Abstract
Our aim was to evaluate and compare the diagnostic performance of three methods commonly used for GDM screening: fasting plasma glucose (FPG), two-step 50 g glucose challenge test (GCT), and 75 g glucose tolerance test (GTT) in a randomized study design to predict GDM in the first trimester and determine the best approach in predicting GDM. In a non-blind, parallel-group prospective randomized controlled study; 736 singleton pregnant women underwent FPG testing in the first trimester and randomly assigned to two groups; two-step 50 g GCT and 75 g GTT. GDM diagnosis was made according to Carpenter–Coustan or ADA (American Diabetes Association) criteria in two-step 50 g GCT and 75 g GTT groups, respectively. Subsequent testing was performed by two-step 50 g GCT at 24–28 weeks for screen negatives. After excluding the women who were lost to follow-up or withdrawn as a result of pregnancy loss, 486 pregnant women were recruited in the study. The FPG, two-step GCT, and one-step GTT methods identified GDM in 25/486 (5.1 %), 15/248 (6.0 %), and 27/238 (11.3 %) women, respectively. Area under ROC curves were 0.623, 0.708, and 0.792, respectively. Sensitivities were 47.17, 68.18, and 87.1 %, respectively. Specificities were 77.37, 100, and 100 %, respectively. Positive predictive values were 20.33, 100, and 100 %, respectively. Negative predictive values were 92.29, 97, and 98.1 %, respectively. Until superior screening alternatives become available, the 75 g GTT may be preferred for GDM screening in the first trimester.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with onset or first recognition during pregnancy, whether or not the condition persisted after pregnancy and not excluding the possibility that unrecognized glucose intolerance may have antedated or begun concomitantly with the pregnancy [1–3]. GDM affects 1–14 % of all pregnant women, causing increased short- and long-term maternal and perinatal complications [4, 5]. There is a lack of international uniformity in the approach of screening and diagnosis for GDM [1, 4, 6–13]. While a glucose challenge test is commonly employed, glucose dosages and diagnostic thresholds vary greatly [6].
The International Association of Diabetes and Pregnancy Study Groups (IADPSG) and American Diabetes Association (ADA) recommended a simplified “one-step” approach to the screening and diagnosis of GDM with 75 g glucose tolerance test (GTT) [1, 6]. Screening and diagnosis of GDM is commonly delayed until 24–28 weeks of pregnancy with the rationale that the diabetogenic effects of pregnancy increase with gestation. IADPSG recently suggested earlier screening for GDM and fasting plasma glucose (FPG) levels at the first prenatal visit between 92 and 126 mg/dl were defined as GDM [6]. Also, ADA and IADPSG recommended that women with FPG ≥126 mg/dl or HbA1C ≥6.5 % or random plasma glucose ≥200 mg/dl at initial visit should receive a diagnosis of overt diabetes [1, 6]. Previous studies investigating the potential value of first-trimester screening for early prediction of GDM reported promising results [14–16]. Effective early identification of the high-risk group for subsequent development of GDM is likely to improve pregnancy outcome because with appropriate dietary advice and pharmacological interventions the incidence of the disease and associated maternal and perinatal complications could potentially be reduced [14]. Additionally, early testing would identify women with undiagnosed preexisting diabetes.
To our knowledge, this is the first study evaluating and comparing the diagnostic performance of FPG, two-step 50 g glucose challenge test (GCT), and 75 g GTT in a randomized study design to predict GDM in the first trimester.
Materials and methods
We carried out a prospective, non-blind, parallel-group randomized controlled trial comparing three screening tests for GDM in the first trimester. The study was conducted at the antenatal outpatient clinic between 31 December 2010 and 31 December 2011; in accordance with the revised CONSORT guidelines [17]. This tertiary health care referral institution serves to a population with a broad ethnic and socioeconomic base and manages approximately 18,000 deliveries per year. The study protocol was approved by the institutional human ethics committee (#8-20/12/2010).
