Abstract
The soluble amyloid protein procurer α (sAPPα) and β (sAPPβ) have been postulated as promising new cerebrospinal fluid (CSF) biomarkers for Alzheimer’s disease (AD) and multiple other neurodegenerative diseases, but have failed to meet expectations with their often discordant and even contradictory findings to date. The aim of the study was to systematically explore this issue. Cochrane Library, PubMed, and CNKI were systematically searched without language or date restrictions. This network meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and also adhered to the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines. Twenty studies, comprising ten groups, were eligible and included. Overall, 19 eligible studies with 1634 patients contributed to the analysis of CSF sAPPα levels and 16 eligible studies with 1684 patients contributed to the analysis of CSF sAPPβ levels. CSF sAPPβ levels are significantly higher in AD than in corticobasal syndrome (CBS) and progressive supranuclear palsy (PSP); higher in Control than in Depression, CBS and PSP; higher in Parkinson’s disease dementia (PDD) than in CBS and PSP; higher in mild cognitive impairment progressed to AD dementia during the follow-up period (pMCI) than in Depression and PSP; higher in stable mild cognitive impairment (sMCI) than in Depression. With regard to CSF sAPPα levels, there were no significant difference among groups. However, surprisingly, the resultant rankings graphically showed that pMCI populations have the highest levels of CSF sAPPα and sAPPβ. Furthermore, it seemed there was a positive correlation between CSF sAPPα and sAPPβ levels. The measurement of CSF sAPPα and sAPPβ levels may provide an alternative method for the diagnosis of early-stage AD, pMCI, which is conducive to preventive therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurodegenerative diseases such as Alzheimer’s disease (AD) are characterized by the progressive loss of region-specific neurons in the brain. Although the high failure rate of drug research and development for AD, the amyloid cascade hypothesis was still the focus of pharmaceutical giants’ attention (Kodamullil et al. 2017). According to the hypothesis, the cleavage of amyloid protein procurer (APP) generates amyloid-β (Aβ), soluble APPβ (sAPPβ) (Chow et al. 2010), and soluble APPα (sAPPα) (O’Brien and Wong 2011). Generally, sAPPα involves numerous physiological functions in the brain (Habib et al. 2017), impedes the generation of Aβ (Deng et al. 2015). By comparison, sAPPβ contributes to degeneration in AD (Nikolaev et al. 2009). Furthermore, sAPPα has shown 100-fold more potent than sAPPβ in protecting hippocampal neurons against excitotoxicity and Aβ toxicity (Furukawa et al. 1996).
Currently, it is noteworthy that more and more studies have reported CSF sAPPα and sAPPβ levels in multiple other neurodegenerative diseases, such as dementia with Lewy bodies (DLB) (Mulugeta et al. 2011), frontotemporal dementia (FTD) (Steinacker et al. 2009; Gabelle et al. 2011; Perneczky et al. 2011; Magdalinou et al. 2015; Alcolea et al. 2017), Parkinson’s disease dementia (PDD) (Mulugeta et al. 2011; Magdalinou et al. 2015), progressive supranuclear palsy (PSP) (Magdalinou et al. 2015; Alcolea et al. 2017), and even in corticobasal syndrome (CBS) (Magdalinou et al. 2015; Alcolea et al. 2017), mild cognitive impairment (MCI) (Post et al. 2006; Fellgiebel et al. 2009; Hertze et al. 2010; Perneczky et al. 2011; Alexopoulos et al. 2012; Lewczuk et al. 2012; Araki et al. 2017), depression (Post et al. 2006; Hertze et al. 2010), and the healthy elderly (Lannfelt et al. 1995; Peskind et al. 1997; Fellgiebel et al. 2009; Hertze et al. 2010; Mulugeta et al. 2011; Alexopoulos et al. 2012; Lewczuk et al. 2012; Popp et al. 2012; Miyajima et al. 2013; Taverna et al. 2013; Tsolakidou et al. 2013; Cuchillo-Ibañez et al. 2015; Magdalinou et al. 2015; Moriya et al. 2015; Alcolea et al. 2017; Araki et al. 2017). Nevertheless, up to now, the CSF sAPPα and sAPPβ levels of AD (Perneczky et al. 2014) and these neurodegenerative diseases are still discordant and even contradictory. How exactly are the CSF sAPPα and sAPPβ levels alter in these diseases?
Comparing to traditional meta-analysis, network meta-analysis (NMA) allows for the synthesis of direct and indirect evidence to compare multiple diseases in a single analysis simultaneously. Therefore, we conducted an NMA to systematically evaluate the changes of CSF sAPPα and sAPPβ levels in AD and multiple other neurodegenerative diseases.
