Abstract
Bisphenol A (BPA) is a chemical that is used in a variety of consumer products, and exposure to BPA is widespread among the general population. Recent studies have suggested that BPA may affect the thyroid and related pathways. However, human studies are still limited. The aim of this study was to determine the relationship between BPA exposure and thyroid function. We obtained survey data and blood samples from The Thai National Health Examination Survey IV 2009, a nationally representative cross-sectional survey using a multistage, stratified sampling of the Thai population. A total of 2,340 subjects aged 18–94 years were sampled for the present study. Serum BPA, TSH, FT4, and related covariates were measured. BPA was log-transformed prior to analysis. BPA was detected in 52.8 % of serum samples with a median concentration of 0.33 (range 0–66.91) ng/mL. We excluded subjects who tested positive for thyroid autoantibody and then stratified the remaining subjects by gender; the analysis showed a significantly negative correlation between serum BPA and FT4 levels in males (r = −0.14, P < 0.001). In contrast, no association was observed in females. BPA was not associated with TSH in either gender. This gender-related discrepancy is possibly related to androgen-related differences in the metabolism of BPA. Our preliminary results provide evidence of a negative association between BPA and FT4 levels. Additional detailed studies are needed to investigate the temporal relationship and potential public health implications of such an association.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bisphenol A (BPA or 4,4′-isopropylidenediphenol), a xenoestrogen compound, is a pervasive endocrine-disrupting chemical (EDC) used in the manufacturing industry as an important intermediate in the production of polycarbonate plastics and epoxy resins. BPA is found in a wide variety of products, such as food and beverage containers (including baby and water bottles), dental fillings and sealants, water supply pipes, coatings, flame-retardant materials, recycled paper, and cash register receipts. Exposure to BPA, not only primarily through food and drinking water, but also through dermal exposure and inhalation of household dusts, is confirmed by the presence of urinary BPA in more than 90 % of the US population [1]. Historically, BPA was thought to be rapidly degraded and excreted from the body, but new studies point to the potential bioaccumulation of BPA in adipose tissue [2, 3]. BPA has been reported in concentrations of 1–10 ng/mL in the serum of pregnant women, in the amniotic fluid of their fetuses, and in cord serum taken at birth [4, 5]. In addition, BPA concentrations of up to 100 ng/g were reported in the placentas [5]. BPA exposure has the potential to influence numerous, wide-ranging, and complex outcomes. To date, health complications related to BPA exposure include diabetes mellitus, increased adiposity, altered brain development and sexual differentiation, altered behavior, neoplasia and preneoplastic lesions of the mammary and prostate glands, suppressed immune response, and reproductive problems [6]. Some of these proposed relationships are still in the very early stages of exploration.
Moreover, BPA interferes with thyroid hormone pathways. From in vitro studies, BPA binds to the thyroid hormone receptor and acts as an antagonist to T3 at the thyroid hormone receptor, thus inhibiting thyroid hormone receptor-mediated transcriptional activity [7, 8]. BPA fed to pregnant rats was associated with increased thyroid hormone levels in the offspring, which was compatible with thyroid resistance syndrome [8, 9]. Accordingly, studies have also shown that BPA is able to block T3-induced metamorphosis of tadpoles [10] and differentiation of mouse oligodendrocytes [11]. An in vivo study reported that BPA suppressed TSH release from bullfrog pituitary glands in a manner that is independent of both the thyroid hormone feedback mechanism and the estrogenic activity of BPA [12]. BPA was also shown to decrease thyroperoxidase activity in the FTC-238/hTPO recombinant cell line [13]. However, other animal studies [14–16] have found no or contrasting effects on thyroid hormone levels after BPA exposure. A non-significant inverse relationship between urinary BPA and total T4 was observed in previous study of US population [17]. Also an inverse association between urinary BPA and thyroid-stimulating hormone (TSH) was reported in male patients [18]. Urinary BPA was detected in 94.3 % of the population in several Asian countries [19]. However, studies relating BPA and thyroid function in Asian population are still lacking. The aim of the present study was to investigate the relationship between BPA exposure and thyroid function measurements collected in the fourth Thai National Health Examination Survey (NHES IV) 2009.