Participants
Women admitted for routine antenatal care during the study period were informed about the study by their obstetricians and were invited to participate in the trial. Eligible participants were 3,726 pregnant women between 11 and 14 weeks of gestation. Pregnant women with multiple gestation, taking medications which could affect blood insulin and glucose levels, having hypertension or concomitant serious systemic disease, pregestational known diabetes (Type 1–2), and FPG levels ≥126 mg/dl were excluded from the study in order to screen a rather low-risk population. After excluding 2,990 women (521 did not meet inclusion criteria; 1,095 declined to participate and 1,374 had other reasons or had a significant communication barrier) a written informed consent was obtained from 736 women.
Methods
Intervention allocation
Participants were randomly assigned using computer-generated allocations to one of two parallel groups initially in 1:1 ratio, to screen for GDM either using the two-step method (50 g GCT ± 100 g GTT) or using the one-step method (75 g GTT) as illustrated in Fig. 1. Block randomization with blocks of four was used to ensure that each group consisted of an equal number of participants as defined previously [18]. During the creation of the allocation list, blocks were chosen randomly using computer-generated random numbers. Research assistant and the women were not blinded for allocation after randomization due to the nature of the study. Participants who experienced fetal loss before 24–28 weeks were withdrawn.
We have screened all women for fasting plasma glucose levels with a fasting state of 8–14 h at the first prenatal visit. If fasting state was not appropriate, the test was administered on the next day. The FPG levels between 92 and 126 mg/dl were defined as GDM as recommended by IADPSG [6]. At the second antenatal visit at 11–14 weeks, for participants allocated in two-step group, 50 g GCT was performed regardless of the fasting state as described by ACOG for 24–28 weeks [7]. The 1-h venous plasma glucose concentration of ≥140 mg/dl was considered as a positive screening result. GDM was diagnosed when an abnormal GCT (≥140 mg/dl) was followed by two or more abnormal values on a 3-h 100 g GTT performed within 1 week using the Carpenter–Coustan criteria (0-h 95 mg/dl, 1-h 180 mg/dl, 2-h 155 mg/dl, and 3-h 140 mg/dl) [19, 20]. GDM was also diagnosed with a GCT value of 200 mg/dl or higher. For participants allocated in one-step group, 75 g GTT was performed with a fasting state of 8–14 h and GDM was diagnosed with one or more abnormal glucose values using the ADA and IADPSG criteria (0-h 92 mg/dl, 1-h 180 mg/dl, and 2-h 153 mg/dl) [1, 6].
The primary aim of the study was to compare diagnostic strategies and report test characteristics (specificity and sensitivity, PPV and NPV) of the first-trimester screening tests for GDM and the secondary aim was to offer a first-trimester screening test with high predictive value for GDM.
Quantitative determinations of blood glucose were done by enzymatic method on Roche automated clinical chemistry analyzer (Hitachi 912 analyzer, Roche Diagnostics GmbH, Germany). Glucose was assayed using a commercial glucose oxidase kit (Glucose GOD-PAP, Roche Diagnostics GmbH, Germany). Measuring range was 2–450 mg/dl (0.11–25 mmol/l) and intra- and inter-assay coefficient of variation (CV) values was 0.9 and 1.8 %, respectively.
Statistical evaluation
For the power calculation, we accepted the GDM prevalence as 5 % for our population and the effect size as 0.3 [21, 22]. The sample size calculation for the entire study population of 3,726 women: a two sample comparison with a 5 % level of significance (alpha) and power of 0.95 gave a study population of 220 women in each group. This sample size was able to detect a 0.5 standard deviation (SD) difference in continuous variables given the same power and significance level. Assuming a 20 % dropout rate, we needed to include approximately 528 pregnant women. Sample size calculations were performed using the G*Power v3.1.5 general power analysis program [23].
Demographic data and glucose results were presented as mean with SD or median with range for continuous variables, and as number with percentage for categorical variables. The Student’s (Independent samples) t test or the Mann–Whitney U-test were used to compare continuous variables as appropriate, and the χ2- test or Fisher’s exact test was used to compare categorical variables. Two-tailed P value of <0.05 was considered statistically significant. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) with their associated 95 % CI were determined for each method. The detection and false-positive rates in the prediction of GDM were estimated by using receiver operating characteristic (ROC) curves analysis, and diagnostic power of these screening tests to predict GDM in early pregnancy were assessed by comparison of area under ROC (AUROC) curves. The statistical software package SPSS (SPSS Inc., Chicago, Ill., USA) was used for data analyses.
