Abstract
Sexual dimorphisms account for differences in clinical manifestations or incidence of infectious or autoimmune diseases and malignancy between females and males. Females develop enhanced innate and adaptive immune responses than males and are less susceptible to many infections of bacterial, viral, parasitic, and fungal origin and malignancies but in contrast, they are more prone to develop autoimmune diseases. The higher susceptibility to infections in males is observed from birth to adulthood, suggesting that sex chromosomes and not sex hormones have a major role in sexual dimorphism in innate immunity. Sex-based regulation of immune responses ultimately contributes to age-related disease development and life expectancy. Differences between males and females have been described in the expression of pattern recognition receptors of the innate immune response and in the functional responses of phagocytes and antigen presenting cells. Different factors have been shown to account for the sex-based disparity in immune responses, including genetic factors and hormonal mediators, which contribute independently to dimorphism in the innate immune response. For instance, several genes encoding for innate immune molecules are located on the X chromosome. In addition, estrogen and/or testosterone have been reported to modulate the differentiation, maturation, lifespan, and effector functions of innate immune cells, including neutrophils, macrophages, natural killer cells, and dendritic cells. In this review, we will focus on differences between males and females in innate immunity, which represents the first line of defense against pathogens and plays a fundamental role in the activation, regulation, and orientation of the adaptive immune response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexual dimorphisms have been documented in immunity and indeed, clinical manifestations of infectious or autoimmune diseases and malignancy differ between females and males [1, 2]. Today, the general hypothesis is that females develop stronger innate and adaptive immune responses than males [1, 2]. Importantly, females respond better to various types of vaccination and are less susceptible to many infections caused by bacteria, viruses, parasites, and fungi, such as Staphylococus spp., Mycbacterium tuberculosis, parainfluenza virus, respiratory syncytial virus, hepatitis B virus, Entomoeba histolytica, and Aspergillosis fumigatus [3, 4]. Accordingly, epidemiological studies revealed that males had a higher mortality rates to various infection diseases [5]. The higher susceptibility to infections in males is observed from birth to adulthood. For instance, the incidence of sepsis or meningitis is more frequent in male newborns and the susceptibility to tuberculosis is greater in males from infancy to childhood, suggesting that sex chromosomes and not sex hormones have an important role during this period of life [3]. In addition, these sex-based differences in immune responses are associated with a higher incidence of autoimmune diseases and malignancies in females compared with males (Fig. 1) [1].
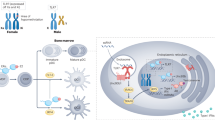
Changes in immune responses and susceptibility to diseases in males and females. Males are more prone to infections and malignancies, whereas females are more susceptible to autoimmune diseases. Several immunological factors vary between women and men. For instance, (1) pDCs from females produce higher amounts of IFN-α after stimulation with TLR7 ligands, resulting in stronger secondary activation of CD8+ T cells, (2) monocytes from males produce higher level of pro-inflammatory cytokines after LPS stimulation, (3) female hormones delay neutrophil apoptosis, (4) macrophages polarization may differ between males and females, and (5) decidual NK cells are involved in tissue remodeling during the development of the placenta
Sex differences in immune responses between males and females are not restricted to mammals and disparities have been reported from insects to lizards and birds. In all these species, males present lower immune responses than females [1]. For instance, in Drosophila melanogaster, X-linked genetic variation in genes involved in the immune response was associated with differences in gene expression and in defense against bacterial infections between males and females [6].
Therefore, a general rule is that a greater susceptibility to infection is observed in males with diverse underlying causes. Indeed, different factors were showed to account for the sex-based disparity in immune responses, including genetic factors and hormonal mediators [1, 3] (Fig. 2, Table 1).
X-linked genes involved in innate immunity. Several genes involved in the innate immune response are located on the X chromosome, including genes involved in the process of macrophage differentiation from hematopoietic stem cell (i.e., IL3RA, GATA1) and macrophage polarization (i.e., IL13RA), genes required for the activation of the intracellular oxidative burst in phagocytes (i.e., CYBB), a regulator of the actin cytoskeleton (i.e., WAS), genes involved in virus recognition (i.e., TLR7, TLR8), and a gene involved in the TLR/IL-1R signaling pathways (i.e., IRAK1). In addition, X chromosome carries also different genes involved in adaptive immunity such as IL2RG, FOXP3, and CD40L
In this review, we will focus on differences between males and females in innate immunity, which represents the first line of defense against pathogens and plays a fundamental role in the activation, regulation, and orientation of the adaptive immune response.
Sex-Based Differences in Innate Immunity
The activation of innate immune responses is initiated by the recognition of pathogens and damaged tissues by a set of germline-encoded molecules called pattern recognition molecules (PRMs) expressed or released by innate immunity cells and other cell types, that discriminate self versus non-self and modified self. PRMs recognize motifs found in microorganisms or in damaged tissues, which are called pathogen-associated molecular patterns (PAMPs) [7] or danger-associated molecular patterns (DAMPs), respectively. Similarly to adaptive immune responses, also innate defense mechanisms consist of a cellular and a humoral arm. Thus, based on their localization, these PRMs are divided in cell-associated molecules and humoral molecules. Cellular PRMs belong to different functional and structural groups, which include the toll-like receptors (TLRs), scavenger receptors, lectin receptors, and G protein-coupled receptors for formyl peptides [8]. These molecules are mainly responsible of activating signaling pathways leading to activation of gene expression associated with the activation of local and systemic inflammatory responses, leukocyte recruitment, activation and survival, or to microbe phagocytosis and killing. The humoral arm of innate immunity is also diverse; it includes collectins (e.g., mannose-binding lectin, surfactant protein A and D, C1q), ficolins, and pentraxins and mainly acts by activating the complement system and the opsonization of microbes [8]. The cellular and humoral arms of innate immunity cooperate and synergize in the primary defense against pathogens leading to the activation, regulation, and orientation of the adaptive immune response [8].
Differences in Response to TLR Agonists and Sepsis
The activity of the innate immune response was reported to vary between males and females [1]. For instance, the expression of PRMs and signaling pathways activated after detection of PAMPs differs between males and females [1, 9, 10]. These observations were mostly related to sex hormones and to genetic mediators (see below) [7]. For instance, estrogen and/or testosterone can modulate the differentiation, maturation, and effector functions of innate immune cells and some genes encoding for innate immune molecules are located on the X chromosome [1] (Fig. 2). For instance, genes encoding for TLR7 or TLR8 that sense single stranded RNAs as their natural ligand and also small synthetic molecules such as imidazoquinolines and nucleoside analogs and IRAK1, a key molecule of the TLR-dependent signaling pathway, are located on the X chromosome. An increasing number of studies has suggested that the production of IFN-α induced by TLR7 agonist was higher in female plasmacytoid dendritic cells (pDCs) compared with male cells [11,12,13,14,15]. Importantly, pDCs from human and mouse females displayed higher basal levels of IFN regulatory factor (IRF) 5, which has been shown to be a central mediator of TLR7 signaling (see below) [15, 16]. However, further studies showed that both sex hormones and the X chromosome could independently contribute in this inequality between males and females (see below) [17].
Peripheral blood mononuclear cells (PBMC) from human males produced lower amounts of IFN-α after stimulation with TLR7 ligands and greater amounts of the immunosuppressive cytokine IL-10 in response to TLR7 ligands [9]. In addition to TLR7, several studies report differential responsiveness to TLR4 in female compared with male macrophages, however, with contrasting results. It has been reported that male macrophages had higher expression of cell surface TLR4 and responded to lipopolysaccharide (LPS), a TLR4 ligand, with a higher production of both IL-1β and CXCL10 and with a lower production of the prostaglandin PGE(2) than female-derived macrophages [18]. In contrast, more recently, others found that the tissue-resident leukocyte populations in female mice and rats are more numerous and have a greater density of pathogen/injury-sensing TLRs compared with those in males [19].