Materials and methods
The NHES IV 2009 was a nationally representative cross-sectional survey using a multistage, stratified sampling of the Thai population. The survey was approved by the Ethical Review Committee for Research in Human Subjects, Ministry of Public Health. All participants provided written informed consent. The sampling method has been described in detail elsewhere [20]. Briefly, the sampling units in each of the four stages of selection included: (1) five provinces in each of the four regions and Bangkok; (2) three to five districts in each province; (3) 13–14 electoral units or villages from urban and rural areas; and (4) eight to ten men and women from each electoral unit or village. The final sample size was targeted at 21,960 individuals and the final sample included 20,450 individuals (93.1 % of the target). According to the data from International Council for the Control of Iodine Deficiency Disorders (ICCIDD), Thailand is one of the countries that have sufficient iodine status [21]. However, populations in some rural areas in the Northern part of Thailand still have mild iodine deficiency [22].
Data collection
Data collection included a face-to-face interview conducted in the community and a subsequent health examination and blood sample collection. Demographic data, medical history, and lists of current medications were obtained during the interview using standard questionnaires. Any individuals reporting prior thyroid disorders or use of medications that alter thyroid function e.g., lithium, amiodarone, and recent iodinated contrast exposure were excluded. In the field survey, a brief physical examination was performed by certified field research assistants. Body mass index (BMI) was calculated using the following formula: weight in kg divided by height in meters squared. Pregnant women and patients with a reported history of thyroid disorder were excluded from the study. BPA levels may be affected by seasonal variation [23]. However, this seasonal effect is minimal in Thailand which is located in tropical area with warm-hot climate year-round. Venous blood samples were obtained from participants and then centrifuged; the serum was frozen and transferred to the central laboratory center in the Faculty of Medicine, Ramathibodi Hospital, Mahidol University for analysis. A subsample of serum for measurement of BPA, TSH, free thyroxine (FT4), thyroid peroxidase antibody (TPOAb), and thyroglobulin antibody (TgAb) levels was randomly selected according to age group (15–29, 30–44, 45–59, 60–69, 70–79, and ≥80 years), sex, and region (urban and rural). In each stratum, 25 individuals were randomly selected using computerized software. A total of 2,700 were selected, of which 2,586 serum samples were available.
Serum analysis
Serum BPA levels were determined by competitive ELISA (IBL International GmbH, Hamburg, Germany) with a detection limit of 0.3 ng/mL. The assay has intra-assay and inter-assay precisions of 7.0 and 13.6 %, respectively. Serum TSH, FT4, TPOAb, and TgAb were measured by electrochemiluminescence immunoassay on a Cobas e 411 analyzer (Roche Diagnostics GmbH, Mannheim, Germany). The assays have intra-assay precisions of 3.6, 1.31, 9.2, and 6.1 %, respectively. The corresponding normal ranges of serum TSH, FT4, TPOAb, and TgAb are 0.27–4.2 mIU/L, 0.93–1.71 ng/dL, 0–34 IU/mL, and 0–115 IU/mL. Serum BPA samples were collected at the same time as serum TSH, FT4, TPOAb, and TgAb.