Results
3,726 women were eligible at our antenatal clinic during the study period. Of these, 736 women agreed to participate and were randomized for the study. 239 women were lost to follow-up (mostly due to choice of prenatal care in a different hospital) and 11 were withdrawn as they experienced a pregnancy loss and finally 486 pregnant women were recruited in the study (Fig. 1). Figure 1 displays the flow of women in the present study.
Participant characteristics are presented in Table 1. The three groups were comparable regarding maternal age, BMI, educational level, gravidity, parity, previous spontaneous abortion, family history of diabetes, previous GDM, or macrosomia (P > 0.05).
736 women underwent FPG screening and 613 women had FPG <92 mg/dl, while 123 women had FPG between 92 and 126 mg/dl. Two-step 50 g GCT were performed in 377 women, of which 327 were screen negative (<140 mg/dl), 5 were GDM (>200 mg/dl) and 55 were screen positive (>140 mg/dl). These 55 women underwent 100 g GTT and 10 of the women were diagnosed as GDM. There were 349 women randomized to one-step 75 g GTT, of whom 27 were diagnosed as GDM. The women with negative GDM screening test results (n = 444) in the first trimester were rescreened between 24 and 28 weeks. In the two-step group 37 women were screen positive and after 100 g OGTT and 7 women were diagnosed as GDM at 24–28 weeks. Five of these 7 women were screen (+) in the first trimester but had normal 100 g OGTT. While, there were 4 more women diagnosed as GDM in the one-step group between 24 and 28 weeks.
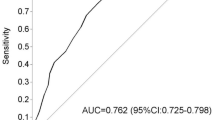
Among the 486 women studied, a total of 53 women (10.9 %) were diagnosed as GDM in a two-step and one-step group by using ACOG [7] or IADPSG [6] criteria, respectively (Table 2). The FPG, two-step, and one-step methods identified GDM in 25/486 (5.1 %), 15/248 (6.0 %), and 27/238 (11.3 %) women in the first trimester, respectively. FPG testing false positively diagnosed 98 women as GDM according to IADPSG criteria. Table 3 displays results of the comparison of the three screening tests in terms of accuracy measures. Comparison of accuracy measures resulted with the highest sensitivity and diagnostic odds ratios for 75 g GTT (sensitivity: 87.09 %; 95 % CI 69.23–95.78 and diagnostic odds ratio: 2.41; 95 % CI 1.75–3.32). The FPG test had more false-positive test results and was, therefore, less specific (77.37 %; 95 % CI 73.07–81.23) than two other tests. Positive predictive value of FPG was very weak (20.33 %; 95 % CI 13.81–28.72) compared to the other two tests. Negative predictive values for all tests were comparable 92.29 %; 95 % CI 88.91–94.72, 97 %; 95 % CI 93.54–98.65 and 98.1 %; 95 % CI 94.89–99.39 for the FPG, two-step GCT, and one-step GTT respectively. The negative likelihood ratio of 75 g GTT was by far the best and the positive likelihood ratio FPG test was the most weak of the three screening tests. Area under ROC curve of FPG testing was 0.623; 95 % CI 0.538–0.707, 50 g GCT was 0.708; 95 % CI 0.617–0.798 and 75 g GTT was 0.792; 95 % CI 0.709–0.876.
Discussion
In the present study, the incidence of GDM was 10.9 % when the first- and second-trimester screening results were evaluated and screening with 75 g GTT detected 87.09 % of GDM women, while two-step GCT detected 68.18 % of GDM in the first trimester. Thus, the majority of the cases were detected in the first trimester. The present study shows that the area under the curve was larger for the 75 g GTT, indicating that the 75 g GTT was a better predictor for GDM than FPG test and two-step GTT. Sensitivity of the 75 g GTT was almost twofold higher compared with FPG testing. The FPG test had more false-positive test results and was, therefore, less specific than two other tests.