Different clinical studies in patients with sepsis have demonstrated that females had a better outcome compared with males [20,21,22,23,24,25]. For instance, a study revealed that in patients with surgical septic shock, hospital-mortality rate was 70% in male patients (23 out of 33 patients) and 26% in female patients (5 out of 19) [24]. More recently, a retrospective analysis of 373,370 patients from the US National Trauma Data Bank reported that male gender was independently associated with post-traumatic sepsis [25]. In addition, the male gender was identified as an independent risk factor for the development of major infections in surgical patients [26].
The disparities in the innate immune response between males and females, such as a distinctive expression of pro-inflammatory and anti-inflammatory cytokines between male and female patients after surgery, have been suspected to play a role in these clinical outcome differences [27, 28]. In a preclinical model of polymicrobial infection induced by cecal ligation and puncture (CLP) in mice, the administration of β-glucan, a structural component of the fungal cell wall, after the onset of CLP enhanced survival in female mice over a 10-day period and only for 24 h in males [29]. This protection observed in females was associated with decreased IL-10 and IL-6 levels and reduced bacterial burden in the liver compared with male mice [29]. As discussed below, sex hormones were involved in the difference of outcome between genders in sepsis [24, 30]. For instance, an enhanced level of female hormone estrogens associated with a reduced level of the male hormone testosterone was associated with increased patients’ death in men [30]. The survival rate may be influenced by gender also in the course of septic complications after trauma. For instance, in a mouse model of trauma/hemorrhage and CLP, females survived better than males [31]. These data suggest that unbalanced production of pro-inflammatory mediators during early hyper-responsive or late hypo-responsive phases of sepsis depending on the sex may be crucial for patients’ outcome in sepsis.
Differences in Innate Immune Cells
Neutrophils
Neutrophils are the predominant leukocyte subset in the human peripheral blood and play a major role in defense against microbial pathogens through their phagocytic activity [32, 33]. Accordingly, primary immunodeficiencies associated with neutropenia or neutrophil dysfunction lead to recurrent infections and life-threatening conditions (see below) [32, 34]. A number of studies have shown that neutrophils are engaged into complex bidirectional cross-talks with other leukocytes [35]. For instance, neutrophils can shape the inflammatory and immune responses through the production of cytokines and chemokines [35] and are a major source of humoral fluid phase PRM, thus contributing to the humoral arm of innate immunity [7]. More recently, evidences for the existence of neutrophil subsets with functional and phenotypic heterogeneity have emerged in both humans and mice [33, 35,36,37]. Therefore, in addition to their involvement in the elimination of pathogens, neutrophils play a fundamental role in the activation, orientation, and regulation of both the innate and adaptive immune responses and are key players in the resolution or exacerbation of diverse pathologic conditions such as infections, chronic inflammation, autoimmunity, and cancer [32, 33].
In addition to primary immunodeficiencies, different studies have suggested that sex hormones can induce disparities in the biology and/or effector functions of neutrophils between males and females [38]. For instance, hormones control the granulopoiesis and/or the lifespan of neutrophils (see below) [38]. Interestingly, the number of circulating neutrophils in women is increased during pregnancy and during the luteal phase of the menstrual cycle, compared with non-pregnant women and with women in the follicular phase of the normal ovarian cycle, respectively [38]. Circulating neutrophils have a short lifespan and become rapidly apoptotic, leading to their clearance by phagocytes. A rapid and efficient elimination of apoptotic neutrophils by macrophages participates in the maintenance of the tissue homeostasis [39]. For instance, increased levels of apoptotic neutrophils associated with a defective elimination of apoptotic bodies have been observed in patients with systemic lupus erythematous and associated with disease activity [40]. As described below, female sex hormones can affect neutrophil lifespan and the spontaneous apoptosis of neutrophils after in vitro incubation was significantly delayed in women compared with age-matched men [41]. In addition to female hormones, neutropenia associated with impaired immune response against infections was observed in mice deficient for the androgen receptor, because it is essential for the G-CSF signaling [42].
Monocytes and Macrophages
Monocytes, which account for about 5 to 10% of the circulating white blood cells, are an important source of cytokines and key players of innate immune responses. Monocyte-released cytokines, IL-6 in particular, are involved in the acute phase systemic response and the recruitment of other inflammatory cells of adaptive immunity. IL-6 is one of the main cytokines involved in chronic inflammation-related monocyte functions. After entering the blood from the bone marrow, they remain a few hours in the blood and migrate to tissues, where they mature into macrophages. Tissue resident macrophages protect tissues and maintain homeostasis, whereas inflammatory macrophages are recruited at inflammatory sites and contribute to the inflammatory response. It has recently been shown that in the mouse, macrophage progenitors colonize peripheral tissues during embryogenesis and differentiate into tissue resident macrophages (Kupffer cells in liver, microglia in brain, Langerhans cells in the skin, and alveolar macrophages in lung), which will self-maintain throughout life [43, 44]. In contrast, adult bone marrow-derived monocytes are the source of inflammatory macrophages and only in part contribute to replenish resident macrophages in the gut, heart, and dermis [45,46,47,48].
The number and function of innate cells have been reported to differ between males and females in both rodents and humans, but with contrasting results [18, 19]. Innate cells isolated from females generally show a more intense response to inflammatory stimuli. A higher number of pleural and peritoneal macrophages, a more efficient phagocytosis and higher levels of TLR2, TLR3, and TLR4 have been observed in female than in male mice [19]. However, as reported above, male macrophages have been shown to better respond to TLR4 ligands [18]. In a recent study from the Human Functional Genomics Project, a major project that assessed the variability of human cytokine responses to a large panel of microbial and metabolic stimuli in a group of 500 healthy volunteers; female sex was associated with higher circulating levels of IL-1Ra, lower IL-18 binding protein (IL-18BP), and unaffected IL-6 [49]. Regarding myelomonocytic cells, the production of pro-inflammatory cytokines released from monocytes (IL-1β, TNF-α, IL-6, IFN-γ) was higher in men after stimulation with several stimuli (e.g., LPS or Candida albicans conidia) and although the use of oral contraceptives did not have strong effects on cytokine production capacity in vitro, women using oral contraceptives showed a further decreased IFN-γ and TNF-α response after LPS stimulation [49]. Interestingly, the majority of the cytokines and mediators that differed between men and women did not correlate with progesterone and testosterone concentrations, excluding a potential role of hormones in explaining the gender differences [49].
Plasticity and diversity are key properties of cells of the monocyte-macrophage lineage [50,51,52]. Macrophages can undergo polarized classical M1 activation in response to interferon-γ (IFN-γ) and LPS, or alternative M2 activation driven by IL-4 or IL-13. In addition, they are involved in complex bidirectional interactions with other cell types, such as fibroblasts, mesenchymal stem cells, endothelial cells, and T, B, and NK cells, which contribute to their diversity. Indeed, M1- and M2- polarized macrophages are extremes of a continuum in a universe of functional states [53, 54]. A study has reported a differential polarization of macrophages in male and female mice infected with Coxsackievirus B3, which causes severe myocarditis in male but not female mice [55]. Macrophages infiltrating the myocardium from infected male mice expressed high levels of classically activated M1 markers and female macrophages were associated with M2 phenotype [55]. Adoptive-transfer experiments revealed that the excessive presence of M1 macrophages may cause damage to the host and that M2 macrophages were protected against infection-induced myocarditis, suggesting a role for macrophage polarization in defining the sex-related susceptibility to viral myocarditis. Moreover, the higher incidence of asthma observed in female mice was also associated with higher polarization of macrophages to a M2 phenotype when compared to male mice [56, 57].