Statistical analysis
In total, we possessed complete data for serum BPA, TSH, FT4, TPOAb, and TgAb for 2,586 subjects aged 15 years and older randomly sampled from the NHES IV. One hundred and seven subjects with outlying high or low levels of TSH and FT4 levels were excluded from our analysis. Owing to significant differences reported elsewhere in thyroid hormone levels in adolescents compared to adults [24], we compared the levels of TSH and FT4 between adults and adolescents in this study and found significant differences. Adolescents aged 15–17 years from the study population were separately analyzed. Finally, the data from 2,340 adult subjects were analyzed. Since thyroid autoantibodies including TPOAb and TgAb may cause abnormal thyroid function, we also analyzed the data for participants with positive thyroid autoantibodies separately. Data were presented as mean ± SD or median and interquartile range (IQR). Comparisons of the differences between means and medians were analyzed using the t test and the Mann–Whitney U test, respectively. Since the distribution of BPA concentrations was right-skewed, the data were natural logarithm (ln)-transformed prior to analysis. TSH is usually right-skewed. But after excluded the outliers, TSH was in normal distribution. Spearman’s rank correlation was performed to assess the relationships between two variables. Multivariable linear regression was used to examine the association of FT4 or TSH level, as a dependent variable, with BPA and related covariates in both male and female subjects. Statistical significance was established at P < 0.05. All of the analyses were conducted using SPSS software version 16.0 (Chicago, IL, USA).
Results
The study sample included 2,340 Thai adults (55 % in urban area). Demographic data and subject characteristics are shown in Table 1. Overall, BPA was detected in 52.8 % of serum samples with a median concentration of 0.33 (IQR 0.08–0.75; maximum concentration of 66.9) ng/mL. Positive TPOAb and TgAb were presented in 368 (15.7 %) and 280 (12 %) of the subjects, respectively. Levels of FT4 were positively correlated with male gender (r = 0.15, P < 0.0001) and inversely correlated with age (r = −0.1, P < 0.0001), BMI (r = −0.08, P < 0.0001), ln(BPA) (r = −0.1, P < 0.0001), and TgAb (r = −0.05, P = 0.01). TSH had positive relationships with age (r = 0.06, P < 0.01), BMI (r = 0.05, P = 0.01), and urban residence area (r = 0.05, P < 0.01) and inverse relationship with male (r = −0.07, P < 0.01). There were correlations between BPA and age, male, FT4. No correlation between BPA and BMI was observed.
Comparison between male and female subjects
Table 2 shows the characteristics of male and female subjects. Overall, men had lower BMI, TSH, TgAb levels, and percentage of positive TPOAb and TgAb, and higher serum BPA and FT4 concentrations compared to women. No differences were observed in age or TPOAb levels between two genders. When the analysis of subjects was stratified by gender, a weak inverse relationship between serum ln(BPA) and FT4 was observed in men (r = −0.16, P < 0.001; n = 1,159) and in women (r = −0.06, P = 0.03; n = 1,181). However, this relationship in women did not remain after multivariable regression analysis (Table 3). There were no associations between serum ln(BPA) and TSH among male and female subjects.
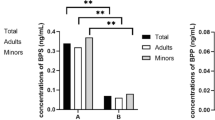
Owing to the significant correlation between TgAb and FT4 levels in the present study, we conducted an additional analysis of the data in subjects negative for TgAb. We observed an inverse association between ln(BPA) and FT4 in male subjects (r = −0.14, P < 0.001; n = 1,080). In contrast, no association was found in females (r = −0.06, P = NS; n = 980). Additionally, there were similar relationships between ln(BPA) and FT4 when we excluded subjects with positive TPOAb and/or TgAb. To assess the robustness of the associations between BPA concentrations and serum FT4, we categorized BPA concentrations into quartiles. Patterns of negative relationships between BPA and FT4 were observed (Fig. 1). In the multivariable regression analysis, there were significant inverse associations between FT4 level and serum BPA among men after adjustment for age and BMI. Adjusted regression coefficients for change in serum FT4 related to serum BPA were 0.1 in males (Table 3).
The analysis was also conducted among the adolescents (15–17 years old). The sample size available for analysis was much smaller than for the adults (N = 139). When stratifying the analysis among adolescents by gender, there was an inverse relationship between ln(BPA) and FT4 in male adolescents but no significant association was identified by multivariate regression analysis.