Previously, two similar studies reported that there is a significant correlation between the results of the first- and early third-trimester 50 g glucose screening tests [24, 25]. These studies concluded that the third-trimester glucose screening may be unnecessary for women with the first-trimester glucose screening test values of <110 and <99 mg/dl, respectively. For those women with positive 50 g GCT, Nahum and Huffaker [24] suggested postponing the GTT to the third trimester, Bhattacharya [25] suggested the GTT directly after positive screening in the first trimester. For the women with intermediate glucose levels (99–139 mg/dl) GTT has been recommended in the early third trimester [25]. Currently early identification and treatment of women with GDM is the goal for improving maternal fetal and neonatal outcomes. A number of studies have addressed the issue of an optimal screening method for detecting GDM, but very few investigators have performed large, prospective population-based studies evaluating screening and diagnostic testing for GDM [3, 11]. The lack of consensus regarding diagnostic criteria, glucose load, cutoff values of screening tests, and selection of historical risk indicators make these studies difficult to compare. Agreeing on the screening and diagnostic tests for GDM seems difficult. IADPSG consensus panel proposed that decision of performing blood testing with any of HBA1c, FPG, or random PG at the first prenatal visit for evaluation of glycemia on all pregnant women or only on women with characteristics indicating a high risk for diabetes is to be made on the basis of the background frequency of abnormal glucose metabolism in the population and on local circumstances [6].
As the prevalence of GDM is increasing, cost evaluation of glucose screening and treating GDM are valuable [26]. It has been traditionally accepted that screening for GDM in the first trimester is not cost-effective since the majority of the pregnant women will not manifest diabetes until the third trimester and those with normal screening results in the first trimester would need to be rescreened. There are studies reporting that a decrease in adverse perinatal outcomes might offset the costs of screening [27].
Studies evaluating the first-trimester GDM screening are usually based on only high-risk women [28–30]. Low-risk women represent only 10 % of most populations and identifying these cases may add complexity to the screening process [31]. Although, selective screening may limit false-positive rates and probably the cost, the potential for missing a significant proportion of cases makes this approach unattractive. The overall prevalence of GDM in the literature varies from 1 to 14 % [5]. According to the Turkish Diabetes Epidemiology Study (TURDEP-I) which was a cross-sectional, population-based survey conducted in between 1997 and 1998 and included 24,788 women, crude prevalence of diabetes was 7.2 % in Turkey [32]. The prevalence of diabetes was increased to 16.5 % in the TURDEP-II study which was also conducted in the same clinics with the same design included 26,499 women in 2010 [33]. These two studies showed that the prevalence of DM in Turkey has increased 229 % during this 12-year period. In our country, universal screening is the standard care; thus, our study was not designed to evaluate this issue and screening was recommended for all pregnant women irrespective of the risk factors.
Using FPG as a screening test for GDM offers some advantages over the glucose challenge test. FPG is easy to administer, well-tolerated, inexpensive, reliable, and reproducible and has been reported to vary little throughout the gestation [34]. A retrospective study from Riskin–Mashiah et al. [16] evaluated the first-trimester FPG level as a screening test for GDM and compared it with body mass index. They concluded that higher first-trimester fasting glucose levels, within the normoglycemic range, constitute an independent risk factor for the development of GDM among young pregnant women. In contrast, Sacks et al. [15] reported that poor specificity (high false-positive rate) of FPG screening test at the first prenatal visit makes it an inefficient screening test for gestational diabetes although it has good patient compliance. Agarwal and Dhatt [35] also reported similar high false-positive rates for FPG as a screening test in early pregnancy and concluded that glucose screening in early pregnancy can detect most cases of GDM, but that FPG is not suitable, since in early stages, as with type 2 diabetes, hyperglycemia is only post-prandial. Also, ADA does not support the IADPSG recommendation to use a FPG of 92 mg/dl or more for the diagnosis of GDM in early pregnancy [1]. Similarly, in the present study, low sensitivity and low specificity of the FPG limit its use as a screening test for GDM in the first trimester. There is also debate about which FPG level would be most predictive for GDM in early pregnancy. Based on their study Zhu et al. [36] concluded that for Chinese population a FPG of 92 m/dl should not be used as a criteria to diagnose GDM and suggested a FPG value between 6.1 and 6.99 mmol/L (109–126 mg/dl) for GDM diagnosis.
Lapolla et al. [37] reported that the new IADPSG criteria identified a group of women previously classified as normal but whose outcomes were similar to the women who are diagnosed as GDM by the previous criteria. Also, the study by Riskin-Mashiah et al. [38] reported that high first-trimester fasting glucose levels (currently considered a non-diabetic range) increase the risk of adverse pregnancy outcomes and they suggested that early detection and treatment of women at high risk for these complications might improve pregnancy outcome. As evaluation of perinatal outcome was not the aim of the study it has not been investigated.