Methods
Search Strategy and Selection Criteria
We did the NMA using a frequentist model, according to the PRISMA guidelines (Moher et al. 2009), the MOOSE guidelines (Stroup et al. 2000), and the Cochrane Handbook. Two of us (Tang, W., Wang, Y.) searched PubMed, CNKI, and the Cochrane Library, without language or date restrictions, using keywords and MeSH terms: Alzheimer disease, dementia, neurodegenerative disease, vascular dementia, frontotemporal degeneration, dementia with Lewy bodies, Parkinson disease with dementia, progressive supranuclear palsy, corticobasal syndrome, mild cognitive impairment, depression, soluble amyloid protein precursor, and cerebrospinal fluid. The PubMed search string was (((Alzheimer disease) OR dementia OR depression OR (neurodegenerative disease) OR (vascular dementia) OR (mild cognitive impairment) OR (frontotemporal degeneration) OR (dementia with Lewy bodies) OR (Parkinson disease with dementia) OR (progressive supranuclear palsy) OR (corticobasal syndrome)) AND (soluble amyloid protein precursor) AND (cerebrospinal fluid)). Furthermore, we manually screened the reference lists of studies with potential relevance and review articles.
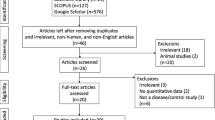
Since MCI can be subdivided into sMCI (stable mild cognitive impairment) and pMCI (mild cognitive impairment progressed to AD dementia during the follow-up period), we include sMCI and pMCI populations in our research. The included studies should meet the following criteria: (1) Provided detailed procedures and criteria for the diagnosis of AD and multiple other neurodegenerative diseases. (2) Reported mean, SD or SEM of CSF sAPPα or sAPPβ levels. (3) Included both females and males. Exclusion criteria were: (1) Letters, commentaries, editorials, reviews. (2) Unable to judge whether it were sAPPα or sAPPβ levels for lack of related information. Additionally, if multiple publications existed for the same study, we included the publication with more complete outcome data. In the end, the remaining 20 studies were included in the NMA (Fig. 1).
Data Analysis
Two investigators (Tang and Wang) initially screened the titles and abstracts, subsequently reviewed full-text versions of the potentially eligible studies. Disagreements between the investigators concerning the decision to exclude or include a study were resolved through discussions. If necessary, we sought the suggestions of another two investigators (Cheng and Yao) for further discussion. Two investigators (Yao and Zhou) independently extracted the data from the primary texts and supplementary materials, which were recorded on a standard spreadsheet that contained fields for: title, first author, year of publication, country, study design, groups, number of patients in each group, age, number of men and women, concentrations and assessment methods for CSF sAPPα and sAPPβ levels (appendix 1). Two investigators (Tang and Wang) independently assessed the quality of evidence using both the Newcastle–Ottawa scale (NOS) (Wells et al. 2011) and the Cochrane Collaboration’s tool. Disagreements were resolved by consensus, and if necessary, consultation with two other investigators (Yao and Guan).
We did two types of meta-analysis. First, we used the traditional pairwise meta-analysis to analyze direct comparisons. A random effects model was applied because of more conservative estimated effects. As all results were extracted as continuous outcomes, we calculated the summary effect sizes as standardized mean differences (SMD), with 95% CI. We assessed the heterogeneity among studies with the Cochran’s Q test and the I2 statistic (DerSimonian and Laird 2015). Secondly, we did random-effects NMA. The resultant rankings are presented graphically with surface under the cumulative ranking (SUCRA) probabilities. Large SUCRA scores indicate a higher CSF sAPPα or sAPPβ levels.
Additionally, meta-regression was used to explore the possible factors that could significantly affect the results. Moreover, three sensitivity analyses were undertaken. First, exclude studies published before 2010. Second, exclude studies with more than one item indicating a high risk of bias assessed by the Cochrane risk of bias tool. Third, exclude studies in which any group contained fewer than 15 participants.
For the traditional meta-analysis, we used Cochrane Collaboration review manager software, version 5.3.5, and STATA, version 14.1. For NMA we used STATA, version 14.1. The NMA was not registered.
Results
After screening 305 publications, we identified 20 eligible studies (Fig. 1), comprising ten groups (AD, pMCI, sMCI, DLB, FTD, PDD, PSP, CBS, Depression, and no neurological disorders Control), a total of 1 899 participants. We didn’t find the studies about CSF sAPPα or sAPPβ level in VaD populations. Characteristics of the included studies are presented in Table 1. The methodological quality assessment of the included studies was presented in eTable 1, appendix 2. The risk of bias graph and summary for included studies were presented in eFig. 1, 2, appendix 2.
A total of 19 eligible studies were included in the comparison of CSF sAPPα levels among the 10 groups. We did direct comparisons with regard to the CSF sAPPα levels (eTable 2, 3, appendix 2). The CSF sAPPα levels were significantly lower in CBS than in AD (SMD = −0.85; 95% CI −1.53, −0.18), lower in PSP than in AD (SMD = −0.77; 95% CI −1.30, −0.23), higher in Control than in CBS (SMD = 0.95; 95% CI 0.28, 1.61), higher in PDD than in CBS (SMD = 0.96; 95% CI: 0.30, 1.63), lower in PSP than in Control (SMD = −0.87; 95% CI −1.39, −0.35), higher in pMCI than in Control (SMD = 1.77; 95% CI 0.18, 3.36), higher in pMCI than in FTD (SMD = 1.53; 95% CI 0.79, 2.27), higher in sMCI than in FTD (SMD = 0.80; 95% CI 0.19, 1.41), lower in PSP than in PDD (SMD = −0.88; 95% CI −1.40, −0.37), lower in sMCI than in pMCI (SMD = −0.52; 95% CI −0.96, −0.07). Additionally, Fig. 2a graphically represents the network of eligible comparisons for CSF sAPPα levels of the NMA. However, the 95% CI and 95% predictive interval (PrI) for the SMD includes zero for all comparisons, indicating a lack of statistically significant difference among the 10 groups regarding CSF sAPPα levels (eTable 2, eFig. 3, appendix 2). Besides, plots of the SURCA, rank probability, ranking plot, and contribution plot were presented in eFig. 4, 5, 6, and eTable 4, appendix 2. It is noteworthy that the pMCI populations ranked the best in terms of CSF sAPPα levels. Moreover, as shown in eFig. 7, appendix 2, there were no statistically significant inconsistency in most loops within the NMA.
Network of eligible comparisons for CSF sAPPα and sAPPβ levels network meta-analysis. The size of nodes is proportional to the total sample size of each subject, and the width of lines is proportional to the number of studies compared in every pair of populations. AD Alzheimer’s disease, CBS corticobasal syndrome, Control no neurological disorders control, DLB dementia with Lewy bodies, FTD frontotemporal dementia, PDD Parkinson’s disease dementia, PSP progressive supranuclear palsy, pMCI MCI progressed to AD dementia during the follow-up period, sMCI stable mild cognitive impairment
We did direct comparisons with regard to the CSF sAPPβ levels in 16 eligible studies (eTable 5, 6, appendix 2). The CSF sAPPβ levels were significantly lower in CBS than in AD (SMD = −1.07; 95% CI −1.81, −0.33), lower in Control than in AD (SMD = −0.50; 95% CI −0.88, −0.11), lower in PSP than in AD (SMD = −1.04; 95% CI −1.88, −0.21), higher in Control than in CBS (SMD = 1.02; 95% CI: 0.62, 1.43), lower in Depression than in Control (SMD = −0.51; 95% CI −1.01, −0.02), lower in PSP than in Control (SMD = −1.03; 95% CI −1.37, −0.68), higher in pMCI than in Control (SMD = 1.99; 95% CI 0.28, 3.70), higher in pMCI than in Depression (SMD = 0.56; 95% CI 0.09, 1.03), higher in pMCI than in FTD (SMD = 1.49; 95% CI 0.75, 2.23), higher in sMCI than in FTD (SMD = 0.83; 95% CI 0.22, 1.45), lower in PSP than in PDD (SMD = −0.73; 95% CI −1.24, −0.23). It is noteworthy that the CSF sAPPβ levels were nearly statistically significantly lower in sMCI than in pMCI (SMD = −0.53; 95% CI −1.07, 0.02). Moreover, Fig. 2b graphically represents the network of eligible comparisons for CSF sAPPβ levels of the NMA. eTable 5 and eFig. 8, appendix 2, displayed that the CSF sAPPβ levels were significantly lower in CBS than in AD (SMD = −388.10; 95% CI −535.73, −240.46), lower in PSP than in AD (SMD = −238.18; 95% CI −298.20, −178.17), higher in Control than in CBS (SMD = 97.89; 95% CI 20.74, 175.03), higher in PDD than in CBS (SMD = 80.00; 95% CI 6.09, 153.91), lower in Depression than in Control (SMD = −71.88; 95% CI −140.42, −3.35), lower in PSP than in Control (SMD = −79.89; 95% CI −128.59, −31.18), higher in pMCI than in Depression (SMD = 56.00; 95% CI 12.36, 99.64), higher in sMCI than in Depression (SMD = 44.00; 95% CI 3.68, 84.32), lower in PSP than in PDD (SMD = −62.00; 95% CI −105.40, −18.60), higher in pMCI than in PSP (SMD = 64.00; 95% CI 1.54, 126.47). Plots of the SURCA, rank probability, ranking plot, and contribution plot were presented in eFig. 9, 10, 11, and eTable 7, appendix 2. The pMCI populations ranked the best in terms of CSF sAPPβ levels as well. Nonetheless, the results should be interpreted with caution because only the NMA comparisons between the pMCI populations with Depression populations and PSP populations reached statistical significance. Besides, inconsistency plot was given in eFig. 12, appendix 2.
Separate contributions to the overall results of sAPPα and sAPPβ were shown in Fig. 3. Moreover, we ranked the 10 groups according to both dimensions of sAPPα and sAPPβ (Fig. 4). pMCI populations have higher levels of CSF sAPPα and sAPPβ, as it lay in the upper right corner. In contrast, DLB, FTD, PSP, and CBS populations tended to have lower levels as they mostly in the lower left corner of the figure. Furthermore, it seemed there was a positive correlation between CSF sAPPα and sAPPβ levels.
The meta-regression with sAPPα (AD vs. Control) indicated that the detection method and total NOS score could not lead to significant changes in the results, except for publication year (P = 0.005). By comparison, the meta-regression with sAPPβ (AD vs. Control) indicated that publication year, detection method and total NOS score could not lead to significant changes in the results. Moreover, for other comparisons, it was impossible to undertake meta-regression further owing to the limited number of studies.
For sAPPα, sensitivity analysis of sample size and quality of the included studies showed that most results were stable, whereas sensitivity analysis of publication year showed more changes (eTable 2, appendix 2). In contrast, for sAPPβ, the results of sensitivity analysis were diametrically opposite (eTable 5, appendix 2). Owing to the fewer number of studies included in each comparison, we only did subgroup analysis to investigate the effect of detection method to AD vs. Control. There was no significant difference of CSF sAPPα levels between AD and Control in ELISA subgroup and MSD (a multiplexing assay of Meso Scale Discovery) subgroup (eFig. 13, appendix 2). However, the CSF sAPPα levels were significantly lower in AD than Control in WB (western blotting) subgroup (SMD = −1.83; 95% CI −3.08, −0.58), higher in IP (immunoprecipitation) +ELISA subgroup (SMD = 1.26; 95% CI 0.41, 2.10). On the other hand, because of the fewer included studies in the two subgroups mentioned above, we could not make a definitive conclusion. By contrast, in eFig. 14, appendix 2, the CSF sAPPβ levels were statistically higher in AD than Control in ELISA subgroup (SMD = 0.99; 95% CI 0.11, 1.88). Nonetheless, there was no significant difference in IP + ELISA subgroup and MSD subgroup, although CSF sAPPβ levels were significantly higher in AD subgroup overall (SMD = 0.50; 95% CI 0.11, 0.88). Finally, visual inspection of funnel plots for sAPPα and sAPPβ did not show distinct asymmetry (eFig. 15, 16, appendix 2).
Discussion
According to the Global Burden of Disease Study 2016, the number of individuals with AD and other dementias was approximately 43.8 million worldwide, comprising 27.0 million women and 16.8 million men (GBD 2015 Disease and Injury Incidence and Prevalence Collaborators 2016; GBD 2016 Dementia Collaborators 2019). Moreover, as population age, the number was projected to increase to over 131 million by 2050. In addition, global deaths due to AD and other dementias were about 1.9 million in 2015 (GBD 2015 Mortality and Causes of Death Collaborators 2016), increased to approximately 2.4 million in 2016 (GBD 2016 Causes of Death Collaborators 2017). AD and other dementias ranked fourth among the leading causes of death globally, increasing sources of health burden (GBD 2016 Causes of Death Collaborators 2017). AD belongs to a large group of neurodegenerative diseases which characterized by progressive cognitive impairment and synaptic damage with neuronal loss. Nowadays, AD is at the forefront of biomedical research. Various hypotheses have emerged to explain underlying pathology, of which amyloid hypothesis is a dominant one.
CSF sAPPα and sAPPβ have been postulated as promising new CSF biomarkers for AD and multiple other neurodegenerative diseases, but have failed to meet expectations with their often contradictory findings. Except for comparisons between AD and healthy elderly control, the number of studies that analyzed other neurodegenerative diseases was still relatively small. To our knowledge, to date, although there was a traditional pairwise meta-analysis from Olsson et al., which only compared CSF sAPPα and sAPPβ levels between pMCI and sMCI, no significant difference was found (Olsson et al. 2016). What is more, for some comparisons, there was no direct comparative research. Hence, traditional pairwise meta-analysis is insufficient to elaborate on the changes of CSF sAPPα and sAPPβ levels in AD and multiple other neurodegenerative diseases. By contrast, NMA allows for a more comprehensive assessment, increasing the precision of estimates and producing a relative ranking of all diseases for the study results. However, there was no NMA performed to systematically explore this issue up to now.
In this study, we provided a relative rank order based on CSF sAPPα and sAPPβ levels. In terms of sAPPβ, AD, as expected, did rank higher compared to CBS, PSP, FTD, PDD, DLB, Control, Depression, and sMCI, but ranked lower than pMCI unexpectedly. The elevated levels of CSF sAPPβ in AD and pMCI populations may be attributed to the increased β-secretase activities or/and levels in the brains (Cheng et al. 2014). It has been reported that populations with pMCI had higher CSF β-secretase activities and levels compared to sMCI, AD, and healthy elderly control (Zetterberg et al. 2008). On the other hand, it was observed that populations with MCI had a higher inflammatory response compared to AD (Tarkowski et al. 2003), and the expression of β-secretase can be upregulated by free radicals and inflammatory cytokines (Tamagno et al. 2005; Sastre et al. 2006). Previously, magnetic resonance imaging researches indicated that, compared to populations with pMCI, ventricular size of AD was enlarged because of the reduction of regional brain volume (de Leon et al. 2004; Kantarci et al. 2007). The enlarged ventricular size could increase CSF volume. Accordingly, the concentrations of β-secretase and sAPPβ would be more diluted in populations with AD compared to pMCI. By contrast, in terms of CSF sAPPα, AD only ranked higher than CBS, PSP, DLB, and FTD, but lower than PDD, Control, sMCI, Depression, and pMCI. The differences among CBS, PSP, DLB, FTD, AD, PDD, Control, sMCI, Depression, and pMCI were not statistically significant, revealing similar levels of CSF sAPPα. As for the relatively increased concentrations of sAPPα in pMCI populations, to our knowledge, there was no clear explanation up to now. Perhaps, APP processing by α-secretase was increased in parallel with that by β-secretase, which may be a normal protective response of brain, as sAPPα possesses neurotrophic and neuroprotective activities (Nhan et al. 2015). Of course, further study is required to provide profound explanations.
In addition, our findings are consistent with the previous studies (Gabelle et al. 2010; Lewczuk et al. 2010; Mulugeta et al. 2011; Alexopoulos et al. 2012) that showed a positive correlation between CSF sAPPα and sAPPβ levels. The enzymes involved in the amyloidogenic and non-amyloidogenic pathway may be precisely regulated by some kind of common upstream mechanism which was upregulated in neurodegenerative diseases, particularly at the early stage of AD (pMCI), leading to the release of sAPPα and sAPPβ increased together. Of course, there may be other reasons. For instance, the presence of sAPPf (Efthimiopoulos et al. 1996; Tezapsidis et al. 1998) (a soluble full-length APP containing an intact cytoplasmic domain), sAPP homodimers (sAPPα/sAPPα, sAPPβ/sAPPβ, and sAPPf/sAPPf), and sAPP heterodimers (sAPPf/sAPPα, sAPPf/sAPPβ, and sAPPα/sAPPβ) should be taken into consideration when detect the levels of CSF sAPPα and sAPPβ (Cuchillo-Ibañez et al. 2015). Because the 6E10 antibody, a widely used antibody that recognizes an epitope present in sAPPα but absent in sAPPβ, will detect not only sAPPα but also sAPPf (Cuchillo-Ibañez et al. 2015). Moreover, the differences of assay kits and assay procedures could also significantly affect the detection (van Waalwijk van Doorn et al. 2016). Up to now, human CSF sAPPα and sAPPβ has mainly been evaluated by WB, ELISA, and MSD. Twenty studies about CSF sAPPα and sAPPβ levels were included in our study, 3 of which were detected by WB, 9 of which were detected by ELISA, 8 of which were detected by MSD. Specifically, for WB, the antibody used to detect CSF sAPPα was 6E10, recognizing the N-terminal part; for ELISA, specific anti-APP antibodies (IBL, Gunma, Japan) were used to detect CSF sAPPα or sAPPβ, recognizing the C-terminal part, except for (Peskind et al. 1997); for MSD, the antibody used to detect CSF sAPPα was 6E10 as well, except for Taverna et al. (used 6E10 and 14D6, simultaneously) (Taverna et al. 2013). Compared to the 6E10 antibody (SMD = 0.28; 95% CI −0.28, 0.85), 14D6 (SMD = 0.75; 95% CI 0.17, 1.33) is a sAPPα-specific antibody, allowing a better separation of AD from healthy elderly control, although needs larger cohorts to verify (Taverna et al. 2013). Notably, the factors mentioned above may be the reasons of discordant and even contradictory findings between researches as well.
It cannot be ignored that this study has some potential limitations. First, evidence in this NMA only originated from Germany (8 studies), Japan (3 studies), Spain (2 studies), Sweden (2 studies), Norway (1 article), Switzerland (1 article), UK (1 article), France (1 article), and USA (1 article). Further studies from other countries are welcomed to explore the effect of race on the results. Second, this NMA was conducted to evaluate the changes of CSF sAPPα and sAPPβ levels in AD and some of the neurodegenerative diseases, but not all of them. Therefore, other neurodegenerative diseases, such as VaD should be deeply researched in the future. Third, except for the lack of information about random sequence generation, blinding, and other useful information, a large portion of the included studies did not assess the sensitivity and specificity of sAPPα and sAPPβ antibodies. In addition to AD vs. Control, for other comparisons, it was impossible to undertake meta-regression further owing to the limited number of studies included. On all accounts, the main strength of our NMA is its extensive and comprehensive literature search and overview of all data. To date, it provides the first systematic overview of the changes of CSF sAPPα and sAPPβ levels in AD, pMCI, sMCI, FTD, PDD, PSP, CBS, Depression, and no neurological disorders Control.
In conclusion, our NMA findings demonstrated that the measurement of CSF sAPPα and sAPPβ levels may be helpful in the diagnosis of early-stage AD, which is conducive to preventive therapy. In the future, a multicentre randomized trial with optimal and standard detection methods, as well as a large sample size, to verify our findings is warranted.
Data Availability
All data of the articles included in our research had been published online and are available to investigators.
Abbreviations
- AD:
-
Alzheimer’s disease
- CBS:
-
Corticobasal syndrome
- CSF:
-
Cerebrospinal fluid
- DLB:
-
Dementia with Lewy bodies
- FTD:
-
Frontotemporal dementia
- NMA:
-
Network meta-analysis
- PDD:
-
Parkinson’s disease dementia
- pMCI:
-
Mild cognitive impairment progressed to AD dementia during the follow-up period
- PSP:
-
Progressive supranuclear palsy
- sAPPα:
-
Soluble amyloid protein procurer α
- sAPPβ:
-
Soluble amyloid protein procurer β
- sMCI:
-
Stable mild cognitive impairment
- VaD:
-
Vascular dementia
References
Alcolea, D., Vilaplana, E., Suárez-Calvet, M., Illán-Gala, I., Blesa, R., Clarimón, J., et al. (2017). CSF sAPPβ, YKL-40, and neurofilament light in frontotemporal lobar degeneration. Neurology,89(2), 178–188.
Alexopoulos, P., Tsolakidou, A., Roselli, F., Arnold, A., Grimmer, T., Westerteicher, C., et al. (2012). Clinical and neurobiological correlates of soluble amyloid precursor proteins in the cerebrospinal fluid. Alzheimer’s and Dementia,8(4), 304–311.
Araki, W., Hattori, K., Kanemaru, K., Yokoi, Y., Omachi, Y., Takano, H., et al. (2017). Re-evaluation of soluble APP-α and APP-β in cerebrospinal fluid as potential biomarkers for early diagnosis of dementia disorders. Biomarker Research,5, 28.
Cheng, X., He, P., Lee, T., Yao, H., Li, R., & Shen, Y. (2014). High activities of BACE1 in brains with mild cognitive impairment. The American Journal of Pathology,184(1), 141–147.
Chow, V. W., Mattson, M. P., Wong, P. C., & Gleichmann, M. (2010). An overview of APP processing enzymes and products. NeuroMolecular Medicine,12(1), 1–12.
Cuchillo-Ibañez, I., Lopez-Font, I., Boix-Amorós, A., Brinkmalm, G., Blennow, K., Molinuevo, J. L., et al. (2015). Heteromers of amyloid precursor protein in cerebrospinal fluid. Molecular Neurodegeneration,10, 2.
Deng, J., Habib, A., Obregon, D. F., Barger, S. W., Giunta, B., Wang, Y. J., et al. (2015). Soluble amyloid precursor protein alpha inhibits tau phosphorylation through modulation of GSK3β signaling pathway. Journal of Neurochemistry,135(3), 630–637.
de Leon, M. J., DeSanti, S., Zinkowski, R., Mehta, P. D., Pratico, D., Segal, S., et al. (2004). MRI and CSF studies in the early diagnosis of Alzheimer’s disease. Journal of Internal Medicine,256(3), 205–223.
DerSimonian, R., & Laird, N. (2015). Meta-analysis in clinical trials revisited. Contemporary Clinical Trials,45(Pt A), 139–145.
Efthimiopoulos, S., Vassilacopoulou, D., Ripellino, J. A., Tezapsidis, N., & Robakis, N. K. (1996). Cholinergic agonists stimulate secretion of soluble full-length amyloid precursor protein in neuroendocrine cells. Proceedings of the National academy of Sciences of the United States of America,93(15), 8046–8050.
Fellgiebel, A., Kojro, E., Müller, M. J., Scheurich, A., Schmidt, L. G., & Fahrenholz, F. (2009). CSF APPs alpha and phosphorylated tau protein levels in mild cognitive impairment and dementia of Alzheimer’s type. Journal of Geriatric Psychiatry and Neurology,22(1), 3–9.
Furukawa, K., Sopher, B. L., Rydel, R. E., Begley, J. G., Pham, D. G., Martin, G. M., et al. (1996). Increased activity-regulating and neuroprotective efficacy of alpha-secretase-derived secreted amyloid precursor protein conferred by a C-terminal heparin-binding domain. Journal of Neurochemistry,67(5), 1882–1896.
Gabelle, A., Roche, S., Gény, C., Bennys, K., Labauge, P., Tholance, Y., et al. (2010). Correlations between soluble α/β forms of amyloid precursor protein and Aβ38, 40, and 42 in human cerebrospinal fluid. Brain Research,1357, 175–183.
Gabelle, A., Roche, S., Gény, C., Bennys, K., Labauge, P., Tholance, Y., et al. (2011). Decreased sAβPPβ, Aβ38, and Aβ40 cerebrospinal fluid levels in frontotemporal dementia. Journal of Alzheimer’s Disease,26(3), 553–563.
GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. (2016). Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet,388(10053), 1545–1602.
GBD 2015 Mortality and Causes of Death Collaborators. (2016). Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet,388(10053), 1459–1544.
GBD 2016 Causes of Death Collaborators. (2017). Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet,390(10100), 1151–1210.
GBD 2016 Dementia Collaborators. (2019). Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. The Lancet. Neurology,18(1), 88–106.
Habib, A., Sawmiller, D., & Tan, J. (2017). Restoring soluble amyloid precursor protein α functions as a potential treatment for alzheimer’s disease. Journal of Neuroscience Research,95(4), 973–991.
Hertze J, Minthon L, Zetterberg H, Vanmechelen E, Blennow K, Hansson O (2010) Evaluation of CSF biomarkers as predictors of Alzheimer’s disease: A clinical follow-up study of 4.7 years. Journal of Alzheimer’s Disease 21(4):1119–1128
Kantarci, K., Weigand, S. D., Petersen, R. C., Boeve, B. F., Knopman, D. S., Gunter, J., et al. (2007). Longitudinal 1H MRS changes in mild cognitive impairment and Alzheimer’s disease. Neurobiology of Aging,28(9), 1330–1339.
Kodamullil, A. T., Zekri, F., Sood, M., Hengerer, B., Canard, L., McHale, D., et al. (2017). Trial watch: Tracing investment in drug development for Alzheimer disease. Nature Reviews Drug Discovery,16(12), 819.
Lannfelt, L., Basun, H., Wahlund, L. O., Rowe, B. A., & Wagner, S. L. (1995). Decreased alpha-secretase-cleaved amyloid precursor protein as a diagnostic marker for Alzheimer’s disease. Nature Medicine,1(8), 829–832.
Lewczuk, P., Kamrowski-Kruck, H., Peters, O., Heuser, I., Jessen, F., Popp, J., et al. (2010). Soluble amyloid precursor proteins in the cerebrospinal fluid as novel potential biomarkers of Alzheimer’s disease: a multicenter study. Molecular Psychiatry,15(2), 138–145.
Lewczuk, P., Popp, J., Lelental, N., Kölsch, H., Maier, W., Kornhuber, J., et al. (2012). Cerebrospinal fluid soluble amyloid-β protein precursor as a potential novel biomarkers of Alzheimer’s disease. Journal of Alzheimer’s Disease,28(1), 119–125.
Magdalinou, N. K., Paterson, R. W., Schott, J. M., Fox, N. C., Mummery, C., Blennow, K., et al. (2015). A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. Journal of Neurology, Neurosurgery and Psychiatry,86(11), 1240–1247.
Miyajima, M., Nakajima, M., Ogino, I., Miyata, H., Motoi, Y., & Arai, H. (2013). Soluble amyloid precursor protein α in the cerebrospinal fluid as a diagnostic and prognostic biomarker for idiopathic normal pressure hydrocephalus. European Journal of Neurology,20(2), 236–242.
Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ,339, b2535.
Moriya, M., Miyajima, M., Nakajima, M., Ogino, I., & Arai, H. (2015). Impact of cerebrospinal fluid shunting for idiopathic normal pressure hydrocephalus on the amyloid cascade. PLoS ONE,10(3), e0119973.
Mulugeta, E., Londos, E., Hansson, O., Ballard, C., Skogseth, R., Minthon, L., et al. (2011). Cerebrospinal fluid levels of sAPPα and sAPPβ in lewy body and alzheimer’s disease: Clinical and neurochemical correlates. International Journal of Alzheimer’s Disease,2011, 495025.
Nhan, H. S., Chiang, K., & Koo, E. H. (2015). The multifaceted nature of amyloid precursor protein and its proteolytic fragments: Friends and foes. Acta Neuropathologica,129(1), 1–19.
Nikolaev, A., McLaughlin, T., O’Leary, D. D., & Tessier-Lavigne, M. (2009). APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature,457(7232), 981–989.
O’Brien, R. J., & Wong, P. C. (2011). Amyloid precursor protein processing and Alzheimer’s disease. Annual Review of Neuroscience,34, 185–204.
Olsson, B., Lautner, R., Andreasson, U., Öhrfelt, A., Portelius, E., Bjerke, M., et al. (2016). CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. The Lancet. Neurology,15(7), 673–684.
Perneczky, R., Alexopoulos, P., & Kurz, A. (2014). Soluble amyloid precursor proteins and secretases as Alzheimer’s disease biomarkers. Trends in Molecular Medicine,20(1), 8–15.
Perneczky, R., Tsolakidou, A., Arnold, A., Diehl-Schmid, J., Grimmer, T., Förstl, H., et al. (2011). CSF soluble amyloid precursor proteins in the diagnosis of incipient Alzheimer disease. Neurology,77(1), 35–38.
Peskind, E. R., Leverenz, J., Farlow, M. R., Ito, R. K., Provow, S. A., Siegel, R. S., et al. (1997). Clinicopathologic correlations of soluble amyloid beta-protein precursor in cerebrospinal fluid in patients with Alzheimer disease and controls. Alzheimer Disease and Associated Disorders,11(4), 201–206.
Popp, J., Lewczuk, P., Kölsch, H., Meichsner, S., Maier, W., Kornhuber, J., et al. (2012). Cholesterol metabolism is associated with soluble amyloid precursor protein production in Alzheimer’s disease. Journal of Neurochemistry,123(2), 310–316.
Post, A., Ackl, N., Rücker, M., Schreiber, Y., Binder, E. B., Ising, M., et al. (2006). Toward a reliable distinction between patients with mild cognitive impairment and Alzheimer-type dementia versus major depression. Biological Psychiatry,59(9), 858–862.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. (2000) Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283(15):2008–2012
Sastre, M., Dewachter, I., Rossner, S., Bogdanovic, N., Rosen, E., Borghgraef, P., et al. (2006). Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma. Proceedings of the National academy of Sciences of the United States of America,103(2), 443–448.
Steinacker, P., Hendrich, C., Sperfeld, A. D., Jesse, S., Lehnert, S., Pabst, A., et al. (2009). Concentrations of beta-amyloid precursor protein processing products in cerebrospinal fluid of patients with amyotrophic lateral sclerosis and frontotemporal lobar degeneration. Journal of Neural Transmission,116(9), 1169–1178.
Tamagno, E., Parola, M., Bardini, P., Piccini, A., Borghi, R., Guglielmotto, M., et al. (2005). Beta-site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. Journal of Neurochemistry,92(3), 628–636.
Tarkowski, E., Andreasen, N., Tarkowski, A., & Blennow, K. (2003). Intrathecal inflammation precedes development of Alzheimer’s disease. Journal of Neurology, Neurosurgery and Psychiatry,74(9), 1200–1205.
Taverna, M., Straub, T., Hampel, H., Rujescu, D., & Lichtenthaler, S. F. (2013). A new sandwich immunoassay for detection of the α-secretase cleaved, soluble amyloid-β protein precursor in cerebrospinal fluid and serum. Journal of Alzheimer’s disease,37(4), 667–678.
Tezapsidis, N., Li, H. C., Ripellino, J. A., Efthimiopoulos, S., Vassilacopoulou, D., Sambamurti, K., et al. (1998). Release of nontransmembrane full-length Alzheimer’s amyloid precursor protein from the lumenar surface of chromaffin granule membranes. Biochemistry,37(5), 1274–1282.
Tsolakidou, A., Alexopoulos, P., Guo, L. H., Grimmer, T., Westerteicher, C., Kratzer, M., et al. (2013). β-Site amyloid precursor protein-cleaving enzyme 1 activity is related to cerebrospinal fluid concentrations of sortilin-related receptor with A-type repeats, soluble amyloid precursor protein, and tau. Alzheimer’s and Dementia,9(4), 386–391.
van Waalwijk van Doorn, L. J., Koel-Simmelink, M. J., Haußmann, U., Klafki, H., Struyfs, H., Linning, P., et al. (2016). Validation of soluble amyloid-β precursor protein assays as diagnostic CSF biomarkers for neurodegenerative diseases. Journal of Neurochemistry,137(1), 112–121.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. (2011) Tugwell The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [online]. The Ottawa Hospital Rresearch Institute. Available at: http://www.ohri.ca/programs/clinical epidemiology/oxford.asp. Accessed May 2, 2019.
Zetterberg, H., Andreasson, U., Hansson, O., Wu, G., Sankaranarayanan, S., Andersson, M. E., et al. (2008). Elevated cerebrospinal fluid BACE1 activity in incipient Alzheimer disease. Archives of Neurology,65(8), 1102–1107.
Acknowledgments
This work was funded in part by the Anhui Provincial Natural Science Foundation, Key Research and Development Plan “A” (Grant Numbers 1804h08020236); the Key Project of University Natural Science Foundation of Anhui Province (Grant Numbers KJ2018A0206).
Funding
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical Approval
The manuscript is a retrospective report that does not require ethics committee approval at our institution.
Informed Consent
Written informed consent was obtained from all participants involved in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tang, W., Wang, Y., Cheng, J. et al. CSF sAPPα and sAPPβ levels in Alzheimer’s Disease and Multiple Other Neurodegenerative Diseases: A Network Meta-Analysis. Neuromol Med 22, 45–55 (2020). https://doi.org/10.1007/s12017-019-08561-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-019-08561-7