Institutional Review Board at the Kyungpook National University Hospital.
Table 1 presents demographic characteristics and dietary habits of the participants. Among the total 180 participants, 70 were male and 110 were female. The mean ages of the male and female participants were 54.8 and 57.0 years, and average BMIs were 24.6 and 23.8 kg/m2, respectively. Twenty-five participants were smokers, and 70 participants consumed alcohol. Fifty participants had MS without T2DM, 40 participants had T2DM without MS, and 90 participants had neither MS nor T2DM.
MS was diagnosed based on the concomitant presence of at least three of the following five features: waist circumference ≥ 90 cm (men) or ≥ 80 cm (women); arterial pressure ≥ 130/85 mmHg or the receipt of antihypertensive medication; triglyceride (Tg) levels ≥ 150 mg/dL; fasting blood glucose ≥ 110 mg/dL; and high-density lipoprotein levels < 40 mg/dL (men) or < 50 mg/dL (women). T2DM was defined as fasting blood glucose level ≥ 126 mg/dL or the receipt of antidiabetic medication.
Food frequency questionnaire
Individual dietary intake over the past 12 months was assessed using a 106-item food frequency questionnaire (FFQ). This FFQ was developed for the Korea Genome Epidemiology Study and was validated for large population-based studies in Korea (Ahn et al. 2007). Participants recorded serving size (small, medium, or large) and the frequency of consumption of specific foods and beverages. We used nine predefined frequency categories: never or seldom, once a month, 2–3 times a month, 1–2 times a week, 3–4 times a week, 5–6 times a week, once a day, twice a day, and > 3 times a day. Images of food item serving sizes were provided for easy reference. The consumption frequency of each item was weighted according to the reported serving size. Items reported with a serving size of “small” were assigned a weightage of 0.5, and items reported with a serving size of “large” were assigned a weightage of 1.5. The weighted frequencies were uniformly converted to servings per day. Food items included in the FFQ were categorized into nine food groups: meats, seafood, grains, vegetables, fruits, seaweeds, beans, dairy products, and eggs. For a detailed investigation, meats and seafood were subcategorized into beef, pork, and chicken and fish and shellfish, respectively. Drinking and smoking were treated as dichotomous variables without considering quantity or frequency.
Measurement of OCPs and PCBs
The analytical method described by Kang et al. (2008) was applied with some modifications. Two milliliters of serum samples were spiked with 13C-labeled OCP (ES-5349; Cambridge Isotope Labs, USA) and PCB (P48M-ES; Wellington Laboratory, Canada) standards and allowed to equilibrate. The samples were denatured and diluted with equal amounts of formic acid and deionized water and then extracted with C18 SPE cartridges (Waters, USA). The eluates were applied to a silica gel and Florisil cartridge (Waters, USA), dried, and then transferred to vials. Before the instrumental analysis, the samples were reconstituted with 13C-labeled standards (EC-5350; Cambridge Isotope Labs, USA and P48M-RS, Wellington Laboratory, Canada). A high-resolution mass spectrometer (JMS-800D; JEOL, Japan) interfaced with a 6890 N gas chromatograph (Agilent Technologies, USA) was employed to measure the levels of the analytes. Total lipids were estimated using the following formula (Phillips et al. 1989): total lipids (mg dL−1) = 2.27 × total cholesterol + Tg + 62.3.
Quality control
The resolution of high-resolution mass spectrometry was maintained higher than 10,000 in all selective ion monitoring ranges, and the linearity (r 2) of relative response factors were higher than 0.99. The instrumental limit of detection (LOD) was employed, and LODs were calculated as three times the signal to noise ratio of the chromatogram. The isotope dilution method was used for OCP and PCB determinations, and the recoveries of 13C-labeled compounds were within 50–120%. A method blank sample was analyzed for every 11 samples, and the blank contaminations were less than 5% of the average concentrations. The blank levels were corrected by subtracting from measured sample levels. The accuracy of the measurement was tested analyzing standard reference materials (SRM 1957 and SRM 1958 from NIST, USA), and the measured concentrations were within the given ranges.
Statistical analysis
PASW Statistics for Windows, Version 18.0 (SPSS Inc., Chicago, USA) was used for all statistical analyses. The correlations among the variables were tested using Spearman’s rank correlation, and the Kruskal–Wallis test and Mann–Whitney U test were used to compare OCP and PCB levels among different drinking, smoking, and disease groups. Values below the LOD were considered to be zero for the Spearman’s rank correlation, Kruskal–Wallis test, and Mann–Whitney U test.
Multiple linear regression (MLR) analysis was performed to comprehensively investigate associations of age, gender, BMI, T2DM, MS, and dietary habits with serum OCP and PCB levels. The variables were input in a hierarchical order in the MLR analysis: age, gender, BMI, drinking, and smoking in the first group; and dietary habits in the second group. Before analysis, all variables, except gender, smoking, and drinking, were log transformed to correct skewed data, and values below the LOD were considered to be LOD/2. The optimal model for each compound was selected based on the change in the adjusted coefficient of determination (R 2*) and F. We confirmed that the variance inflation factors of all variables were < 5.0, and the results of the Durbin–Watson test were within 2.0 ± 0.5.
Results and discussion
Serum OCP and PCB levels
The concentrations of OCPs and PCBs in the serum of participants are shown in Fig. 1 and Table S1 in Supporting Information. Among 22 OCP compounds, p,p’-dichlorodiphenyldichloroethylene (p,p’-DDE) was detected at the highest level, followed by β-hexachlorocyclohexane (β-HCH), p,p’-dichlorodiphenyltrichloroethane (p,p-DDT), trans-nonachlor, hexachlorinated benzene (HCB), oxychlordane, and heptachlor epoxide. These seven OCP compounds accounted for 93.2% of total OCP levels. The serum levels of all 22 OCPs ranged from 38.8 to 4600 ng g−1 lipid, with mean and median levels of 638 and 483 ng g−1 lipid, respectively. Among the 34 PCB congeners, PCB 153 was detected at the highest level, followed by PCB 118, 138, 180, and 187. The serum levels of the 34 PCBs ranged from 35.6 to 1680 ng g−1 lipid, with mean and median levels of 294 and 483 ng g−1 lipid, respectively.
Concentrations of major OCP and PCB compounds in the serum of the participants. a Serum OCP levels. b Serum PCB levels. β-HCH β-hexachlorocyclohexane; HCB hexachlorinated benzene; p,p’-DDE p,p’-dichlorodiphenyldichloroethylene; p,p’-DDT p,p’-dichlorodiphenyltrichloroethane; p,p’-DDD p,p’-dichlorodiphenyldichloroethane; OCP organochlorine pesticides; PCB polychlorinated biphenyls
These results provide valuable information on the background serum OCP and PCB levels of populations living in a coastal/rural of Korea, because only a few studies have reported serum levels of OCPs and PCBs in Korea, as discussed above. The serum levels of most of OCP and PCB compounds from this study were higher than those for adult from urban areas in Korea, but the distributions of OCPs and PCBs were similar (Kang et al. 2008; Park et al. 2014, 2007). However, the OCPs and PCBs in the serum of prepubertal Korean children were much lower than those from this study, likely because of the association of serum POP levels with age (Park et al. 2016).
The OCP and PCB levels in this study were relatively higher than those in studies conducted in Japan (Tsukino et al. 2006), the UK (Thomas et al. 2006), and the USA (CDC 2005), but lower than those reported in a Romanian population (Dirtu et al. 2006). Finally, serum OCP levels were lower and serum PCB levels were higher in the present study than in studies conducted in China (Bi et al. 2007, Lee et al. 2007). Regional differences in serum OCP and PCB levels can be explained by different dietary habits and different contamination levels in these countries, both in food and in the environment.
According to the results of the Spearman’s rank correlation analysis among the major OCP and PCB congeners, serum levels of the OCPs and PCBs were significantly and positively correlated with one another (ρ = 0.257–0.969, p < 0.01). The highest correlation coefficient was between PCB 153 and PCB 180 (ρ = 0.969). The correlations between OCPs and PCBs were substantially strong (ρ = 0.257–0.864), with the highest correlation observed between trans-nonachlor and PCB 138 (ρ = 0.864). The correlations between heptachlor epoxide and other contaminants were relatively weak (ρ = 0.257–0.503) but significant (p < 0.01). The significant, strong correlations among OCPs and PCBs implied similar exposure routes and toxicokinetics of the chemicals. After the manufacture and usage of OCPs and PCBs were banned in Korea a few decades ago, environmental levels and sources of direct exposure have been decreasing, and therefore, dietary exposure became the major source of exposure to OCPs and PCBs. Meanwhile, the correlations of some metabolites, such as heptachlor epoxide, with the other compounds were relatively low, presumably because of different exposure routes.
Association of age, gender, and BMI with serum OCP and PCB levels
The results of the MLR analysis are presented in Table 4, and the bivariate associations of gender, age, and BMI with serum OCP and PCB levels and food intakes are shown in Table S2 in Supporting Information. The results of the MLR and Spearman’s rank correlation showed that age was positively associated with some serum OCP and PCB levels. It is well known that increased age is associated with a higher body burden of POPs due to greater exposure before the ban of POP production in addition to bodily accumulation of POPs over a longer lifetime (Hirai et al. 2005; Ben Hassine et al. 2014; Mori et al. 2014). In addition, age-associated deteriorating metabolism (Laden et al. 1999) and lower dietary intake in older groups in this study could result in lower serum OCP and PCB levels (Table S2 in Supporting Information).
According to the results of the MLR and Mann–Whitney U test, serum levels of most OCP and PCB congeners differed significantly by gender and were higher in male than female participants. In general, men have higher body POP burdens than women because women lose POPs through parity and breastfeeding (Hardell et al. 2010, Mori et al. 2014). In addition, as men generally consume more foods than women do, it may be speculated that higher dietary POP exposure could increase POP levels in the male body.
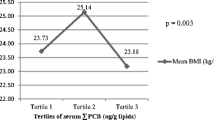
BMI was positively associated with serum OCP and PCB levels in this study, while previous reports on the association of BMI with POP levels have been inconsistent. A larger body size can increase the half-life of POPs by preserving them in fat, according to the toxicokinetics of POPs (Jandacek and Tso 2001). In previous studies in adults, positive associations between BMI and serum POP levels were found (Lee et al. 2007; Cao et al. 2011). On the other hand, as BMI is linked to fat storage of lipophilic compounds in the human body, larger body size can act as a larger reservoir to dilute POP concentration. In the studies of adolescents and pregnant women, whose bodies are growing, BMI and serum OCP and PCB levels had negative correlations (Nawrot et al. 2002; Dhooge et al. 2010; Humblet et al. 2010). The participants in this study were middle-aged, and BMI was positively associated with serum OCP and PCB levels, in accordance with previous studies. In addition, it is also possible that higher dietary intake may have contributed to higher POP exposure, since there is a significant association between BMI and food intake (Table S2 in Supporting Information).
Association of MS and T2DM with serum OCP and PCB levels
Table 2 compares serum OCP and PCB levels between healthy participants and those with MS or T2DM; levels were higher in participants with MS or T2DM than in healthy participants, although the dietary habits among the participant groups were not significantly different (data not shown). Participants with MS showed significantly higher serum levels of β-HCH, heptachlor epoxide, and trans-nonachlor than healthy participants and compared to healthy participants; those with T2DM had significantly higher serum levels of all individual OCPs, total OCPs, and PCB 105, 118, 138, and 146.
The associations between MS and T2DM with POP levels were identified in a few previous studies (Lee et al. 2010, 2011a). The causal relationship, however, has only recently begun to be clarified. As discussed above, increased body fat may extend the half-lives of POPs, or other confounders, such as dietary, socioeconomic, and genetic factors, might affect both the prevalence of MS and T2DM and serum POP levels. Recent studies have suggested toxic mechanisms that lead to the development of MS and T2DM (Lee et al. 2014), including gene expression alterations (Arzuaga et al. 2009, Lyche et al. 2011), chronic inflammation in adipose tissue (Arsenescu et al. 2008), interactions with gut microbiota (Lee et al. 2011b), global DNA hypomethylation (Rusiecki et al. 2008), and mitochondrial dysfunction (Lim et al. 2010). In-depth research is required to understand the comprehensive association among these diseases and serum POP levels.
It should be pointed out that the data from patients with MS or T2DM in this study might have measurement biases. Porta et al. (2009) pointed out that the lipid adjustment method reported by Phillips et al. (1989), which was used in this study, might inaccurately estimate serum POP levels in patients with high serum lipid levels, such as patients with MS or T2DM. In addition, a simulation study by Schisterman et al. (2005) noted that lipid-standardization of serum POP concentrations could be biased when additional factors influence both serum PCB levels and serum lipid contents. Lastly, as patients with diseases tend to modify their lifestyles after diagnosis, the FFQ data might not reflect the long-term dietary habits of the subjects. Therefore, we subdivided the subjects into three subgroups for partial correlation and MLR analyses: participants with MS, participants with T2DM, and participants who did not have MS or T2DM (healthy participants).
Association between serum OCP and PCB levels and dietary habits
Although there have been a few studies reporting the internal burden of OCPs and PCBs in Korean populations, the influence of dietary intake on the body burden of OCPs and PCBs has not been thoroughly investigated. To our knowledge, this is the first study to investigate associations between serum OCP and PCB levels and dietary intake in the Korean population, which can help improve our understanding of the influence of dietary OCP and PCB exposure.
The partial correlation coefficients between log-transformed serum OCP and PCB levels and dietary intake for the healthy participants, adjusted for age, gender, BMI, drinking, and smoking, are shown in Table 3, and the results of the MRL analysis are shown in Table 4. In general, the intake of animal foods, including meats and seafood, was positively correlated with serum OCP and PCB levels, while the intake of phytogenic foods, including grains, fruits, and vegetables, was negatively correlated with serum OCP and PCB levels.
The association between animal food consumption and elevated serum POP levels has been reported by many previous studies, including those by Arrebola et al. (2009), Tsukino et al. (2006), and Gasull et al. (2011). The positive associations between animal foods and serum POP levels were attributed to the high POP levels in animal foods (Moser and McLachlan 2001) as a result of biomagnification. The results of this study could also be explained by high PCB levels in animal food items in the previous market basket surveys in Korea (Son et al. 2012; Shin et al. 2015). In addition, the digestible fat in animal foods helps to absorb POPs in the gastrointestinal tract; this enhances the formation of bile salt micelles that carry POPs to the intestinal lumen, and the absorption of digestible fat accelerates POP absorption by increasing the fugacity of the ingesta and decreasing the POP fugacity in intestinal cells (Kelly et al. 2004).
The scarce positive associations of phytogenic foods with serum OCP and PCB levels resulted from the low OCP and PCB levels in phytogenic foods (Son et al. 2012). Furthermore, it was noteworthy that phytogenic food intake did not just increase serum OCP and PCB levels, but rather decreased them. If total daily OCP and PCB intake governed serum OCP and PCB levels, serum OCP and PCB levels should be positively associated with the intake of phytogenic foods, because intake of grains and vegetables accounted for the majority of total PCB consumption in the Korean population (Son et al. 2012; Shin et al. 2015). The negative association was likely attributable to the non-digestible fraction of phytogenic foods that inhibit the absorption of POPs in the gastrointestinal tract and enhance their fecal excretion (Kelly et al. 2004; Jandacek and Tso 2001).
The results of the Kruskal–Wallis test showed smoking was positively associated with serum OCP and PCB levels, whereas those of the MLR did not show a significant association (Table S3 in Supporting Information and Table 4). The positive association between smoking and serum POP levels was previously reported by Mcgraw and Waller (2009), whereas a negative association was reported by Hsu et al. (2009), Ibarluzea et al. (2011), and Arrebola et al. (2012). Arrebola et al. (2012) found a positive association of smoking habit with p,p’-DDT, and a negative association with its metabolite, p,p’-DDE, suggesting that exposure to polycyclic aromatic hydrocarbons in cigarette smoke might enhance cytochrome P450 activity and stimulate POP metabolism. The insignificant association found in this study with the MRL, unlike the result observed with the Kruskal–Wallis test, implied that smoking was not directly associated with serum POP levels, but related with other dietary habits.
Compared to non-drinkers, participants who currently or previously consumed alcohol showed higher serum levels of most OCPs and PCBs (Table S4 in Supporting Information and Table 4). A positive correlation between POP levels and alcohol consumption has been observed in various human samples, including milk, serum, and adipose tissue (Dewailly et al. 1996; Ibarluzea et al. 2011; Mcgraw and Waller 2009). Dewailly et al. (1996) suggested that the association between elevated POP levels and alcohol consumption might be a result of impaired hepatic metabolic rates and enhanced POP absorption by increased solubility in the gastrointestinal tract.
The FFQ approach employed in this study may have some limitations. First, although the FFQ used in this study was verified (Ahn et al. 2007), the quantitative analysis of food intake in the Korean population still had some uncertainties because Korean people consume mixed foods and share dishes. In addition, the number of subjects included in this study might be insufficient to make conclusions. Nonetheless, the results consistently showed significant associations between dietary habits and serum OCP and PCB levels. Our findings suggested that using food matrices rather than the total amount of OCPs and PCBs in food items is more important in estimating the actual influence of dietary intake on internal levels of OCPs and PCBs. In addition, the previous total OCP and PCB daily intake studies might have overestimated the health effect of dietary OCP and PCB exposure on subjects mainly exposed to OCPs and PCBs through phytogenic foods, such as in the Korean population. In addition, the increase in OCP and PCB exposure should be considered in future risk assessments in the Korean population due to the increasing intake of meats.
Influence of dietary habits on serum OCP and PCB levels
The results of the correlation analyses in participants with MS or T2DM showed just a few significant findings (data not shown). The intake of fruits with HCB, beans with HCB, PCB 138, and PCB 153, and shellfish with HCB showed positive correlations in those with MS (Table S5 in Supporting Information). Only the intake of fruits with β-HCH and beef with HCB showed negative correlations in participants with T2DM. The MLR models for participants with MS (Table 5) showed fewer independent variables compared to those for healthy participants. Gender differences and drinking remained significant in all models, whereas age was significant only in some PCB models. Like the results of healthy participants, the intake of grains and vegetables were negatively associated, and the intake of shellfish and pork showed a positive association. A positive association of OCP and PCB levels with bean intake and a negative association with the intake of dairy products were also identified. The results of the MLR analysis for participants with T2DM were not significant (p > 0.05).
Despite the potential uncertainties, the results of the MLR analysis for participants with MS could help assess the risk of dietary OCP and PCB exposure. The differences between healthy participants and those with MS could suggest that MS might alter the influence of dietary intake on serum OCP and PCB levels. The positive association of bean intake with serum OCP and PCB levels is particularly noteworthy because bean-rich meals have been recommended as dietetic therapy for MS patients. Several studies have reported alterations in metabolism due to intake of beans. For example, Nagasawa et al. (2003) found that the intake of soy protein isolate increased plasma adiponectin and decreased PAI-1 expression, fatty acid synthase mRNA, and plasma Tg levels. Soy-based diet supplements reduced plasma levels of total cholesterol, Tg, non-high-density lipoprotein cholesterol, and free fatty acids (Dyrskog et al. 2005). In addition, soy intake altered lipid peroxidation in postmenopausal women with MS (Azadbakht et al. 2007), and dietary soy protein isolate affected the peroxisome proliferator-activated receptor, liver-X receptor, sterol regulatory element-binding protein signaling, and the attenuation of MS (Ronis et al. 2009). The data from participants with MS might have significant biases; therefore, further research is necessary to verify these results.
Conclusions
This study reported serum OCP and PCB levels in a Korean population. As previous reports on serum OCP and PCB levels in Korean populations have been insufficient, this study provides valuable information on serum contamination levels in a general Korean population. In addition, this study found significant associations of the OCP and PCB levels with age, gender, BMI, MS, T2DM, and dietary habits, which was the first study to elucidate factors associated with internal OCP and PCB exposure based on toxicokinetics and OCP and PCB levels in regional food items. Although this study has several limitations and uncertainties and therefore the conclusions may not be generalized, the findings in this study can improve our understanding on the risk and mitigation of the dietary OCP and PCB exposures and their associations with body conditions. The results of this study must be verified in carefully designed studies with larger numbers of subjects.
References
Ahn Y, Kwon E, Shim JE, Park MK, Joo Y, Kimm K, Park C, Kim DH (2007) Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr 61(12):1435–1441
Arrebola J, Martinolmedo P, Fernandez M, Sanchezcantalejo E, Jimenezrios J, Torne P, Porta M, Olea N, (2009) Predictors of concentrations of hexachlorobenzene in human adipose tissue: Amultivariate analysis by gender in Southern Spain Environ Int 35:27–32
Arrebola JP, Mutch E, Rivero M, Choque A, Silvestre S, Olea N, Ocaña-Riola R, Mercado L a (2012) Contribution of sociodemographic characteristics, occupation, diet and lifestyle to DDT and DDE concentrations in serum and adipose tissue from a Bolivian cohort. Environ Int 38:54–61
Arsenescu V, Arsenescu RI, King V, Swanson H, Cassis LA (2008) Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ Health Perspect 116:761–768
Arzuaga X, Ren N, Stromberg A, Black EP, Arsenescu V, Cassis LA, Majkova Z, Toborek M, Hennig B (2009) Induction of gene pattern changes associated with dysfunctional lipid metabolism induced by dietary fat and exposure to a persistent organic pollutant. Toxicol Lett 189:96–101
ATSDR (2000) Toxicological profile for polychlorinated biphenyls (PCBs)
ATSDR (2002) Toxicological profile for DDT, DDE, and DDD
ATSDR (2005) Toxicological profile for alpha-, beta-, gamma-, and delta-hexachlorocyclohexane
Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, Hu FB, Willett WC (2007) Dietary soya intake alters plasma antioxidant status and lipid peroxidation in postmenopausal women with the metabolic syndrome. Br J Nutr 98:807–813
Ben Hassine S, Hammami B, Ben Ameur W, El Megdiche Y, Barhoumi B, El Abidi R, Ridha Dri M (2014) Concentrations of organochlorine pesticides and polychlorinated biphenyls in human serum and their relation with age, gender, and BMI for the general population of Bizerte, Tunisia. Environ Sci Pollut Res 21:6303
Bi X, Thomas GO, Jones KC, Qu W, Sheng G, Martin FL, Fu J (2007) Exposure of electronics dismantling workers to polybrominated diphenyl ethers, polychlorinated biphenyls, and organochlorine pesticides in South China. Environ Sci Technol 41:5647–5653
Cao L-L, Yan C-H, Yu X-D, Tian Y, Zhao L, Liu J-X, Shen X-M (2011) Relationship between serum concentrations of polychlorinated biphenyls and organochlorine pesticides and dietary habits of pregnant women in Shanghai. Sci Total Environ 409:2997–3002
CDC (2005) Third national report on human exposure to environmental chemicals
Dewailly E, Ayotte P, Laliberté C, Weber JP, Gingras S, Nantel AJ (1996) Polychlorinated biphenyl (PCB) and dichlorodiphenyl dichloroethylene (DDE) concentrations in the breast milk of women in Quebec. Am J Public Health 86:1241–1246
Dhooge W, Den Hond E, Koppen G, Bruckers L, Nelen V, Van De Mieroop E, Bilau M, Croes K, Baeyens W, Schoeters G et al (2010) Internal exposure to pollutants and body size in Flemish adolescents and adults: associations and dose-response relationships. Environ Int 36:330–337
Dirtu AC, Cernat R, Dragan D, Mocanu R, Van Grieken R, Neels H, Covaci A (2006) Organohalogenated pollutants in human serum from Iassy, Romania and their relation with age and gender. Environ Int 32:797–803
Dyrskog S, Jeppesen P, Colombo M, Abudula R, Hermansen K (2005) Preventive effects of a soy-based diet supplemented with stevioside on the development of the metabolic syndrome and type 2 diabetes in Zucker diabetic fatty rats. Metabolism 54(9):1181–1188
El-Shahawi MS, Hamza A, Bashammakh AS, Al-Saggaf WT (2010) An overview on the accumulation, distribution, transformations, toxicity and analytical methods for the monitoring of persistent organic pollutants. Talanta 80:1587–1597
Escher BI, Hermens JLM (2004) Peer reviewed: internal exposure: linking bioavailability to effects. Environ Sci Technol 38:455A–462A
Gasull M, Bosch M, Basea D, Puigdomènech E, Pumarega J, Porta M (2011) Empirical analyses of the influence of diet on human concentrations of persistent organic pollutants : a systematic review of all studies conducted in Spain. Environ Int 37:1226–1235
Hardell E, Carlberg M, Nordström M, van Bavel B (2010) Time trends of persistent organic pollutants in Sweden during 1993-2007 and relation to age, gender, body mass index, breast-feeding and parity. Sci Total Environ 408:4412–4419
Hirai T, Fujimine Y, Watanabe S, Nakano T (2005) Congener-specific analysis of polychlorinated biphenyl in human blood from Japanese. Environ Geochem Health 27(1):65–73
Hsu J-F, Lee C-C, Su H-J, Chen H-L, Yang S-Y, Liao P-C (2009) Evaluation of background persistent organic pollutant levels in human from Taiwan: polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls. Environ Int 35:33–42
Humblet O, Williams PL, Korrick S a, Sergeyev O, Emond C, Birnbaum LS, Burns JS, Altshul L, Patterson DG, Turner WE, Lee MM, Revich B, Hauser R (2010) Predictors of serum dioxin, furan, and PCB concentrations among women from Chapaevsk, Russia. Environ Sci Technol 44:5633–5640
Ibarluzea J, Alvarez-Pedrerol M, Guxens M, Marina LS, Basterrechea M, Lertxundi A, Etxeandia A, Goñi F, Vioque J, Ballester F, Sunyer J (2011) Sociodemographic, reproductive and dietary predictors of organochlorine compounds levels in pregnant women in Spain. Chemosphere 82:114–120
Jandacek RJ, Tso P (2001) Factors affecting the storage and excretion of toxic lipophilic xenobiotics. Lipids 36:1289–1305
Kang J, Park H, Chang Y, Choi J-W (2008) Distribution of organochlorine pesticides (OCPs ) and polychlorinated biphenyls ( PCBs ) in human serum from urban areas in Korea. Chemosphere 73:1625–1631
Kelly B, Gobas FAPC, S.McLachlan M (2004) Intestinal absorption and biomagnification of organic contaminants in fish, wildlife, and humans. Environ Toxicol Chem 23:2324–2336
Laden F, Neas LM, Spiegelman D, Hankinson SE, Willett WC, Ireland K, Wolff MS, Hunter DJ, Wafter C (1999) Predictors of plasma concentrations of DDE and PCBs in a group of U.S. women. Environ Health Perspect 107:75–81
Lee S, Dai Q, Zheng W, Gao Y, Blair A, Tessari JD, Tian B, Shu X, Tian Ji B (2007) Association of serum concentration of organochlorine pesticides with dietary intake and other lifestyle factors among urban Chinese women. Environ Int 33:157–163
Lee D-H, Steffes MW, Sjödin A, Jones RS, Needham LL, Jacobs DR (2010) Low dose of some persistent organic pollutants predicts type 2 diabetes: a nested case-control study. Environ Health Perspect 118:1235–1242
Lee D-H, Steffes MW, Sjödin A, Jones RS, Needham LL, Jacobs DR (2011a) Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PLoS One 6:e15977
Lee H-S, Lee J-C, Lee I-K, Moon H-B, Chang Y-S, Jacobs DR, Lee D-H (2011b) Associations among organochlorine pesticides, Methanobacteriales, and obesity in Korean women. PLoS One 6:e27773
Lee D-H, Porta M, Jacobs DR, Vandenberg LN (2014) Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr Rev 35:557–601
Lim S, Cho YM, Park KS, Lee HK (2010) Persistent organic pollutants, mitochondrial dysfunction, and metabolic syndrome. Ann N Y Acad Sci 1201:166–176
Lohmann R, Breivik K, Dachs J, Muir D (2007) Global fate of POPs: current and future research directions. Environ Pollut 150:150–165
Lyche JL, Nourizadeh-Lillabadi R, Karlsson C, Stavik B, Berg V, Skåre JU, Alestrøm P, Ropstad E (2011) Natural mixtures of POPs affected body weight gain and induced transcription of genes involved in weight regulation and insulin signaling. Aquat Toxicol 102:197–204
Mcgraw JE, Waller DP (2009) Fish ingestion and congener specific polychlorinated biphenyl and p,p’-dichlorodiphenyldichloroethylene serum concentrations in a great lakes cohort of pregnant African American women. Environ Int 35:557–565
Metcalf R, Kapoor I, Lu P (1973) Model ecosystem studies of the environmental fate of six organochlorine pesticides. Environ Health Perspect 44:197–207
Moon H, Choi H-G (2009) Human exposure to PCDDs, PCDFs and dioxin-like PCBs associated with seafood consumption in Korea from 2005 to 2007. Environ Int 35:279–284
Mori C, Kakuta K, Matsuno Y, Todaka E, Watanabe M, Hanazato M, Kawashiro Y, Fukata H (2014) Polychlorinated biphenyl levels in the blood of Japanese individuals ranging from infants to over 80 years of age. Environ Sci Pollut Res 21:6434
Moser GA, McLachlan MS (2001) The influence of dietary concentration on the absorption and excretion of persistent lipophilic organic pollutants in the human intestinal tract. Chemosphere 45:201–211
Nagasawa A, Fukui K, Kojima M, Kishida K, Maeda N, Nagaretani H, Hibuse T (2003) Divergent effects of soy protein diet on the expression of adipocytokines. Biochem Biophys Res Commun 311:909–914
Nawrot TS, Staessen JA, Den Hond EM, Koppen G, Schoeters G, Fagard R, Thijs L, Winneke G, Roels H a (2002) Host and environmental determinants of polychlorinated aromatic hydrocarbons in serum of adolescents. Environ Health Perspect 110:583–589
Park H, Lee S, Kang J, Chang Y (2007) Congener-specific approach to human PCB concentrations by serum analysis. Chemosphere 68:1699–1706
Park H, Park E, Chang Y-S (2014) Ten-year time trend of dioxins in human serum obtained from metropolitan populations in Seoul. Korea Sci Total Environ 470-471:1338–1345
Park SH, Hong YS, Ha EH, Park H (2016) Serum concentrations of PCBs and OCPs among prepubertal Korean children. Environ Sci Pollut Res 23:3536
Phillips DL, Pirkle JL, Burse VW, Bernert JT, Henderson LO, Needham LL (1989) Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol 18:495–500
Porta M, Jariod M, López T, Pumarega J, Puigdomènech E, Marco E, Malats N, Grimalt JO, Real FX (2009) Correcting serum concentrations of organochlorine compounds by lipids: alternatives to the organochlorine/total lipids ratio. Environ Int 35:1080–1085
Ronis MJ, Chen Y, Badeaux J, Badger TM (2009) Dietary soy protein isolate attenuates metabolic syndrome in rats via effects on PPAR, LXR, and SREBP signaling. J Nutr 139(8):1431–1438
Rusiecki JA, Baccarelli A, Bollati V, Tarantini L, Moore LE, Bonefeld-Jorgensen EC (2008) Global DNA hypomethylation is associated with high serum-persistent organic pollutants in Greenlandic Inuit. Environ Health Perspect 116:1547–1552
Schisterman EF, Whitcomb BW, Buck Louis GM, Louis T a (2005) Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ Health Perspect 113:853–857
Shin ES, Nguyen KH, Kim J, Kim CI, Chang YS (2015) Progressive risk assessment of polychlorinated biphenyls through a total diet study in the Korean population. Environ Pollut 207:403–412
Son M-H, Kim J, Park H, Kim M, Paek O, Chang Y (2012) Assessment of the daily intake of 62 polychlorinated biphenyls from dietary exposure in South Korea. Chemosphere 89:957–963
Storelli MM, Barone G, Perrone VG, Giacominelli-Stuffler R (2011) Polychlorinated biphenyls (PCBs), dioxins and furans (PCDD/Fs): occurrence in fishery products and dietary intake. Food Chem 127:1648–1652
Thomas GO, Wilkinson M, Hodson S, Jones KC (2006) Organohalogen chemicals in human blood from the United Kingdom. Environ Pollut 141:30–41
Trudel D, Scheringer M, Goetz NV, Hungerb K (2011) Total consumer exposure to polybrominated diphenyl ethers in North America and Europe. Environ. Sci. Technol. 45:2391–2397
Tsukino H, Hanaoka T, Sasaki H, Motoyama H, Hiroshima M, Tanaka T, Kabuto M, Turner W, Patterson DG, Needham L, Tsugane S (2006) Fish intake and serum levels of organochlorines among Japanese women. Sci Total Environ 359:90–100
Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee D-H, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP (2012) Hormones and endocrine-disrupting chemicals: low-dose effects and non-monotonic dose responses. Endocr Rev 33:378–455
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NO. NRF-2017R1A2B3012681) and a research grant (PE17040) from the Korea Polar Research Institute (KOPRI).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Roland Kallenborn
Electronic supplementary material
ESM 1
(DOCX 56 kb)
Rights and permissions
About this article
Cite this article
Kim, JT., Kang, JH., Chang, YS. et al. Determinants of serum organochlorine pesticide and polychlorinated biphenyl levels in middle-aged Korean adults. Environ Sci Pollut Res 25, 249–259 (2018). https://doi.org/10.1007/s11356-017-0382-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0382-7