As we had designed this study before the publication of the recent IADPSG recommendations and recent literature [6, 39], HbA1C levels were not measured. Therefore, it is hard to determine whether the screen positives were truly GDM or previously undiagnosed diabetes. This is one of the potential weaknesses of the present study. One must also notice that 75 g GTT, which is directly a diagnostic test, is not a screening test, so more women will be diagnosed with GDM since only one abnormal value is necessary for the diagnosis. Another drawback of our study is although we assumed a dropout rate of 20 % in the study design, dropout rates of each arm inevitably reached 35.01 and 30.65 %, respectively. As dropout rates were similar between study arms, we excluded the data of these participants and “per protocol” analysis was performed.
Conclusions
We recommend that despite easy implementation and low cost, FPG measurement is not appropriate as a screening test for GDM in the first trimester. Until superior screening alternatives become available, the 75 g GTT may be preferred for GDM screening in the first trimester. We cannot recommend early universal screening, as there are no randomized controlled studies showing that early detection of GDM improves pregnancy outcome. However, as our study is a preliminary study, larger randomized clinical trials evaluating the maternal and fetal outcome of women with GDM diagnosed early in pregnancy in comparison with those diagnosed at 24–28 gestational weeks are required in order to verify the real utility of early screening of GDM.
References
American Diabetes A, Standards of medical care in diabetes–2013. Diabetes Care 36(Suppl 1), S11–S66 (2013). doi:10.2337/dc13-S011
C.J. Nolan, Controversies in gestational diabetes. Best Pract. Res. Clin. Obstet. Gynaecol. 25(1), 37–49 (2011). doi:10.1016/j.bpobgyn.2010.10.004
D. Farrar, L. Duley, D.A Lawlor, Different strategies for diagnosing gestational diabetes to improve maternal and infant health. Cochrane Database Syst. Rev. 10, CD007122 (2011). doi:10.1002/14651858.CD007122.pub2
Canadian Diabetes Association, Clinical practice guidelines for the prevention and management of diabetes in Canada. Can. J. Diabetes 32(suppl 1), 1–201 (2008)
ADA, Diagnosis and classification of diabetes mellitus. Diabetes Care 34(Suppl 1), S62–S69 (2011). doi:10.2337/dc11-S062
B.E. Metzger, S.G. Gabbe, B. Persson, T.A. Buchanan, P.A. Catalano, P. Damm, A.R. Dyer, A. Leiva, M. Hod, J.L. Kitzmiler, L.P. Lowe, H.D. McIntyre, J.J. Oats, Y. Omori, M.I. Schmidt, International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33(3), 676–682 (2010)
ACOG, Committee opinion no. 504: screening and diagnosis of gestational diabetes mellitus. Obstet. Gynecol. 118(3), 751–753 (2011). doi:10.1097/AOG.0b013e3182310cc3
J.D. Walker, NICE guidance on diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period. NICE clinical guideline 63. London, March 2008. Diabet. Med. 25(9), 1025–1027 (2008). doi:10.1111/j.1464-5491.2008.02532.x
ACOG, Practice bulletin gestational diabetes. Number 30, September 2001. Obstet. Gynecol. 98(3), 525–538 (2001)
K.G. Alberti, P.Z. Zimmet, Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 15(7), 539–553 (1998). doi:10.1002/(SICI)1096-9136(199807)15:7<539:AID-DIA668>3.0.CO;2-S
J. Tieu, P. Middleton, A.J. McPhee, C.A. Crowther, Screening and subsequent management for gestational diabetes for improving maternal and infant health. Cochrane. Database Syst. Rev. 7, CD007222 (2010). doi:10.1002/14651858.CD007222.pub2
D.R. Coustan, Point: the American Diabetes Association and the International Association of Diabetes and Pregnancy study groups recommendations for diagnosing gestational diabetes should be used worldwide. Clin. Chem. 58(7), 1094–1097 (2012). doi:10.1373/clinchem.2012.186239
M. van Leeuwen, M.D. Louwerse, B.C. Opmeer, J. Limpens, M.J. Serlie, J.B. Reitsma, B.W. Mol, Glucose challenge test for detecting gestational diabetes mellitus: a systematic review. BJOG 119(4), 393–401 (2012). doi:10.1111/j.1471-0528.2011.03254.x
W. Plasencia, R. Garcia, S. Pereira, R. Akolekar, K.H. Nicolaides, Criteria for screening and diagnosis of gestational diabetes mellitus in the first trimester of pregnancy. Fetal Diagn. Ther. 30(2), 108–115 (2011). doi:10.1159/000324684
D.A. Sacks, W. Chen, G. Wolde-Tsadik, T.A. Buchanan, Fasting plasma glucose test at the first prenatal visit as a screen for gestational diabetes. Obstet. Gynecol. 101(6), 1197–1203 (2003)
S. Riskin-Mashiah, A. Damti, G. Younes, R. Auslender, First trimester fasting hyperglycemia as a predictor for the development of gestational diabetes mellitus. Eur. J. Obstet. Gynecol. Reprod. Biol. 152(2), 163–167 (2010). doi:10.1016/j.ejogrb.2010.05.036
K.F. Schulz, D.G. Altman, D. Moher, C. Group, CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Obstet. Gynecol. 115(5), 1063–1070 (2010). doi:10.1097/AOG.0b013e3181d9d421
D.G. Altman, J.M. Bland, How to randomise. BMJ 319(7211), 703–704 (1999)
M.W. Carpenter, D.R. Coustan, Criteria for screening tests for gestational diabetes. Am. J. Obstet. Gynecol. 144(7), 768–773 (1982)
J.P. Vandorsten, W.C. Dodson, M.A. Espeland, W.A. Grobman, J.M. Guise, B.M. Mercer, H.L. Minkoff, B. Poindexter, L.A. Prosser, G.F. Sawaya, J.R. Scott, R.M. Silver, L. Smith, A. Thomas, A.T. Tita, NIH consensus development conference: diagnosing Gestational diabetes mellitus. NIH consens state Sci Statements 29(1), 1–31 (2013)
C. Erem, N. Cihanyurdu, O. Deger, C. Karahan, G. Can, M. Telatar, Screening for gestational diabetes mellitus in northeastern Turkey (Trabzon City). Eur. J. Epidemiol. 18(1), 39–43 (2003)
D. Karcaaltincaba, O. Kandemir, S. Yalvac, S. Guvendag-Guven, A. Haberal, Prevalence of gestational diabetes mellitus and gestational impaired glucose tolerance in pregnant women evaluated by National Diabetes Data Group and Carpenter and Coustan criteria. Int. J. Gynaecol. Obstet. 106(3), 246–249 (2009). doi:10.1016/j.ijgo.2009.04.004
F. Faul, E. Erdfelder, A.G. Lang, A. Buchner, G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39(2), 175–191 (2007)
G.G. Nahum, B.J. Huffaker, Correlation between first- and early third-trimester glucose screening test results. Obstet. Gynecol. 76(4), 709–713 (1990)
S.M. Bhattacharya, Glucose screening test results in first and early third trimester of pregnancy: is there any correlation? J. Obstet. Gynaecol. Res. 28(6), 304–307 (2002)
S.J. Meltzer, J. Snyder, J.R. Penrod, M. Nudi, L. Morin, Gestational diabetes mellitus screening and diagnosis: a prospective randomised controlled trial comparing costs of one-step and two-step methods. BJOG 117(4), 407–415 (2010). doi:10.1111/j.1471-0528.2009.02475.x
D.A. Scott, E. Loveman, L. McIntyre, N. Waugh, Screening for gestational diabetes: a systematic review and economic evaluation. Health Technol. Assess. 6(11), 1–161 (2002)
M.M. Agarwal, G.S. Dhatt, J. Punnose, G. Koster, Gestational diabetes in a high-risk population: using the fasting plasma glucose to simplify the diagnostic algorithm. Eur. J. Obstet. Gynecol. Reprod. Biol. 120(1), 39–44 (2005). doi:10.1016/j.ejogrb.2004.07.034