Discussion
Thyroid hormone is essential for normal brain development in mammals; insufficient levels of this hormone result in substantial cognitive defects. In addition, thyroid dysfunction is associated with adverse effects on the cardiovascular system, serum lipids, bone, and kidney. BPA is one of the highest-volume chemicals produced worldwide, and evidence is emerging to support a suggestive inverse relationship between urinary BPA and FT4 and TSH levels [17, 18]. Several population-level studies have estimated the prevalence of detectable urinary BPA as approximately 91–99.8 % from data in North America [1, 25] and multiple Asian countries such as China, India, Japan, Korea, Kuwait, Malaysia, and Vietnam [19, 26]. We detected BPA (≥0.3 ng/mL) in approximately 53 % of serum samples obtained from the Thai NHES IV 2009. The blood test we conducted may underestimate the true prevalence of BPA in this population because of rapid degradation of BPA in the body [27]. Although detection of BPA in serum may underestimate the total population burden of this potential toxicant, we identified a potential inverse correlation between serum BPA and FT4 levels in adult subjects. Epidemiological studies showed that even a borderline hypothyroxinemia in pregnant women may increase the risk of delayed neurodevelopment in the offspring [28–30]. Thus, even minimal association between BPA exposure and FT4 change in the present study may have detrimental consequences for health.
Our results were consistent with previous studies that BPA can affect thyroid function. Previous in vivo studies suggest that BPA or its halogenated derivatives may interact as agonists or antagonists on the thyroid hormone receptor via genomic [7, 31, 32] and non-genomic [33] pathways. In the previous instance, the suppression of thyroid hormone receptor transcription occurred at low concentrations of BPA [33]. In another study, developmental exposure to BPA in rats produced an endocrine profile similar to that observed in thyroid resistance syndrome [9]. Specifically, T4 levels were elevated during development in the pups of BPA-treated animals, but TSH levels were not different from controls [9]. Further, BPA suppressed the release of TSH from amphibian pituitary [12]. In a recent in vivo study, BPA exposure induced the TSH gene in the larval stage of zebrafish [34]. BPA has also been reported to decrease thyroperoxidase activity [13]. It was hypothesized that BPA was transferred from placenta to fetus via transthyretin, one of thyroid hormone binding proteins, which results in lesser concentrations of T4 in fetal blood [23, 35]. Small study by Sugiura-Ogasawara et al. [36] found no differences in mean levels of serum BPA between patients with and without hypothyroidism. In contrast, BPA had a weak inverse association with TSH but not correlated with FT4 or TT3 in male patients from infertility clinic [18]. A population-based study showed a suggestive inverse relationship between BPA and TT4 [17]. Recent small study in BPA-exposed workers reported positive association between BPA and FT3 while no association with FT4 and TSH [37]. In the present study, there was an inverse correlation between BPA and FT4 among the male adults. This pattern of low FT4 and normal TSH is compatible with central hypothyroidism. A possible explanation is the evidence that BPA can suppress TSH secretion from amphibian pituitary [12]. Recent BPA exposure study in pregnant ewes, which are long-gestation species, found lower T4 level in pregnant and newborns compared with controls [38]. Maternal exposure to BPA might be the potential risk for neonatal hypothyroidism. However, these inconsistent and limited data suggest that BPA may alter thyroid signaling in multiple mechanisms, but more detailed investigations are needed.
Interestingly, we also observed a gender-related difference in the relationship between BPA and FT4 levels. Only in male subjects, serum FT4 had a negative correlation with BPA exposure. Previous animal study revealed that BPA exposure during pregnancy was related to alterations in free T4 among male pups only [16]. Human data from CHAMACOS study in the United States also found the association of maternal BPA exposure and neonatal TSH in males but not females [39]. The experimental studies proposed that men may not metabolize BPA as efficiently as women. The mRNA expression of the uridinediphosphate-glucuronosyltransferase 2B1 (UGT 2B1) which glucuronidates BPA has been reported to be lower in males than in female rats [40, 41]. In addition, BPA exposure was found to suppress the expression of UGT 2B1 in males but not in female rats [42]. The gender-related discrepancy is possibly related to differences in androgen-related metabolism of BPA. Our results were different from a previous human study that found consistent associations between urinary BPA concentrations and total T4 and TSH in both genders [17]. Indeed, we also found an inverse relationship in the female population. However, upon carrying out regression analysis that association was no longer significant. Further experimental and human studies on sex-difference of association of BPA and thyroid function are needed.
There are several limitations to the present study. First, the study is cross-sectional in nature due to the availability of only a single hormone level measurement from each participant. Thus, we are unable to make any conclusions regarding causation in the relationship between BPA exposure and FT4. One sample per subject may not be representative of the average body burden of BPA, because these chemicals are metabolized rapidly. Second, we measured BPA concentrations in serum. The near-complete renal elimination of absorbed BPA has made urinary measurement of BPA a useful biomarker of human exposure. Subsequent studies should employ methodologies with greater sensitivity, selectivity, and precision, such as mass spectrometry in the urine sample. EDCs may affect thyroid homeostasis through various mechanisms, and multiple chemicals have been reported to interfere with thyroid function by each of the identified mechanisms [43, 44]. Owing to the additive or synergistic nature of EDCs with different mechanisms on thyroid hormone metabolisms, an investigation of cumulative risk that accounts for these interactions is warranted. Future research requires more complete data concerning thyroid hormone levels and other factors that impact thyroid function, including iodine status and other EDCs.
Conclusions
Our preliminary results support the inverse association between BPA and FT4 levels. Long-term, detailed studies are needed to establish a temporal relationship between BPA and thyroid hormone, as well as the potential public health implications of such an association.
References
A.M. Calafat, X. Ye, L.Y. Wong, J.A. Reidy, L.L. Needham, Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ. Health Perspect. 116(1), 39–44 (2008)
G.A. Csanady, H.R. Oberste-Frielinghaus, B. Semder, C. Baur, K.T. Schneider, J.G. Filser, Distribution and unspecific protein binding of the xenoestrogens bisphenol A and daidzein. Arch. Toxicol. 76(5–6), 299–305 (2002)
M.F. Fernandez, J.P. Arrebola, J. Taoufiki, A. Navalon, O. Ballesteros, R. Pulgar, J.L. Vilchez, N. Olea, Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod. Toxicol. 24(2), 259–264 (2007)
Y. Ikezuki, O. Tsutsumi, Y. Takai, Y. Kamei, Y. Taketani, Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum. Reprod. 17(11), 2839–2841 (2002)
G. Schonfelder, W. Wittfoht, H. Hopp, C.E. Talsness, M. Paul, I. Chahoud, Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect. 110(11), A703–A707 (2002)
B.S. Rubin, Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 127(1–2), 27–34 (2011)
K. Moriyama, T. Tagami, T. Akamizu, T. Usui, M. Saijo, N. Kanamoto, Y. Hataya, A. Shimatsu, H. Kuzuya, K. Nakao, Thyroid hormone action is disrupted by bisphenol A as an antagonist. J. Clin. Endocrinol. Metab. 87(11), 5185–5190 (2002)
H. Sun, O.X. Shen, X.R. Wang, L. Zhou, S.Q. Zhen, X.D. Chen, Anti-thyroid hormone activity of bisphenol A, tetrabromobisphenol A and tetrachlorobisphenol A in an improved reporter gene assay. Toxicol. In Vitro 23(5), 950–954 (2009)
R.T. Zoeller, R. Bansal, C. Parris, Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology 146(2), 607–612 (2005)