Female antigen presenting cells (APCs) appear more efficient possibly because they express higher concentrations of MHC Class II and co-stimulatory molecules in some contexts [58]. In agreement, a gene expression analysis study demonstrated that women overexpressed several TLR-associated genes that activate the interferon pathway in response to immunization with a yellow fever virus vaccine [59].
Natural Killer Cells
Natural killer (NK) cells are a population of circulating and tissue-resident lymphocytes involved in the early phases of immune responses against microbial pathogens by exhibiting cytotoxic functions against virally infected or neoplastic cells and by secreting several cytokines and chemokines. NK cells develop from a lymphoid precursor resident in the bone marrow, but the final maturation of NK cell precursors can occur in the periphery. During development and activation, NK cells acquire multiple cell surface activating and inhibitory receptors that finely control their functional activation [60].
The most relevant difference among sexes for NK cell number and function relates to the role of NK cells during pregnancy. NK cells are the most abundant class of lymphocytes found in the maternal uterus where their number reaches 70 to 80% of the total leukocytes in the first trimester of pregnancy, then start to decline, and return to basal levels at the end of pregnancy. Numeric variations of uterine NK cells have been also described during the menstrual cycle, with their number increasing in the proliferative phase and reaching the maximal level in the late secretory phase, in response to hormone-induced decidualization [61]. Decidual NK cells are poorly cytotoxic, but they play a crucial role for normal development of placenta and/or its vasculature and uterine tissue remodeling, by producing cytokines, chemokines, and angiogenic factors. It has been shown that peripheral blood NK cell recruitment to the uterus contributes to the accumulation of NK cells during early pregnancy and progesterone plays a crucial role in this event by reprogramming the chemokine receptor profile of peripheral blood NK cells, once exposed to uterine microenvironment [62].
Plasmacytoid Dendritic Cells
Plasmacytoid dendritic cells are innate immune cells present in primary and secondary lymphoid organs and are able to sense a variety of PAMPs, such as the viral single strain RNA via the expression of TLR7 and bacterial CpG nucleotide DNA sequences via the expression of TLR9 [63]. Therefore, pDCs represent the principal source of IFN-α in human blood and play an important role in the defense against infections [63]. Analyses of the production of IFN-α by peripheral blood leukocytes in three independent cohorts of healthy donors revealed that TLR7 stimulation induced higher production of IFN-α in females compared to males [11]. Interestingly, this difference was not observed after TLR9 stimulation and the production of TNF-α after TLR7 stimulation was not altered, suggesting a sex-specific induction of IFN-α in female pDCs via TLR7 [11]. Accordingly, stimulation of purified pDCs with TLR7 ligand revealed higher production of IFN-α in female pDCs compared to male cells [11]. These findings may have direct implications with respect to the pathogenesis of systemic lupus erythematosus (SLE), in which activation of pDCs and IFN-α has been suggested to play a critical role [64, 65]. Accordingly, a strong sex bias was reported in SLE, whose incidence is approximately nine times higher in women relative to men [66].
Studies comparing the course of HIV-1 infection between women and men have demonstrated considerable sex differences in the manifestations of the disease [12, 67, 68]. Indeed, untreated HIV-1-infected women presented 40% less circulating viral RNA than men and, when adjusted for viral load, the response observed in women was associated with a greater activation of CD8+ T cell compared to men [12, 67, 68]. However, the viral load found in women gradually increased after chronic infection to a higher load compared with males and it has been suggested that women have a higher risk of developing the acquired immune deficiency syndrome compared to men for the same level of viral load [1, 67]. Interestingly, the expression of TLR7 in pDCs plays a central role in the activation of the immune system during infection by single-stranded RNA viruses, such as HIV-1, and the elevated expression of IFN-α by pDCs was considered as a major prognostic indicator for the clinical progression [69]. pDCs derived from women produced significantly more IFN-α in response to HIV-1 encoded TLR7 ligands than pDCs derived from men, resulting in stronger secondary activation of CD8+ T cells [12]. However, because of its chronic nature, HIV infection results in prolonged continuous stimulation of pDCs and expression of IFN-α, leading to chronic T cell activation with CCR5 expression, providing more targets for HIV-1 [67, 70]. Therefore, higher expression of IFN-α in infected women leads to a strong initial response that limits the viral infection but, in turn, enhances the disease progression during chronic infection.
Mediators of Sex-Based Differences in Immunity
Genetic Mediators
The major genetic changes involved in the differences existing between males and females innate immune response reside in the X chromosome [3]. In fact, the X chromosome carries different genes involved in innate and adaptive immunity (e.g., TLR7, TLR8, IRAK1, IL2RG, FOXP3, CD40L) and various genetic factors have been suspected to be responsible for the hyper-responsiveness of the female immune system, such as genes escaping X chromosome inactivation and cellular mosaicism [3].
Genetic Mediators in Response to TLR7 Agonists
The mechanism responsible for the higher production of IFN-α in female pDCs has been the object of several investigations. The number of pDCs was not different between women and men and a defective X-inactivation leading to unequal expression of the TLR7 gene in males and females has been suspected [1]. However, initial investigations did not support evidence for significant X-inactivation escape of the human TLR7 gene in female pDCs [11]. The frequencies of described polymorphisms within the genes encoding TLR7, interferon regulatory factor-7 (IRF7), and myeloid differentiation factor-88 (MyD88) were not altered between women and men, suggesting that the difference in IFN-α production between male and female pDCs was mediated by a signaling event downstream of TLR7 [12]. In addition to genetic mediators, the hypothesis that female estrogens could regulate the production of IFN-α by pDCs after engagement of TLR7 has been tested (see below) [13, 14]. Moreover, the basal level of IRF5, which has been shown to be a central mediator of TLR7 signaling, was found higher in pDCs from human and mouse females compared with males [15]. Interestingly, a recent study has revealed that both X-linked factors and sex hormones (see below) contribute to the enhanced expression of IFN-α in female pDCs response to TLR7-ligand by female pDCs [17]. In favor of a role of genetic mediators, the authors used a model of humanized mouse and showed that the frequency of IFN-α-producing pDCs from female human donors was higher compared with male pDCs and was not dependent on the sex of the recipient mice, suggesting that X chromosome dosage contributes to the enhanced production of IFN-α by pDCs [17].
Primary Immunodeficiency
X-linked primary immunodeficiencies are inherited disorders of the immune system affecting almost exclusively males. These diseases can affect both the adaptive and innate arms of immunity and affected individuals are highly susceptible to recurrent bacterial, fungal, and viral infections [71]. Regarding the innate immune system, a mutation in the CYBB gene (also known as NOX2), which is required for the activation of the intracellular oxidative burst and subsequent killing of microorganisms by phagocytes, is responsible of X-linked chronic granulomatous disease (CGD) [71] (Fig. 2). The bactericidal activity of cells from CGD patients is defective and patients are susceptible to catalase-positive bacteria. In addition to CGD, X-linked Wiskott-Aldrich syndrome (WAS), which is due to a mutation in an important regulator of the actin cytoskeleton (i.e., the WAS protein), results in impaired innate and adaptive immune responses [71]. Regarding the cells of the innate immune response, neutrophils, monocytes, macrophages, and DCs isolated from WAS patients displayed defective migration in response to chemotactic stimuli [72]. In addition, the formation of the phagocytic cup was impaired leading to reduced phagocytosis [72] (Fig. 2).