T. Bito, T. Nyari, L. Kovacs, A. Pal, Oral glucose tolerance testing at gestational weeks ≤16 could predict or exclude subsequent gestational diabetes mellitus during the current pregnancy in high risk group. Eur. J. Obstet. Gynecol. Reprod. Biol. 121(1), 51–55 (2005). doi:10.1016/j.ejogrb.2004.11.006
D.M. Jensen, L. Molsted-Pedersen, H. Beck-Nielsen, J.G. Westergaard, P. Ovesen, P. Damm, Screening for gestational diabetes mellitus by a model based on risk indicators: a prospective study. Am. J. Obstet. Gynecol. 189(5), 1383–1388 (2003)
D.R. Danilenko-Dixon, J.T. Van Winter, R.L. Nelson, P.L. Ogburn Jr, Universal versus selective gestational diabetes screening: application of 1997 American Diabetes Association recommendations. Am. J. Obstet. Gynecol. 181(4), 798–802 (1999)
I. Satman, T. Yilmaz, A. Sengul, S. Salman, F. Salman, S. Uygur, I. Bastar, Y. Tutuncu, M. Sargin, N. Dinccag, K. Karsidag, S. Kalaca, C. Ozcan, H. King, Population-based study of diabetes and risk characteristics in Turkey: results of the Turkish diabetes epidemiology study (TURDEP). Diabetes Care 25(9), 1551–1556 (2002)
I. Satman, B. Omer, Y. Tutuncu, S. Kalaca, S. Gedik, N. Dinccag, K. Karsidag, S. Genc, A. Telci, B. Canbaz, F. Turker, T. Yilmaz, B. Cakir, J. Tuomilehto, T.-I.S. Group, Twelve-year trends in the prevalence and risk factors of diabetes and prediabetes in Turkish adults. Eur. J. Epidemiol. 28(2), 169–180 (2013). doi:10.1007/s10654-013-9771-5
T. Siegmund, N.T. Rad, C. Ritterath, G. Siebert, W. Henrich, K.J. Buhling, Longitudinal changes in the continuous glucose profile measured by the CGMS in healthy pregnant women and determination of cut-off values. Eur. J. Obstet. Gynecol. Reprod. Biol. 139(1), 46–52 (2008). doi:10.1016/j.ejogrb.2007.12.006
M.M. Agarwal, G.S. Dhatt, Fasting plasma glucose as a screening test for gestational diabetes mellitus. Arch. Gynecol. Obstet. 275(2), 81–87 (2007). doi:10.1007/s00404-006-0245-9
W.W. Zhu, H.X. Yang, Y.M. Wei, J. Yan, Z.L. Wang, X.L. Li, H.R. Wu, N. Li, M.H. Zhang, X.H. Liu, H. Zhang, Y.H. Wang, J.M. Niu, Y.J. Gan, L.R. Zhong, Y.F. Wang, A. Kapur, Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in china. Diabetes Care 36(3), 586–590 (2013). doi:10.2337/dc12-1157
A. Lapolla, M.G. Dalfra, E. Ragazzi, A.P. De Cata, D. Fedele, New International Association of the Diabetes and Pregnancy Study Groups (IADPSG) recommendations for diagnosing gestational diabetes compared with former criteria: a retrospective study on pregnancy outcome. Diabet. Med. 28(9), 1074–1077 (2011). doi:10.1111/j.1464-5491.2011.03351.x
S. Riskin-Mashiah, G. Younes, A. Damti, R. Auslender, First-trimester fasting hyperglycemia and adverse pregnancy outcomes. Diabetes Care 32(9), 1639–1643 (2009). doi:10.2337/dc09-0688
T. Higgins, HbA1c for screening and diagnosis of diabetes mellitus. Endocrine 43(2), 266–273 (2013). doi:10.1007/s12020-012-9768-y
Acknowledgments
This paper is selected as a poster presentation in the SMFM 33rd Annual Meeting; February 11–16, 2013; San Francisco, USA.
Conflict of interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yeral, M.I., Ozgu-Erdinc, A.S., Uygur, D. et al. Prediction of gestational diabetes mellitus in the first trimester, comparison of fasting plasma glucose, two-step and one-step methods: a prospective randomized controlled trial. Endocrine 46, 512–518 (2014). https://doi.org/10.1007/s12020-013-0111-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-013-0111-z