S. Iwamuro, M. Sakakibara, M. Terao, A. Ozawa, C. Kurobe, T. Shigeura, M. Kato, S. Kikuyama, Teratogenic and anti-metamorphic effects of bisphenol A on embryonic and larval Xenopus laevis. Gen. Comp. Endocrinol. 133(2), 189–198 (2003)
C. Seiwa, J. Nakahara, T. Komiyama, Y. Katsu, T. Iguchi, H. Asou, Bisphenol A exerts thyroid-hormone-like effects on mouse oligodendrocyte precursor cells. Neuroendocrinology 80(1), 21–30 (2004)
M. Kaneko, R. Okada, K. Yamamoto, M. Nakamura, G. Mosconi, A.M. Polzonetti-Magni, S. Kikuyama, Bisphenol A acts differently from and independently of thyroid hormone in suppressing thyrotropin release from the bullfrog pituitary. Gen. Comp. Endocrinol. 155(3), 574–580 (2008)
M. Song, Y.J. Kim, M.K. Song, H.S. Choi, Y.K. Park, J.C. Ryu, Identification of classifiers for increase or decrease of thyroid peroxidase activity in the FTC-238/hTPO recombinant cell line. Environ. Sci. Technol. 45(18), 7906–7914 (2011)
P. Nieminen, P. Lindstrom-Seppa, M. Juntunen, J. Asikainen, A.M. Mustonen, S.L. Karonen, H. Mussalo-Rauhamaa, J.V. Kukkonen, In vivo effects of bisphenol A on the polecat (Mustela putorius). J. Toxicol. Environ. Health A 65(13), 933–945 (2002)
P. Nieminen, P. Lindstrom-Seppa, A.M. Mustonen, H. Mussalo-Rauhamaa, J.V. Kukkonen, Bisphenol A affects endocrine physiology and biotransformation enzyme activities of the field vole (Microtus agrestis). Gen. Comp. Endocrinol. 126(2), 183–189 (2002)
X. Xu, Y. Liu, M. Sadamatsu, S. Tsutsumi, M. Akaike, H. Ushijima, N. Kato, Perinatal bisphenol A affects the behavior and SRC-1 expression of male pups but does not influence on the thyroid hormone receptors and its responsive gene. Neurosci. Res. 58(2), 149–155 (2007)
J.D. Meeker, K.K. Ferguson, Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007–2008. Environ. Health Perspect. 119(10), 1396–1402 (2011)
J.D. Meeker, A.M. Calafat, R. Hauser, Urinary bisphenol A concentrations in relation to serum thyroid and reproductive hormone levels in men from an infertility clinic. Environ. Sci. Technol. 44(4), 1458–1463 (2010)
Z. Zhang, H. Alomirah, H.S. Cho, Y.F. Li, C. Liao, T.B. Minh, M.A. Mohd, H. Nakata, N. Ren, K. Kannan, Urinary bisphenol A concentrations and their implications for human exposure in several Asian countries. Environ. Sci. Technol. 45(16), 7044–7050 (2011)
W. Aekplakorn, S. Chariyalertsak, P. Kessomboon, R. Sangthong, R. Inthawong, P. Putwatana, S. Taneepanichskul, Prevalence and management of diabetes and metabolic risk factors in Thai adults: the Thai National Health Examination Survey IV, 2009. Diabetes Care 34(9), 1980–1985 (2011)
B. de Benoist, M. Andersson, I. Igli, B. Takkouche, H. Allen, Iodine Status Worldwide: WHO Global Database on Iodine Deficiency (WHO, Geneva, 2004)
W. Charoensiriwatana, P. Srijantr, P. Teeyapant, J. Wongvilairattana, Consuming iodine enriched eggs to solve the iodine deficiency endemic for remote areas in Thailand. Nutr. J. 9, 68 (2010)
S.S. Andra, K.C. Makris, Thyroid disrupting chemicals in plastic additives and thyroid health. J. Environ. Sci. Health C 30(2), 107–151 (2012)
M.W. Elmlinger, W. Kuhnel, H.G. Lambrecht, M.B. Ranke, Reference intervals from birth to adulthood for serum thyroxine (T4), triiodothyronine (T3), free T3, free T4, thyroxine binding globulin (TBG) and thyrotropin (TSH). Clin. Chem. Lab. Med. 39(10), 973–979 (2001)
T. Bushnik, D. Haines, P. Levallois, J. Levesque, J. Van Oostdam, C. Viau, Lead and bisphenol A concentrations in the Canadian population. Health Rep. 21(3), 7–18 (2010)
K. Kim, H. Park, W. Yang, J.H. Lee, Urinary concentrations of bisphenol A and triclosan and associations with demographic factors in the Korean population. Environ. Res. 111(8), 1280–1285 (2011)
A.G. Asimakopoulos, N.S. Thomaidis, M.A. Koupparis, Recent trends in biomonitoring of bisphenol A, 4-t-octylphenol, and 4-nonylphenol. Toxicol. Lett. 210(2), 141–154 (2012)
V.J. Pop, E.P. Brouwers, H.L. Vader, T. Vulsma, A.L. van Baar, J.J. de Vijlder, Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin. Endocrinol. (Oxf) 59(3), 282–288 (2003)
P. Berbel, J.L. Mestre, A. Santamaria, I. Palazon, A. Franco, M. Graells, A. Gonzalez-Torga, G.M. de Escobar, Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the first month of gestation: the importance of early iodine supplementation. Thyroid 19(5), 511–519 (2009)
J. Henrichs, J.J. Bongers-Schokking, J.J. Schenk, A. Ghassabian, H.G. Schmidt, T.J. Visser, H. Hooijkaas, S.M. de Muinck Keizer-Schrama, A. Hofman, V.V. Jaddoe, W. Visser, E.A. Steegers, F.C. Verhulst, Y.B. de Rijke, H. Tiemeier, Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J. Clin. Endocrinol. Metab. 95(9), 4227–4234 (2010)
R.A. Heimeier, B. Das, D.R. Buchholz, Y.B. Shi, The xenoestrogen bisphenol A inhibits postembryonic vertebrate development by antagonizing gene regulation by thyroid hormone. Endocrinology 150(6), 2964–2973 (2009)
S. Kitamura, N. Jinno, S. Ohta, H. Kuroki, N. Fujimoto, Thyroid hormonal activity of the flame retardants tetrabromobisphenol A and tetrachlorobisphenol A. Biochem. Biophys. Res. Commun. 293(1), 554–559 (2002)
Z.G. Sheng, Y. Tang, Y.X. Liu, Y. Yuan, B.Q. Zhao, X.J. Chao, B.Z. Zhu, Low concentrations of bisphenol a suppress thyroid hormone receptor transcription through a non-genomic mechanism. Toxicol. Appl. Pharmacol. 259(1), 133–142 (2012)
W.K. Chan, K.M. Chan, Disruption of the hypothalamic-pituitary-thyroid axis in zebrafish embryo-larvae following waterborne exposure to BDE-47, TBBPA and BPA. Aquat. Toxicol. 108, 106–111 (2011)
Y. Wan, K. Choi, S. Kim, K. Ji, H. Chang, S. Wiseman, P.D. Jones, J.S. Khim, S. Park, J. Park, M.H. Lam, J.P. Giesy, Hydroxylated polybrominated diphenyl ethers and bisphenol A in pregnant women and their matching fetuses: placental transfer and potential risks. Environ. Sci. Technol. 44(13), 5233–5239 (2010)
M. Sugiura-Ogasawara, Y. Ozaki, S. Sonta, T. Makino, K. Suzumori, Exposure to bisphenol A is associated with recurrent miscarriage. Hum. Reprod. 20(8), 2325–2329 (2005)
F. Wang, J. Hua, M. Chen, Y. Xia, Q. Zhang, R. Zhao, W. Zhou, Z. Zhang, B. Wang, High urinary bisphenol A concentrations in workers and possible laboratory abnormalities. Occup. Environ. Med. 69(9), 679–684 (2012)
C. Viguie, S.H. Collet, V. Gayrard, N. Picard-Hagen, S. Puel, B.B. Roques, P.L. Toutain, M.Z. Lacroix, Maternal and fetal exposure to bisphenol a is associated with alterations of thyroid function in pregnant ewes and their newborn lambs. Endocrinology 154(1), 521–528 (2013)
J. Chevrier, R.B. Gunier, A. Bradman, N.T. Holland, A.M. Calafat, B. Eskenazi, K.G. Harley, Maternal urinary bisphenol A during pregnancy and maternal and neonatal thyroid function in the CHAMACOS study. Environ. Health Perspect. 121(1), 138–144 (2013)
T. Takeuchi, O. Tsutsumi, Serum bisphenol a concentrations showed gender differences, possibly linked to androgen levels. Biochem. Biophys. Res. Commun. 291(1), 76–78 (2002)
T. Takeuchi, O. Tsutsumi, N. Nakamura, Y. Ikezuki, Y. Takai, T. Yano, Y. Taketani, Gender difference in serum bisphenol A levels may be caused by liver UDP-glucuronosyltransferase activity in rats. Biochem. Biophys. Res. Commun. 325(2), 549–554 (2004)
N. Shibata, J. Matsumoto, K. Nakada, A. Yuasa, H. Yokota, Male-specific suppression of hepatic microsomal UDP-glucuronosyl transferase activities toward sex hormones in the adult male rat administered bisphenol A. Biochem J. 368(Pt 3), 783–788 (2002)
G. Mastorakos, E.I. Karoutsou, M. Mizamtsidi, G. Creatsas, The menace of endocrine disruptors on thyroid hormone physiology and their impact on intrauterine development. Endocrine 31(3), 219–237 (2007)
M.D. Miller, K.M. Crofton, D.C. Rice, R.T. Zoeller, Thyroid-disrupting chemicals: interpreting upstream biomarkers of adverse outcomes. Environ. Health Perspect. 117(7), 1033–1041 (2009)
Acknowledgments
NHES IV was conducted by the National Health Examination Survey Office, Health Systems Research Institute, Thailand. The NHES IV study group includes: National Health Examination Survey Office: Wichai Aekplakorn, Rungkarn Inthawong, Jiraluck Nonthaluck, Supornsak Tipsukum, Yawarat Porrapakkham; Northern region: Suwat Chariyalertsak, Kanittha Thaikla (Chiang Mai University), Wongsa Laohasiriwong, Wanlop Jaidee, Sutthinan Srathonghon, Ratana Phanphanit, Jiraporn Suwanteerangkul, Kriangkai Srithanaviboonchai; North Eastern Region: Pattapong Kessomboon, Somdej Pinitsoontorn, Piyathida Kuhirunyaratn, Sauwanan Bumrerraj, Amornrat Rattanasiri, Suchad Paileeklee, Bangornsri Jindawong, Napaporn Krusun, Weerapong Seeupalat (Khon Kaen University); Southern region: Virasakdi Chongsuvivatwong, Rassamee Sangthong, Mafausis Dueravee (Prince of Songkla University); Central Region: Surasak Taneepanichskul, Somrat Lertmaharit, Vilai Chinveschakitvanich, Onuma Zongram, Nuchanad Hounnaklang, Sukarin Wimuktayon (Chulalongkorn University); Bangkok Region: Panwadee Putwatana, Chalermsri Nuntawan, Karn Chaladthanyagid (Mahidol University). The Thai National Health Examination Survey IV was supported financially by the Health System Research Institute; Bureau of Policy and Strategy, Ministry of Public Health; Thai Health Promotion Foundation; National Health Security Office, Thailand. The assays for TSH, TPOAb, and TgAb were partially supported by Roche Diagnostics Thailand.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sriphrapradang, C., Chailurkit, Lo., Aekplakorn, W. et al. Association between bisphenol A and abnormal free thyroxine level in men. Endocrine 44, 441–447 (2013). https://doi.org/10.1007/s12020-013-9889-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-013-9889-y