Hormonal Mediators
Steroid hormones bound to their receptors interact with specific hormone response elements found in the promoter regions of hormone-responsive gene. This binding directly influences the expression of genes. Interestingly, putative hormone response elements are present in the promoters of some innate immune genes, such as TLR7, MyD88, IRF7, and TLR3 [1, 73]. In addition, hormone receptors can also regulate gene expression without directly binding to DNA but through protein-protein interactions with other DNA-binding transcription factors, such as nuclear factor-κB (NF-κB), specific protein 1 (Sp1), CCAAT/enhancer binding protein β (C/EBPβ), or activator protein 1 (AP-1), which are all involved in the production of pro-inflammatory molecules by innate immune cells [59, 74]. For instance, the repression of IL-6 gene expression is induced through the interaction of estrogen receptor with NF-κB and C/EBPβ [75, 76]. Therefore, it is now accepted that sex hormones such as testosterone and estrogens play an important role in the regulation of the innate immune response [1] (Table 1). Importantly, the effects of sex chromosomes (see above) and sex hormones on immunity and inflammation cannot be considered totally independent since the Sry gene on the chromosome Y induces the development of testis during the embryonic life and the consequent production of testosterone. In particular, sex chromosome complement, by determining whether an ovary or testis develops, exerts indirect hormone-mediated effects on the development of sex-specific traits. However, this does not preclude more direct effects that are independent of gonadal hormones [3]. It has been suggested that sex chromosome complement and sex hormones have a compensatory Ying-Yang effect on the immune responses [77]. For instance, the immune response to an autoantigen was higher in ovariectomized XYSry- mice (mice lacking expression of Sry on the Y chromosome), compared with ovariectomized XX mice, demonstrating that male sex chromosome complement was stimulatory [77]. In contrast, the immune response was reduced after administration of testosterone, suggesting a compensatory effect between male sex chromosome complement and the male hormone testosterone [77]. Moreover, androgens have been shown to have suppressive effects on immune functions, such as on cytokine production and lymphocyte proliferation, following trauma or trauma-hemorrhage and subsequent sepsis [78, 79] (Table 1).
Hormonal Mediators in Neutrophils and Macrophages
As mentioned above, sex hormones have been involved in the control of neutrophil functions and survival [38]. For instance, the addition of progesterone and estradiol in vitro delayed the apoptosis of both female and male neutrophils in a dose-dependent manner [41]. In contrast, the addition of dihydrotestosterone had no significant effect on male and female neutrophil apoptosis, suggesting that only female sex hormones, specifically estradiol and progesterone, can delay the apoptosis of neutrophils [41]. However, further study has demonstrated that androgen receptor knockout (ARKO) mice were neutropenic with reduction of neutrophils and neutrophil precursors in the bone marrow because androgen receptor is essential for G-CSF signaling [42]. Accordingly, ARKO mice presented an increased susceptibility to microbial infection. In normal mice and in patients with prostate cancer, androgen ablation induced by surgical castration did not result in neutropenia but in a moderate reduction of neutrophils, suggesting that androgen receptor is more important than testosterone in neutrophil homeostasis [42].
Estrogen and progesterone have been shown to modulate the chemotactic activity of neutrophils; chemotaxis of neutrophils toward fMLP was enhanced by progesterone and reduced by estradiol [80]. In a model of influenza A virus-infected female mice, the treatment with E2 increased the recruitment of pulmonary neutrophils [81]. In turn, neutrophils enhanced the proportion of influenza virus-specific CD8+ T cells producing both IFN-γ and TNF-α [81]. In contrast, testosterone had no measurable direct effect on neutrophil chemotaxis [80]. However, neutrophils from ARKO mice displayed a reduced production of chemokines (e.g., Ccl2, Ccl3, Ccl4, Cxcl1, Cxcl4, Cxcl7) and cytokines (e.g., IL-6, IL-1β) and a reduced chemotactic response toward Cxcl1 and Cxcl2 [42].
Estradiol-based contraceptives, which are known to increase the susceptibility of women to Candida albicans infections, disrupt the gradient of CXCL1 in the vagina, leading to the blockade of neutrophil migration in the stroma and to subsequent increased risk of vaginal candidiasis [82]. In contrast, progesterone increased the migration of neutrophils. The authors suggest that this regulation of neutrophil chemotaxis in the vaginal lumen may favor reproduction during ovulation (high estrogen level), by sparing sperm from immune attack, and immunity during the luteal phase (high progesterone level) [82].
In addition to the chemotactic activity of neutrophils, it has been proposed that sex hormones modulated the production of reactive oxygen intermediate by neutrophils [38]. In two different trauma models (i.e., thermal injury and trauma-haemorrhagic shock), activation of neutrophils was stronger in male rats compared with proestrus females and castration abrogated neutrophil activation [83]. The authors proposed that increased neutrophil activation in male during non-infectious inflammatory states, such as burn injury, trauma, or haemorrhagic shock, may lead to exacerbated tissue injury [83]. Accordingly, data in literature have reported a sexual dimorphism in the response to injury or illness, with the female sex hormone estradiol conferring protection and the male sex hormone testosterone increasing susceptibility to injury [84].
As discussed above, male and female innate immune cells differ in their response to TLR ligands and hormones may play an important role in this difference. For instance, in vitro treatment of macrophages with the male hormone testosterone reduced the expression of TLR4 in cells and the sensitivity to a TLR4-specific ligand [85]. Moreover, in vivo removal of testosterone through orchidectomy increased macrophage surface expression of TLR4 and enhanced the susceptibility of mice to endotoxic shock [85]. In contrast with testosterone, the female hormone estradiol (E2) has a pro-inflammatory role [59]. E2 enhanced the expression of both TLR4 and CD14 on macrophages and in vivo administration of 17β-estradiol resulted in a marked increase in endotoxin susceptibility [86]. However, data have suggested that high doses of E2 may inhibit the production of inflammatory cytokines (e.g., IL-1β, TNF-α) whereas stimulation with E2 at physiological level enhanced the production [1]. In addition, estrogens have been shown to decrease the number of monocytes, possibly by inducing their apoptosis [87].
Hormonal Mediators in pDCs
Regarding the role of sex hormones in the production of IFN-α by pDCs after engagement of TLR7, progesterone has been shown to inhibit it [88]. Furthermore, the frequency of pDCs producing IFN-α in response to TLR7/8 ligands was reduced in postmenopausal women compared to premenopausal women and treatment of postmenopausal women with estrogen restored the production [12, 13]. However, progesterone plasma level in premenopausal women was positively correlated with the percentage of pDCs producing IFN-α in response to stimulation with a HIV-1-derived TLR7/8 ligand [12]. Interestingly, the adoptive transfer of human progenitor cells in humanized mice (i.e., NOD-SCID-B2m−/− mice were transplanted with CD34+ human progenitor cells) showed that the production of IFN-α by pDCs from either sex was enhanced in female mice [17]. Collectively, these data suggested that sex hormone abundance modulates the ability of pDCs to produce IFN-α in response to TLR7/8 ligands. The cellular mechanisms involved depend on the expression of two estrogen receptors (ER), ERα and ERβ [13, 17]. In mice, ERα-deficient mice revealed that estrogens target directly pDCs to increase the expression of IFN-α [13]. The full activation of ERα requires an interaction between two domains (i.e., activation-function (AF) domains, AF-1 and AF-2), which reside in the N-terminal domain and C-terminal domain of the molecule, respectively [89]. Lack of AF-1 in vivo decreased the activation of pDCs after TLR7 triggering [14]. In addition, the transcriptional regulation of IRF5 was suggested to be under the control of ERα [15]. Therefore, the regulation of IRF5 by estrogen has been proposed as mechanism responsible for the stronger expression of IFN-α in female pDCs [15].
Hormonal Mediators in Conventional DCs
E2 can also modify the differentiation, maturation, and effector functions of conventional DCs, which are potent antigen-presenting cells with an important role in activation and polarization of the adaptive immune response [90]. In vitro addition of E2 was effective in promoting the differentiation of DCs from myeloid precursors [91]. In a murine system, E2 treatment enhanced the differentiation of precursor cells into CD11c+ CD11bint DCs that displayed high cell surface expression of MHC class II and of the costimulatory molecules CD40 and CD86, suggesting that E2 may enhance the functional capability of DCs to mediate the presentation of antigens and to induce the activation of CD4+ T cells [91, 92]. Despite that DCs express both ER subtypes, the activity of E2 on the differentiation of bone marrow-derived DCs acts primarily through ERα [91]. In addition, the treatment of ovariectomized mice with physiological amounts of E2 increased the production of IFN-γ by splenic CD11c+ cells stimulated with IL-12 and IL-18 [92]. Interestingly, E2 can also modulate the activity of human DCs [93]. Indeed, a treatment with E2 increased the secretion of IL-8, CCL2, and IL-6 by human immature monocyte-derived DCs and enhanced the secretion of CCL17, CCL19, and CCL2 by LPS-stimulated human DCs, suggesting that E2 may play a role in the induction and sustenance of inflammatory responses [93]. Accordingly, E2 increased the capacity of mature DCs to stimulate allogenic T cells and enhanced the chemotactic response of mature DCs toward CCL19 [93].
Effects of Menopause and Pregnancy on Innate Immunity
Aging women lose their immunological advantage and show increased susceptibility and mortality to specific infections such as hepatitis, meningococcal, or pneumococcal infections. Estrogens are thought to protect premenopausal women from hepatitis C virus and indeed after menopause, sex differences in hepatitis C infection are lost. Indeed, postmenopausal women have increased rates of fibrosis compared with women of reproductive age because they have lost the protective effects of estrogen [94]. However, immune-pathological effects associated with severe forms of dengue and influenza decrease after menopause [5].
Hormone replacement therapy in women has beneficial effects on the immune system and partly reverts menopause-related immunological changes. Estrogens revert the increased pro-inflammatory cytokine production observed in menopause [95] or postmenopausal increase in NK-cell activity. In particular, plasma IL-6, TNF-α, and IL-1β levels were reduced by the therapy [5, 96].
During healthy pregnancy, the immune system has a dual role: preventing immune mediated rejection of the semi-allogenic fetus and at the same time, protecting the mother and fetus from external pathogens. To achieve these goals, complex molecular cross-talks take place among the maternal endometrium, the fetus, and the placenta. Several factors are involved in these processes, including hormones, growth factors, cytokines, chemokines, adhesion molecules, extracellular matrix components, and matrix-degrading enzymes [97]. In particular, IL-1β and TNF-α have emerged as candidate genes responsible for the activation of the pro-inflammatory cascade at the feto-maternal interface [98]. This complex cross-talk results in the induction of a local inflammatory response and a state of systemic inflammation, as revealed by leukocytosis, endothelium activation, increased activity of innate immune cells, and increased levels of inflammatory cytokines and chemokines, as well as their regulators (e.g., sTNF-RI, sTNF-RII, and IL-1Ra) [99,100,101,102]. This local inflammatory environment regulates trophoblast migration and differentiation, leukocyte influx and activation, complement activation, and angiogenesis in the implantation site. In each reproductive cycle, leukocytes heavily infiltrate the periovulatory follicles, corpus luteum, and endometrium, and contribute to endometrium remodeling and repair. If pregnancy occurs, leukocytes are also involved in embryo implantation and placenta development and in setting the balance between protecting the developing embryo and tolerating its hemiallogeneic tissues [103]. The predominant infiltrating leukocytes in first trimester gestational endometrium are monocyte/macrophages (20–25%) and uterine natural killer (uNK) cells (65–70%) [99]. Chemokines are key mediators for their recruitment, and regulation of their activity through the atipical chemokine receptor 2 (ACKR2/D6) was shown to be involved in protecting the fetus from fetal loss in inflammatory or infectious conditions [104]. In this context, we showed that the long pentraxin PTX3, a humoral component of innate immunity, is involved in pregnancy, in processes which range from ECM assembly, angiogenesis, control of complement activation, and inflammation, to removal of apoptotic cells. PTX3 also emerged as potential biomarker in various pregnancy disorders, namely in pre-eclampsia [98, 105,106,107].
Gender Disparity in Cancer Incidence
Gender disparity in cancer incidence is one consistent finding in epidemiological studies [108]. Males are more prone to develop several cancer types, such as larynx cancer, hypopharynx cancer, Kaposi sarcoma, lip cancer, hepatocellular carcinoma (HCC), bladder cancer, lung cancer, and colon cancer, whereas only few cancers are more common in females, such as breast cancer, thyroid cancer, and anus cancer [108]. Gender differences in cancer incidence are also observed in childhood, suggesting that a genetic mediator plays an important role. Accordingly, in male childhood acute lymphoblastic leukemia, small nucleotide polymorphisms (SNPs) in IFN-γ and IRF4 were associated with protection or increased risk of cancer, respectively [109, 110]. Regarding IRF4, the wild type variant of the SNP showed a repressive effect on the transcription level of IRF4 and aberrant expression of IRF4 was associated with increased risk to develop leukemia in childhood [110, 111]. Based on high-throughput molecular data available through The Cancer Genome Atlas (TCGA) project, a recent study revealed a sex-biased signature of immune gene expression in adult cancer patients [112]. For instance, expression of IL-2 and STAT5 signaling in patients with liver hepatocellular carcinoma were higher in women, suggesting that male and female mount different immune responses in cancer [112].
Environmental factors and lifestyle factors play also an important role in cancer development and may contribute to the gender disparity in cancer incidence, such as smoking-related cancers, which are more common in males [108]. However, after comparable exposure to tobacco smoke, a study had reported that the risk of developing lung cancer was comparable in women and men [113]. Other studies indicated that males have higher morbidity and mortality rates for lung cancer, whereas women present higher risk to develop it [108].
Regarding hormonal mediators, it has been shown that female hormone estrogen receptors are expressed by lung cancer cell lines and are involved in lung carcinogenesis [114]. ER is also expressed by colonocytes, but in contrast with lung, E2 was shown to play a protective role in colorectal cancer through cell growth inhibition [115]. In a mouse model of hepatocellular carcinoma induced by a chemical carcinogen, cancer was observed in 100% of male mice but only in 10 to 30% of female littermates [116]. The pro-inflammatory cytokine IL-6 is part of the hepatic response to systemic inflammation and is a major contributing factor for the development of hepatocellular carcinoma. In this model, the gender disparity was mediated by the inhibition of IL-6 production from Kupffer cells by estrogens [116]. More recently, a study showed that female hormones act via the gonadal-hypophyseal axis to limit hepatocellular carcinoma development [117]. Indeed, a sex-independent production of IL-6 by IL-1β-stimulated Kupffer cells is known to exist and the estrogen-responsive pituitary hormone prolactin acts on hepatocytes to interrupt IL-1β signaling [117]. Therefore, these data may explain why females are less prone to liver cancer than males and why liver incidence increases in postmenopausal women [116, 117]. Finally, in a zebrafish model of liver cancer, increased cortisol expression and a high infiltration level of neutrophils and macrophages in tumor were associated with increased susceptibility to hepatocellular carcinoma and gender disparity [118].
General Conclusions
Immunological advantages of females of several species in resistance to infections are well known since several years (Fig. 1). Generally, females better resist to infections of viral, bacterial, fungal, and parasitic origin, suggesting a more vigorous immune response to pathogens. Males are also more prone to develop several cancer subtypes both in adult and pediatric patients. In contrast, females suffer from autoimmune diseases more than men, possibly because of over-reactive immune responses. However, the cellular and molecular mechanisms regulating sex differences in innate immune responses are still largely unknown.
Better innate resistance to infection of females is observed early in life, indicating that sex chromosomes more than sex hormones have a major role in sex differences in immunity. Indeed, several genes related to immune responses are located on the X chromosome (Fig. 2). In contrast, conflicting results on the effects of sex hormones in sex-dependent differences in innate immune and inflammatory responses have been reported in the literature, possibly because of technical differences in the studies. In particular, the study of the effects of hormonal mediators is hampered by the complexity of the responses depending on the hormonal concentrations and duration of exposure, or immune cell isolation and stimulation techniques, or finally by small sample population studies. However, it is now clearly demonstrated that both sex hormones and X chromosome complement contribute independently to the innate immune response and even if the molecular mechanisms are still only in part defined, the differential responses of innate immunity cells to inflammatory stimuli in the presence of hormones contribute in explaining age and sex differences in innate immune responses and in diseases.
References
Klein SL, Flanagan KL (2016) Sex differences in immune responses. Nat Rev Immunol 16(10):626–638. https://doi.org/10.1038/nri.2016.90
Fish EN (2008) The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol 8(9):737–744. https://doi.org/10.1038/nri2394
Libert C, Dejager L, Pinheiro I (2010) The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol 10(8):594–604. https://doi.org/10.1038/nri2815
vom Steeg LG, Klein SL (2016) SeXX matters in infectious disease pathogenesis. PLoS Pathog 12(2):e1005374. https://doi.org/10.1371/journal.ppat.1005374
Giefing-Kroll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B (2015) How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell 14(3):309–321. https://doi.org/10.1111/acel.12326
Hill-Burns EM, Clark AG (2009) X-linked variation in immune response in Drosophila melanogaster. Genetics 183(4):1477–1491. https://doi.org/10.1534/genetics.108.093971
Jaillon S, Ponzetta A, Magrini E, Barajon I, Barbagallo M et al (2016) Fluid phase recognition molecules in neutrophil-dependent immune responses. Semin Immunol 28(2):109–118. https://doi.org/10.1016/j.smim.2016.03.005
Bottazzi B, Doni A, Garlanda C, Mantovani A (2010) An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu Rev Immunol 28:157–183
Torcia MG, Nencioni L, Clemente AM, Civitelli L, Celestino I et al (2012) Sex differences in the response to viral infections: TLR8 and TLR9 ligand stimulation induce higher IL10 production in males. PLoS One 7(6):e39853. https://doi.org/10.1371/journal.pone.0039853
Asai K, Hiki N, Mimura Y, Ogawa T, Unou K et al (2001) Gender differences in cytokine secretion by human peripheral blood mononuclear cells: role of estrogen in modulating LPS-induced cytokine secretion in an ex vivo septic model. Shock 16(5):340–343
Berghofer B, Frommer T, Haley G, Fink L, Bein G et al (2006) TLR7 ligands induce higher IFN-alpha production in females. J Immunol 177(4):2088–2096
Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK et al (2009) Sex differences in the toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med 15(8):955–959. https://doi.org/10.1038/nm.2004
Seillet C, Laffont S, Tremollieres F, Rouquie N, Ribot C et al (2012) The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor alpha signaling. Blood 119(2):454–464. https://doi.org/10.1182/blood-2011-08-371831
Seillet C, Rouquie N, Foulon E, Douin-Echinard V, Krust A et al (2013) Estradiol promotes functional responses in inflammatory and steady-state dendritic cells through differential requirement for activation function-1 of estrogen receptor alpha. J Immunol 190(11):5459–5470. https://doi.org/10.4049/jimmunol.1203312
Griesbeck M, Ziegler S, Laffont S, Smith N, Chauveau L et al (2015) Sex Differences in plasmacytoid dendritic cell levels of IRF5 drive higher IFN-alpha production in women. J Immunol 195(11):5327–5336. https://doi.org/10.4049/jimmunol.1501684
Schoenemeyer A, Barnes BJ, Mancl ME, Latz E, Goutagny N et al (2005) The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor 7 signaling. J Biol Chem 280(17):17005–17012. https://doi.org/10.1074/jbc.M412584200
Laffont S, Rouquie N, Azar P, Seillet C, Plumas J et al (2014) X-Chromosome complement and estrogen receptor signaling independently contribute to the enhanced TLR7-mediated IFN-alpha production of plasmacytoid dendritic cells from women. J Immunol 193(11):5444–5452. https://doi.org/10.4049/jimmunol.1303400
Marriott I, Bost KL, Huet-Hudson YM (2006) Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: a possible mechanism for gender-based differences in endotoxic shock susceptibility. J Reprod Immunol 71(1):12–27. https://doi.org/10.1016/j.jri.2006.01.004
Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW (2011) Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood 118(22):5918–5927. https://doi.org/10.1182/blood-2011-03-340281
McGowan JE Jr, Barnes MW, Finland M (1975) Bacteremia at Boston City Hospital: occurrence and mortality during 12 selected years (1935-1972), with special reference to hospital-acquired cases. J Infect Dis 132(3):316–335
Bone RC (1992) Toward an epidemiology and natural history of SIRS (systemic inflammatory response syndrome). JAMA 268(24):3452–3455
Fourrier F, Jallot A, Leclerc L, Jourdain M, Racadot A et al (1994) Sex steroid hormones in circulatory shock, sepsis syndrome, and septic shock. Circ Shock 43(4):171–178
Barrow RE, Herndon DN (1990) Incidence of mortality in boys and girls after severe thermal burns. Surg Gynecol Obstet 170(4):295–298
Schroder J, Kahlke V, Staubach KH, Zabel P, Stuber F (1998) Gender differences in human sepsis. Arch Surg 133(11):1200–1205
Kisat M, Villegas CV, Onguti S, Zafar SN, Latif A et al (2013) Predictors of sepsis in moderately severely injured patients: an analysis of the National Trauma Data Bank. Surg Infect 14(1):62–68. https://doi.org/10.1089/sur.2012.009
Offner PJ, Moore EE, Biffl WL (1999) Male gender is a risk factor for major infections after surgery. Arch Surg 134(9):935–938 discussion 938-940
Reade MC, Yende S, D'Angelo G, Kong L, Kellum JA et al (2009) Differences in immune response may explain lower survival among older men with pneumonia. Crit Care Med 37(5):1655–1662. https://doi.org/10.1097/CCM.0b013e31819da853
Angele MK, Pratschke S, Hubbard WJ, Chaudry IH (2014) Gender differences in sepsis: cardiovascular and immunological aspects. Virulence 5(1):12–19. https://doi.org/10.4161/viru.26982
Newsome CT, Flores E, Ayala A, Gregory S, Reichner JS (2011) Improved antimicrobial host defense in mice following poly-(1,6)-beta-D-glucopyranosyl-(1,3)-beta-D-glucopyranose glucan treatment by a gender-dependent immune mechanism. Clin Vaccine Immunol 18(12):2043–2049. https://doi.org/10.1128/CVI.05202-11
Christeff N, Benassayag C, Carli-Vielle C, Carli A, Nunez EA (1988) Elevated oestrogen and reduced testosterone levels in the serum of male septic shock patients. J Steroid Biochem 29(4):435–440
Drechsler S, Weixelbaumer K, Raeven P, Jafarmadar M, Khadem A et al (2012) Relationship between age/gender-induced survival changes and the magnitude of inflammatory activation and organ dysfunction in post-traumatic sepsis. PLoS One 7(12):e51457. https://doi.org/10.1371/journal.pone.0051457
Mantovani A, Cassatella MA, Costantini C, Jaillon S (2011) Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 11(8):519–531
Jaillon S, Galdiero MR, Del Prete D, Cassatella MA, Garlanda C et al (2013) Neutrophils in innate and adaptive immunity. Semin Immunopathol 35(4):377–394. https://doi.org/10.1007/s00281-013-0374-8
Wirths S, Bugl S, Kopp HG (2014) Neutrophil homeostasis and its regulation by danger signaling. Blood 123(23):3563–3566. https://doi.org/10.1182/blood-2013-11-516260
Scapini P, Cassatella MA (2014) Social networking of human neutrophils within the immune system. Blood 124(5):710–719. https://doi.org/10.1182/blood-2014-03-453217
Marini O, Costa S, Bevilacqua D, Calzetti F, Tamassia N et al (2017) Mature CD10+ and immature CD10− neutrophils present in G-CSF-treated donors display opposite effects on T cells. Blood 129(10):1343–1356. https://doi.org/10.1182/blood-2016-04-713206
Coffelt SB, Wellenstein MD, de Visser KE (2016) Neutrophils in cancer: neutral no more. Nat Rev Cancer 16(7):431–446. https://doi.org/10.1038/nrc.2016.52
Bouman A, Heineman MJ, Faas MM (2005) Sex hormones and the immune response in humans. Hum Reprod Update 11(4):411–423. https://doi.org/10.1093/humupd/dmi008
Jeannin P, Jaillon S, Delneste Y (2008) Pattern recognition receptors in the immune response against dying cells. Curr Opin Immunol 20(5):530–537
Kaplan MJ (2011) Neutrophils in the pathogenesis and manifestations of SLE. Nat Rev Rheumatol 7(12):691–699. https://doi.org/10.1038/nrrheum.2011.132
Molloy EJ, O'Neill AJ, Grantham JJ, Sheridan-Pereira M, Fitzpatrick JM et al (2003) Sex-specific alterations in neutrophil apoptosis: the role of estradiol and progesterone. Blood 102(7):2653–2659. https://doi.org/10.1182/blood-2003-02-0649
Chuang KH, Altuwaijri S, Li G, Lai JJ, Chu CY et al (2009) Neutropenia with impaired host defense against microbial infection in mice lacking androgen receptor. J Exp Med 206(5):1181–1199. https://doi.org/10.1084/jem.20082521
Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E et al (2015) Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518(7540):547–551. https://doi.org/10.1038/nature13989
De Kleer I, Willems F, Lambrecht B, Goriely S (2014) Ontogeny of myeloid cells. Front Immunol 5:423. https://doi.org/10.3389/fimmu.2014.00423
Wynn TA, Chawla A, Pollard JW (2013) Macrophage biology in development, homeostasis and disease. Nature 496(7446):445–455. https://doi.org/10.1038/nature12034
Bain CC, Bravo-Blas A, Scott CL, Perdiguero EG, Geissmann F et al (2014) Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol 15(10):929–937. https://doi.org/10.1038/ni.2967
McGovern N, Schlitzer A, Gunawan M, Jardine L, Shin A et al (2014) Human dermal CD14(+) cells are a transient population of monocyte-derived macrophages. Immunity 41(3):465–477. https://doi.org/10.1016/j.immuni.2014.08.006
Molawi K, Wolf Y, Kandalla PK, Favret J, Hagemeyer N et al (2014) Progressive replacement of embryo-derived cardiac macrophages with age. J Exp Med 211(11):2151–2158. https://doi.org/10.1084/jem.20140639
Ter Horst R, Jaeger M, Smeekens SP, Oosting M, Swertz MA et al (2016) Host and environmental factors influencing individual human cytokine responses. Cell 167(4):1111–1124 e1113. https://doi.org/10.1016/j.cell.2016.10.018
Biswas SK, Mantovani A (2010) Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 11(10):889–896. https://doi.org/10.1038/ni.1937
Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122(3):787–795. https://doi.org/10.1172/JCI59643
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8(12):958–969. https://doi.org/10.1038/nri2448
Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW et al (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41(1):14–20. https://doi.org/10.1016/j.immuni.2014.06.008
Mantovani A, Allavena P (2015) The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med DOI. https://doi.org/10.1084/jem.20150295
Li K, Xu W, Guo Q, Jiang Z, Wang P et al (2009) Differential macrophage polarization in male and female BALB/c mice infected with coxsackievirus B3 defines susceptibility to viral myocarditis. Circ Res 105(4):353–364. https://doi.org/10.1161/CIRCRESAHA.109.195230
Melgert BN, Oriss TB, Qi Z, Dixon-McCarthy B, Geerlings M et al (2010) Macrophages: regulators of sex differences in asthma? Am J Respir Cell Mol Biol 42(5):595–603. https://doi.org/10.1165/rcmb.2009-0016OC
Galvan-Pena S, O'Neill LA (2014) Metabolic reprograming in macrophage polarization. Front Immunol 5:420. https://doi.org/10.3389/fimmu.2014.00420
Gubbels Bupp MR (2015) Sex, the aging immune system, and chronic disease. Cell Immunol 294(2):102–110. https://doi.org/10.1016/j.cellimm.2015.02.002
Klein SL, Jedlicka A, Pekosz A (2010) The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis 10(5):338–349. https://doi.org/10.1016/S1473-3099(10)70049-9
Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L et al (2011) Innate or adaptive immunity? The example of natural killer cells. Science 331(6013):44–49. https://doi.org/10.1126/science.1198687
Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C et al (2006) Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 12(9):1065–1074
Carlino C, Stabile H, Morrone S, Bulla R, Soriani A et al (2008) Recruitment of circulating NK cells through decidual tissues: a possible mechanism controlling NK cell accumulation in the uterus during early pregnancy. Blood 111(6):3108–3115
Chistiakov DA, Orekhov AN, Sobenin IA, Bobryshev YV (2014) Plasmacytoid dendritic cells: development, functions, and role in atherosclerotic inflammation. Front Physiol 5:279. https://doi.org/10.3389/fphys.2014.00279
Marshak-Rothstein A (2006) Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol 6(11):823–835. https://doi.org/10.1038/nri1957
Ronnblom L, Eloranta ML, Alm GV (2006) The type I interferon system in systemic lupus erythematosus. Arthritis Rheum 54(2):408–420. https://doi.org/10.1002/art.21571
Whitacre CC (2001) Sex differences in autoimmune disease. Nat Immunol 2(9):777–780. https://doi.org/10.1038/ni0901-777
Ghosh S, Klein RS (2017) Sex drives dimorphic immune responses to viral infections. J Immunol 198(5):1782–1790. https://doi.org/10.4049/jimmunol.1601166
Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB et al (2001) Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med 344(10):720–725. https://doi.org/10.1056/NEJM200103083441003
Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I et al (2005) Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest 115(11):3265–3275. https://doi.org/10.1172/JCI26032
Tsao LC, Guo H, Jeffrey J, Hoxie JA, Su L (2016) CCR5 interaction with HIV-1 Env contributes to Env-induced depletion of CD4 T cells in vitro and in vivo. Retrovirology 13:22. https://doi.org/10.1186/s12977-016-0255-z
Pessach IM, Notarangelo LD (2009) X-linked primary immunodeficiencies as a bridge to better understanding X-chromosome related autoimmunity. J Autoimmun 33(1):17–24. https://doi.org/10.1016/j.jaut.2009.03.003
Bouma G, Burns SO, Thrasher AJ (2009) Wiskott-Aldrich Syndrome: immunodeficiency resulting from defective cell migration and impaired immunostimulatory activation. Immunobiology 214(9–10):778–790. https://doi.org/10.1016/j.imbio.2009.06.009
Hannah MF, Bajic VB, Klein SL (2008) Sex differences in the recognition of and innate antiviral responses to Seoul virus in Norway rats. Brain Behav Immun 22(4):503–516. https://doi.org/10.1016/j.bbi.2007.10.005
Bjornstrom L, Sjoberg M (2005) Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 19(4):833–842. https://doi.org/10.1210/me.2004-0486
Ray A, Prefontaine KE, Ray P (1994) Down-modulation of interleukin-6 gene expression by 17 beta-estradiol in the absence of high affinity DNA binding by the estrogen receptor. J Biol Chem 269(17):12940–12946
Stein B, Yang MX (1995) Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol Cell Biol 15(9):4971–4979
Palaszynski KM, Smith DL, Kamrava S, Burgoyne PS, Arnold AP et al (2005) A yin-yang effect between sex chromosome complement and sex hormones on the immune response. Endocrinology 146(8):3280–3285. https://doi.org/10.1210/en.2005-0284
Wichmann MW, Zellweger R, DeMaso CM, Ayala A, Chaudry IH (1996) Mechanism of immunosuppression in males following trauma-hemorrhage. Critical role of testosterone. Arch Surg 131(11):1186–1191 discussion 1191-1182
Trigunaite A, Dimo J, Jorgensen TN (2015) Suppressive effects of androgens on the immune system. Cell Immunol 294(2):87–94. https://doi.org/10.1016/j.cellimm.2015.02.004
Miyagi M, Aoyama H, Morishita M, Iwamoto Y (1992) Effects of sex hormones on chemotaxis of human peripheral polymorphonuclear leukocytes and monocytes. J Periodontol 63(1):28–32. https://doi.org/10.1902/jop.1992.63.1.28
Robinson DP, Hall OJ, Nilles TL, Bream JH, Klein SL (2014) 17beta-estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific CD8 T cell responses in the lungs. J Virol 88(9):4711–4720. https://doi.org/10.1128/JVI.02081-13
Lasarte S, Samaniego R, Salinas-Munoz L, Guia-Gonzalez MA, Weiss LA et al (2016) Sex hormones coordinate neutrophil immunity in the vagina by controlling chemokine gradients. J Infect Dis 213(3):476–484. https://doi.org/10.1093/infdis/jiv402
Deitch EA, Ananthakrishnan P, Cohen DB, Xu DZ, Feketeova E et al (2006) Neutrophil activation is modulated by sex hormones after trauma-hemorrhagic shock and burn injuries. Am J Physiol Heart Circ Physiol 291(3):H1456–H1465. https://doi.org/10.1152/ajpheart.00694.2005
Angele MK, Schwacha MG, Ayala A, Chaudry IH (2000) Effect of gender and sex hormones on immune responses following shock. Shock 14(2):81–90
Rettew JA, Huet-Hudson YM, Marriott I (2008) Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod 78(3):432–437. https://doi.org/10.1095/biolreprod.107.063545
Rettew JA, Huet YM, Marriott I (2009) Estrogens augment cell surface TLR4 expression on murine macrophages and regulate sepsis susceptibility in vivo. Endocrinology 150(8):3877–3884. https://doi.org/10.1210/en.2009-0098
Bouman A, Schipper M, Heineman MJ, Faas MM (2004) Gender difference in the non-specific and specific immune response in humans. Am J Reprod Immunol 52(1):19–26. https://doi.org/10.1111/j.1600-0897.2004.00177.x
Hughes GC, Thomas S, Li C, Kaja MK, Clark EA (2008) Cutting edge: progesterone regulates IFN-alpha production by plasmacytoid dendritic cells. J Immunol 180(4):2029–2033
Tora L, White J, Brou C, Tasset D, Webster N et al (1989) The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell 59(3):477–487
Lanzavecchia A, Sallusto F (2001) The instructive role of dendritic cells on T cell responses: lineages, plasticity and kinetics. Curr Opin Immunol 13(3):291–298
Paharkova-Vatchkova V, Maldonado R, Kovats S (2004) Estrogen preferentially promotes the differentiation of CD11c+ CD11b(intermediate) dendritic cells from bone marrow precursors. J Immunol 172(3):1426–1436
Siracusa MC, Overstreet MG, Housseau F, Scott AL, Klein SL (2008) 17beta-estradiol alters the activity of conventional and IFN-producing killer dendritic cells. J Immunol 180(3):1423–1431
Bengtsson AK, Ryan EJ, Giordano D, Magaletti DM, Clark EA (2004) 17beta-estradiol (E2) modulates cytokine and chemokine expression in human monocyte-derived dendritic cells. Blood 104(5):1404–1410. https://doi.org/10.1182/blood-2003-10-3380
Baden R, Rockstroh JK, Buti M (2014) Natural history and management of hepatitis C: does sex play a role? J Infect Dis 209(Suppl 3):S81–S85. https://doi.org/10.1093/infdis/jiu057
Kamada M, Irahara M, Maegawa M, Ohmoto Y, Takeji T et al (2001) Postmenopausal changes in serum cytokine levels and hormone replacement therapy. Am J Obstet Gynecol 184(3):309–314. https://doi.org/10.1067/mob.2001.109940
Vural P, Akgul C, Canbaz M (2006) Effects of hormone replacement therapy on plasma pro-inflammatory and anti-inflammatory cytokines and some bone turnover markers in postmenopausal women. Pharmacol Res 54(4):298–302. https://doi.org/10.1016/j.phrs.2006.06.006
Salamonsen LA, Dimitriadis E, Jones RL, Nie G (2003) Complex regulation of decidualization: a role for cytokines and proteases—a review. Placenta 24(Suppl A):S76–S85
Garlanda C, Maina V, Martinez de la Torre Y, Nebuloni M, Locati M (2008) Inflammatory reaction and implantation: the new entries PTX3 and D6. Placenta 29(Suppl B):129–134
Leonard S, Murrant C, Tayade C, van den Heuvel M, Watering R et al (2006) Mechanisms regulating immune cell contributions to spiral artery modification—facts and hypotheses—a review. Placenta 27(Suppl A):S40–S46
Redman CW, Sargent IL (2005) Latest advances in understanding preeclampsia. Science 308(5728):1592–1594
Graham C, Chooniedass R, Stefura WP, Becker AB, Sears MR et al (2017) In vivo immune signatures of healthy human pregnancy: inherently inflammatory or anti-inflammatory? PLoS One 12(6):e0177813. https://doi.org/10.1371/journal.pone.0177813
PrabhuDas M, Bonney E, Caron K, Dey S, Erlebacher A et al (2015) Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol 16(4):328–334. https://doi.org/10.1038/ni.3131
Salamonsen LA, Zhang J, Brasted M (2002) Leukocyte networks and human endometrial remodelling. J Reprod Immunol 57(1–2):95–108
Martinez de la Torre Y, Buracchi C, Borroni EM, Dupor J, Bonecchi R et al (2007) Protection against inflammation- and autoantibody-caused fetal loss by the chemokine decoy receptor D6. Proc Natl Acad Sci U S A 104(7):2319–2324
Salustri A, Garlanda C, Hirsch E, De Acetis M, Maccagno A et al (2004) PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development 131(7):1577–1586
Cetin I, Cozzi V, Pasqualini F, Nebuloni M, Garlanda C et al (2006) Elevated maternal levels of the long pentraxin 3 (PTX3) in preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol 194(5):1347–1353
Cozzi V, Garlanda C, Nebuloni M, Maina V, Martinelli A et al (2012) PTX3 as a potential endothelial dysfunction biomarker for severity of preeclampsia and IUGR. Placenta 33(12):1039–1044. https://doi.org/10.1016/j.placenta.2012.09.009
Dorak MT, Karpuzoglu E (2012) Gender differences in cancer susceptibility: an inadequately addressed issue. Front Genet 3:268. https://doi.org/10.3389/fgene.2012.00268
Morrison BA, Ucisik-Akkaya E, Flores H, Alaez C, Gorodezky C et al (2010) Multiple sclerosis risk markers in HLA-DRA, HLA-C, and IFNG genes are associated with sex-specific childhood leukemia risk. Autoimmunity 43(8):690–697. https://doi.org/10.3109/08916930903567492
Do TN, Ucisik-Akkaya E, Davis CF, Morrison BA, Dorak MT (2010) An intronic polymorphism of IRF4 gene influences gene transcription in vitro and shows a risk association with childhood acute lymphoblastic leukemia in males. