Abstract
While visceral adipose tissue (VAT) associates to obesity, there is debate for subcutaneous adipose tissue (SAT). One explanation may be SAT subcompartments, superficial-SAT (sSAT) and deep-SAT (dSAT), recently recognized as independent depots. Our aim was to establish roles for sSAT/dSAT with obesity by examining the expression of proteins key to adipocyte metabolism. Paired biopsies from sSAT and dSAT of 10 normal-weight (BMI 21.8 ± 0.8 kg/m2) and 11 obese subjects (BMI 44 ± 2.1 kg/m2) were analyzed for differences in insulin sensitivity using adiponectin, GLUT4 and resistin, glucocorticoid metabolism by 11βHSD1 and alterations of the adipokines leptin and TNFα. Between lean and obese subjects, sSAT and dSAT changes for GLUT4, resistin and TNFα were equivalent. Resistin and TNFα increased in both obese SAT sub-compartments; 33-fold (sSAT; P < 0.006) and 18.5-fold (dSAT; P < 0.003) higher resistin, with undetectable in leans to significant TNFα levels in obese. In contrast, GLUT4 showed 5.5-fold (sSAT; P < 0.03) and 7-fold (dSAT; P < 0.03) lower levels in obese, correlating to BMI (r = −0.6423, P = 0.007) and HOMA-IR (r = −0.5882, P = 0.017). Exclusive sSAT-specific differences were observed for adiponectin, leptin, and 11βHSD1. Both sSAT 11βHSD1 and leptin increased in obese, with 11βHSD1 2.5-fold (P = 0.052) and leptin 3.3-fold (P < 0.008) higher, with 11βHSD1 correlating to HOMA-IR (r = 0.5203, P = 0.0323) and leptin to BMI (r = 0.5810, P = 0.01). In contrast, obese had 7-fold (P < 0.02) lower sSAT adiponectin, correlating to BMI (r = −0.5178, P = 0.027) and HOMA-IR (r = −0.4570, P = 0.049). Overall, sSAT and dSAT are distinct abdominal adipose tissue depots with independent metabolic functions. Between the two, sSAT shows clear independent effects that associate to obesity and its metabolic complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Back in the 1950s, it was first proposed that the anatomical distribution of the adipose tissue (AT) was fundamental to the regulation of endocrine and metabolic functions, with android obesity more directly associating to diabetes and atherosclerosis [1]. It is now clear that this anatomical distribution is dependent on the division of abdominal AT into the visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) depots, which show subject-to-subject variation, dependent on age, gender, ethnicity, nutritional intake, and the autonomic regulation of energy homeostasis [2–4]. While the emphasis has been given to the importance of VAT for metabolic disruption, a growing number of studies are now highlighting the potential impact of SAT accumulation. While early clinical evidence implicated SAT with insulin sensitivity and insulin resistance [5], recent studies on metabolic syndrome (MetS) and nascent MetS have demonstrated a role for SAT in early metabolic disruption [6, 7].
One explanation to the discrepant findings for SAT in obesity-associated complications resides in the anatomical notion that SAT is a homogenous compartment. It should rather be considered that SAT is two subcompartments, anatomically distinguished by Scarpas’s fascia: superficial SAT (sSAT), which lays below the epidermal layers, and deep SAT (dSAT), which is adjacent to the preperitoneal AT [8]. These compartments show subject-to-subject variations particularly in association to obesity and insulin resistance [8, 9]. Studies from AT subcompartments in lean subjects and animal models have identified depot-specific expression patterns of key proteins namely associated to obesity and its complications, raising the possibility that SAT subcompartments yield distinct metabolic effects [10–12].
To explore if the SAT subcompartments may differentially contribute to obesity and its comorbidities, this study aimed at evaluating the expression of proteins key to adipocyte activity and metabolism from AT biopsies obtained from lean subjects and severely obese yet otherwise healthy subjects showing key features of obesity related complications.
Methods and procedures
Patients and tissue sampling
Adipose tissue samples from the anterior SAT-subcompartments, sSAT and dSAT, were obtained as previously described [10] from the peri-umbilical level in 10 healthy normal-weight subjects (Lean: 5F/5M, mean ± SEM, BMI 21.8 ± 0.8 kg/m2, age 44.5 ± 2.9 years) and 11 otherwise healthy, severely obese subjects (Obese: 5F/6M, BMI 44.0 ± 2.1 kg/m2, age 38 ± 2.6 years). Briefly, at the time of the procedure, fasting blood samples were collected for biochemical analyses. All hormonal determinations were performed using commercially available assays, as described previously [10]. The study protocol was approved by the individual Institution Ethics Committees, with the aim and design of the study explained to each subject, who in turn gave their informed consent.
RNA isolation and semi-quantitative RT-PCR
The extraction of total RNA from AT biopsies and the semi-quantitative RT-PCR analysis was performed according to methods previously described [10]. Briefly, all the samples were analyzed in duplicate with each primer set and quantified by densitometry using QuantityOne software (Bio-Rad, Hercules, CA). Results are presented as arbitrary units (AU) relative to the expression of the internal control hypoxanthine phosphoribosyl transferase (HPRT).
Protein evaluation
Cell lysates (CL) from AT biopsies were isolated and analyzed according to methodologies previously described [10]. Results from western immunoblot (WIB) analyses were quantified by densitometry using QuantityOne software and normalized to total protein and are presented as AU.
Statistical evaluation
Data are expressed as mean ± standard error of the mean (SEM). Differences between depots and between lean and morbid obese subjects were evaluated using Student’s t test and Mann and Whitney test. Relationships between sSAT and dSAT expression profiles with HOMA-IR and serum concentrations of adiponectin, resistin, leptin, and TNFα were analyzed using Pearson’s regression analysis. Skewed variables were logarithmically transformed before the analysis, where appropriate. Statistical significance was set at P < 0.05. Analyses were performed using Prism (Graphpad Sofware, Inc., San Diego, CA).
Results
Study groups consisted of 10 healthy lean and 11 severely obese subjects (Table 1). According to previous investigations, anthropometric differences that contribute to obesity comorbidities were observed between the two groups, with BMI being positively correlated to HOMA-IR (r = 0.67, P = 0.002) and insulin (r = 0.93, P = 0.0001), leptin (r = 0.84, P = 0.0001) and TNFα levels (0.76, P = 0.0001). Oppositely, there was a negative correlation between BMI and adiponectin levels (r = −0.52, P = 0.02). HOMA-IR was positively correlated to TNFα serum levels (r = 0.73, P = 0.004) and negatively correlated to adiponectin (r = −0.46, P = 0.049).
Glucose metabolism
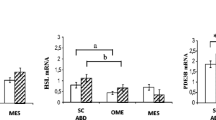
Adiponectin mRNA expression was similar between SAT subcompartments within both study groups (Fig. 1), while it was 1.4-fold higher in obese subjects for both sSAT (P < 0.002) and dSAT (P < 0.001), when compared to controls (Fig. 1). At the protein level, adiponectin expression was increased by 16-fold in sSAT of lean subjects when compared to dSAT (P < 0.008; Fig. 1), while differences were negligible within the obese group. In the obese group, however, adiponectin was 7-fold lower in sSAT than in lean subjects (P < 0.02; Fig. 1).
Our analysis of GLUT4 mRNA expression within or between groups demonstrated no significant change between SAT subcompartments, although a trend toward higher expression levels was evident in sSAT for both obese and leans (Fig. 2). This was paralleled by the finding that the intracellular expression of GLUT4 in sSAT was 8-fold higher (P < 0.05) than in dSAT from lean subjects and, albeit non-significantly, in samples from obese patients (Fig. 2). As a consequence, lean subjects had a 5.5- (P < 0.03) and 7-fold (P < 0.03) higher endogenous GLUT4 expression in sSAT and dSAT, than in obese subjects (Fig. 2).
With respect to resistin, we demonstrated 10-fold higher (P < 0.04) mRNA levels in dSAT of lean subjects compared to sSAT (Fig. 2), while differences between SAT-subcompartments were not significant in the obese group. Compared to lean subjects, however, resistin mRNA expression in obese patients was 15-fold (P < 0.02) higher in the sSAT and 5-fold (P < 0.03) higher in the dSAT (Fig. 2). A similar pattern of expression was also observed at the endogenous protein level. In fact, dSAT from lean subjects expressed 3-fold (P < 0.05) higher endogenous resistin levels than sSAT, while the expression level between SAT depots was comparable in obese subjects (Fig. 2). Of note, sSAT and dSAT from obese patients expressed 33- (P < 0.006) and 18.5-fold (P < 0.003) higher endogenous resistin than controls (Fig. 2).
Glucocorticoid Metabolism
A small yet significant 1.6-fold (P < 0.04) lower 11βHSD1 mRNA expression was observed in sSAT than dSAT from lean subjects (Fig. 3). Between groups, obese had 2.2-fold (P < 0.006) and 3.5-fold (P < 0.007) higher mRNA 11βHSD1 expression for sSAT and dSAT, respectively. The pattern of endogenous 11βHSD1 protein reflected that of mRNA in lean subjects, while obese subjects had 4-fold (P < 0.04) higher sSAT levels when compared to dSAT (Fig. 3). Interestingly, between groups only sSAT expression showed near significant variations, exhibiting 2.5-fold higher levels in obese (Fig. 3).
Adipocytokines
At the mRNA level, a small but significant 1.2-fold (P < 0.04) decrease in leptin mRNA synthesis between sSAT and dSAT was observed in lean subjects, while no differences were observed between depots in the obese subjects (Fig. 4). Furthermore, no significant differences in leptin mRNA expression were observed between groups. In obese patients, a 26-fold (P < 0.003) higher level of endogenous leptin was found in sSAT compared to dSAT which also accounted for a significant difference in sSAT leptin expression between groups, being 6-fold (P < 0.008) higher in obese subjects.
Our analysis documented very low TNFα mRNA levels in all biopsies (Fig. 4) with no significant differences in TNFα mRNA expression between SAT-subcompartments or between lean and obese subjects for dSAT. Conversely, a 1.8-fold (P < 0.03) increase in TNFα mRNA was observed in sSAT of obese subjects compared to controls (Fig. 4). While endogenous TNFα protein was expressed in SAT from obese subjects, it was generally below the limits of detection in the leans, clear evidence of AT TNFα overexpression developing with obesity (Fig. 4).
Correlation studies
Correlation studies for tissue-expressed molecular variables were primarily, but not exclusively related to sSAT. Markers related to glucose metabolism were associated as follows: BMI (r = 0.58, P = 0.015) and HOMA-IR (r = 0.49, P = 0.047) correlated positively to sSAT adiponectin mRNA and negatively to sSAT adiponectin protein (r = −0.54, P = 0.032; and r = −0.46, P = 0.049; respectively), while a stronger correlation was evident between serum levels of adiponectin and its protein expression in dSAT (r = 0.68, P = 0.0021) compared to sSAT (r = 0.52, P = 0.027); sSAT GLUT4 protein was negatively correlated to both BMI (r = −0.64, P = 0.007) and HOMA-IR (r = −0.58, P = 0.017); completing glucose metabolism analysis, resistin mRNA expression was correlated to BMI in both sSAT (r = 0.51, P = 0.025) and dSAT (r = 0.61, P = 0.006), while HOMA-IR correlated exclusively to sSAT resistin mRNA (r = 0.64, P = 0.004). No correlations were otherwise documented between BMI, HOMA-IR or serum resistin levels to tissue protein production. Analysis of 11βHSD1 tissue expression resulted in a positive association between sSAT protein expression and HOMA-IR (r = 0.52, P = 0.032). Finally, analysis of specific tissue adipocytokines, failed to reveal significant correlations between leptin and HOMA-IR, while sSAT protein expression was positively correlated both to BMI (r = 0.58, P = 0.01) as well as serum leptin concentrations (r = 0.69, P = 0.002); TNFα endogenous protein did not correlate to HOMA-IR but was associated to BMI when expressed in both sSAT (r = 0.67, P = 0.002) and dSAT (r = 0.56, P = 0.017), while a significant correlation was evident between sSAT protein expression and circulating TNFα concentrations (r = 0.54, P = 0.02).
Discussion
Abdominal SAT and VAT work, at least in part, to differentially regulate the endocrine and metabolic functions of AT. In a normal physiological state, the SAT subcompartments, sSAT and dSAT, exhibit independent expression profiles of important molecular markers of obesity and its metabolic complications [10]. To evaluate the hypothesis that sSAT and dSAT function as distinct mediators of obesity and its comorbidities, we investigated paired biopsies obtained from sSAT and dSAT for potential differences in molecular markers of insulin resistance, glucocorticoid metabolism, and adipokine production between lean and obese subjects. Our results indicate that sSAT and dSAT alterations in protein expression between lean and obese subjects for GLUT4, resistin, and TNFα were equivalent, while exclusive sSAT-specific differences were observed for adiponectin, leptin, and 11βHSD1 protein.
In diabetes or obesity, circulating concentrations of adiponectin are significantly decreased, and return to normal with weight loss and/or normalization of glucose levels [13]. While the general consensus is adiponectin produced by VAT is directly implicated [14], herein, we show that adiponectin protein is remarkably decreased in sSAT from obese subjects and correlates negatively to both BMI and HOMA-IR. Although the possibility of altered adiponectin trafficking from each compartment cannot be excluded, there appears to be a dysregulation in adiponectin translation in obesity. This may likely be an early event. In fact, Bremer and Jalial [7] have demonstrated a SAT-specific downregulation of adiponectin with nascent MetS. Importantly, the present study shows that it is sSAT-specific adiponectin that is dynamically changing with obesity.
Adipose tissue-specific GLUT4 over-expression in muscle-specific GLUT4 knockout mice can restore whole body insulin sensitivity [15]. In the lean state, the endogenous levels of GLUT4 were found to be lower in dSAT than in sSAT and VAT [10]. This pattern was confirmed in the present study, with a further decrease in both SAT subcompartments seen with obesity. In particular, sSAT endogenous GLUT4 showed a significant correlation to BMI and HOMA-IR. As this study exclusively examines the intracellular expression of GLUT4, the results may be explained by a differential sub-cellular localization and/or a decrease in protein synthesis/turnover of GLUT4 between SAT subcompartments and between lean and obese subjects. Preperitoneal adipocytes from obese subjects respond better to insulin, proposed to be due to a higher GLUT4 and altered trafficking [16]. Our findings suggest that with obesity, both SAT-subcompartments undergo dynamic changes related to synthesis and/or the cellular distribution of GLUT4.
With obesity, we observed a dramatic increase of resistin production in both SAT subcompartments, suggesting that they are equal contributors. While resistin has been proposed to be the link between obesity and insulin resistance [17], low resistin levels detected in other studies and the lack of significance between serum levels, questions its role [10, 18]. Interestingly, adipose-specific GLUT4 over-expression in muscle-specific GLUT4 knockout mice which restored whole body insulin sensitivity, altered resistin but not leptin or adiponectin levels, suggesting that crosstalk may exist between the protein regulation of GLUT4 and resistin [17]. While resistin secretion was not evaluated in the present study, it appears that in the normal population, dSAT is the more important contributor to resistin production [10]. With obesity, the balance changes with both the SAT subcompartments appearing to contribute equally to resistin protein production, perhaps regulated in part by the dynamics of depot-specific GLUT4 expression.
Both 11βHSD1 expression and activity are positively correlated to obesity and insulin resistance with adipocyte-specific glucocorticoid inactivation protecting against diet-induced obesity [19, 20]. Consistent with this, 11β-HSD1 knockout (11β-HSD1−/−) mice chronically fed a high-fat (HF) diet resist MetS in part by preferentially accumulating peripheral rather than visceral fat [21], with distinct depot-specific mechanisms driving this healthier fat pattern and function [22]. Herein, we observed that the augmentation of 11βHSD1 SAT expression in obesity is specific to the sSAT subcompartment of the SAT depot.
With obesity, serum leptin levels are significantly elevated with the SAT-specific expansion contributing directly to the increase in fasting circulating leptin levels [23]. While SAT has been implicated as being responsible for serum leptin levels, Kelley et al, [9] observed that sSAT area correlates strongly to serum leptin levels. While leptin secretion from sSAT and dSAT were not evaluated in the present study, the results are in accordance with the study by Kelley et al, [9], where significantly higher endogenous leptin levels were present in sSAT subcompartment as opposed to dSAT, with sSAT leptin synthesis correlating to obesity.
In the present study, endogenous TNFα protein expression in both SAT-subcompartments correlated positively to BMI with sSAT expression correlating to serum TNFα. Previously, we observed a correlation between dSAT TNFα mRNA and HOMA-IR in lean subjects [10]; however, with the incorporation of obese subjects, this correlation was lost being that TNFα was more tightly associated to BMI. While the emphasis is on the importance of VAT TNFα expression in obesity [24], the dramatic increase in endogenous TNFα expression we observed with obesity in both SAT-subcompartments, cannot exclude a role. TNFα is known to directly regulate the expression and secretion of adiponectin and leptin [25]. Based on this and a negative association between TNFα and adiponectin serum levels, it has been proposed that TNFα and adiponectin serve to antagonize each other and regulate insulin sensitivity [26]. As such, both sSAT and dSAT-specific TNFα may contribute to altered insulin sensitivity and the development of insulin resistance.
In summary, an evaluation of proteins key for AT activity and metabolism in the abdominal SAT depots of a normal and severely obese subjects has revealed that the sSAT and dSAT-subcompartments are molecularly distinct, supporting independent metabolic functions. With obesity, equivalent sSAT and dSAT changes were observed for GLUT4, resistin, and TNFα, with resistin and TNFα increasing, while endogenous GLUT4 expression decreased, results which correlated to HOMA-IR and/or BMI. More significantly, however, was sSAT-specific regulation of adiponectin, leptin, and 11βHSD1, with leptin and 11βHSD1 increasing with obesity while adiponectin decreased, correlating to HOMA-IR and/or BMI. Overall, sSAT and dSAT are clearly distinct abdominal AT depots that undergo dynamic changes with the development of obesity; however, importantly between the two, sSAT shows clear independent effects that associate to obesity. These results further emphasize the importance of now considering SAT as two independent depots.
References
J. Vague, The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout and uric calculous disease. Am. J. Clin. Nutr. 4, 20–31 (1956)
B.L. Wajchenberg, Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr. Rev. 21, 697–738 (2000)
J.A. Nazare, J.D. Smith, A.L. Borel, S.M. Haffner, B. Balkau, R. Ross, C. Massien, N. Alméras, J.P. Després, Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: the international study of prediction of intra-abdominal adiposity and its relationship with cardiometabolic risk/intra-abdominal adiposity. Am. J. Clin. Nutr. 96, 714–726 (2012)
M.J. Lee, Y. Wu, S.K. Fried, Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol. Aspects Med. 34, 1–11 (2013)
C. Maffeis, R. Manfredi, M. Trombetta, S. Sordelli, M. Storti, T. Benuzzi, R.C. Bonadonna, Insulin sensitivity is correlated with subcutaneous but not visceral body fat in overweight and obese prepubertal children. J. Clin. Endocrinol. Metab. 93, 2122–2128 (2008)
A.A. Bremer, S. Devaraj, A. Afify, I. Jialal, Adipose tissue dysregulation in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 96, E1782–E1788 (2011)
A.A. Bremer, I. Jialal, Adipose tissue dysfunction in nascent metabolic syndrome. J. Obes. 2013, 393192 (2013). doi:10.1155/2013/393192
H.G. Alexander, A.E. Dugdale, Fascial planes within subcutaneous fat in humans. Eur. J. Clin. Nutr. 46, 903–906 (1992)
D.E. Kelley, F.L. Thaete, F. Troost, T. Huwe, B.H. Goodpaster, Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 278, E941–E948 (2000)
G.E. Walker, B. Verti, P. Marzullo, G. Savia, M. Mencarelli, F. Zurleni, A. Liuzzi, A.M. Di Blasio, Deep sub-cutaneous adipose tissue (dSAT): a metabolically distinct abdominal adipose depot. Obes. Res. 15, 1933–1943 (2007)
A. Roca-Rivada, J. Alonso, O. Al-Massadi, C. Castelao, J.R. Peinado, L.M. Seoane, F.F. Casanueva, M. Pardo, Secretome analysis of rat adipose tissues shows location-specific roles for each depot type. Proteomics 74, 1068–1079 (2011)
M. Pardo, A. Roca-Rivada, L.M. Seoane, F.F. Casanueva, Obesidomics: contribution of adipose tissue secretome analysis to obesity research. Endocrine 41, 374–383 (2012)
N. Garcia de la Torre, M.A. Rubio, E. Bordiú, L. Cabrerizo, E. Aparicio, C. Hernández, A. Sánchez-Pernaute, L. Díez-Valladares, A. Torres, M. Puente, A.L. Charro, Effects of weight loss after bariatric surgery for morbid obesity on vascular endothelial growth factor-A, adipocytokines, and insulin. J. Clin. Endocrinol. Metab. 93, 4276–4281 (2008)
Y. Matsuzawa, T. Funahashi, T. Nakamura, The concept of metabolic syndrome: contribution of visceral fat accumulation and its molecular mechanism. J. Atheroscler. Thromb. 18, 629–639 (2011)
E. Carvalho, K. Kotani, O.D. Peroni, B.B. Kahn, Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle. Am. J. Physiol. Endocrinol. Metab. 89, E551–E561 (2005)
A. Marette, P. Mauriège, B. Marcotte, C. Atgié, C. Bouchard, G. Thériault, L.J. Bukowiecki, P. Marceau, S. Biron, A. Nadeau, J.P. Després, Regional variation in adipose tissue insulin action and GLUT4 glucose transporter expression in severely obese premenopausal women. Diabetologia 40, 590–598 (1997)
C.M. Steppan, S.T. Bailey, S. Bhat, E.J. Brown, R.R. Banerjee, C.M. Wright, H.R. Patel, R.S. Ahima, M.A. Lazar, The hormone resistin links obesity to diabetes. Nature 409, 292–293 (2001)
R.Z. Yang, Q. Huang, A. Xu, J.C. McLenithan, J.A. Eisen, A.R. Shuldiner, S. Alkan, D.W. Gong, Comparative studies of resistin expression and phylogenomics in human and mouse. Biochem. Biophys. Res. Commun. 310, 927–935 (2003)
P.M. Stewart, J.W. Tomlinson, Cortisol, 11 beta-hydroxysteroid dehydrogenase type 1 and central obesity. Trends Endocrinol. Metab. 13, 94–96 (2002)
E.E. Kershaw, N.M. Morton, H. Dhillon, L. Ramage, J.R. Seckl, J.S. Flier, Adipocyte-specific glucocorticoid inactivation protects against diet-induced obesity. Diabetes 54, 1023–1031 (2005)
N.M. Morton, J.M. Paterson, H. Masuzaki, M.C. Holmes, B. Staels, C. Fievet, B.R. Walker, J.S. Flier, J.J. Mullins, J.R. Seckl, Novel adipose tissue-mediated resistance to diet-induced visceral obesity in 11 beta-hydroxysteroid dehydrogenase type 1-deficient mice. Diabetes 53, 931–938 (2004)
M. Wamil, J.H. Battle, S. Turban, T. Kipari, D. Seguret, R. de Sousa Peixoto, Y.B. Nelson, D. Nowakowska, D. Ferenbach, L. Ramage, K.E. Chapman, J. Hughes, D.R. Dunbar, J.R. Seckl, N.M. Morton, Novel fat depot–specific mechanisms underlie resistance to visceral obesity and inflammation in 11β-hydroxysteroid dehydrogenase type 1–deficient mice. Diabetes 60, 1158–1167 (2011)
M. Cnop, M.J. Landchild, J. Vidal, P.J. Havel, N.G. Knowles, D.R. Carr, F. Wang, R.L. Hull, E.J. Boyko, B.M. Retzlaff, C.E. Walden, R.H. Knopp, S.E. Kahn, The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations : distinct metabolic effects of two fat compartments. Diabetes 51, 1005–1015 (2002)
Y.L. Cao, C.Z. Hu, X. Meng, D.F. Wang, J. Zhang, Expression of TNF-alpha protein in omental and subcutaneous adipose tissue in obesity. Diabetes Res. Clin. Pract. 79, 214–219 (2008)
H. Gottschling-Zeller, M. Birgel, D. Scriba, W.F. Blum, H. Hauner, Depot-specific release of leptin from subcutaneous and omental adipocytes in suspension culture: effect of tumor necrosis factor-alpha and transforming growth factor-beta1. Eur. J. Endocrinol. 141, 436–442 (1999)
A.S. Lihn, B. Richelsen, S.B. Pedersen, S.B. Haugaard, G.S. Rathje, S. Madsbad, O. Andersen, Increased expression of TNF-alpha, IL-6, and IL-8 in HALS: implications for reduced adiponectin expression and plasma levels. Am. J. Physiol. Endocrinol. Metab. 285, E1072–E1080 (2003)
Acknowledgments
We would like to thank Dr. Barbara Verti and Dr. Giulio Savia for their assistance and Professor Antonio Liuzzi for his exclusive support and mentorship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walker, G.E., Marzullo, P., Prodam, F. et al. Obesity modifies expression profiles of metabolic markers in superficial and deep subcutaneous abdominal adipose tissue depots. Endocrine 46, 99–106 (2014). https://doi.org/10.1007/s12020-013-0040-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-013-0040-x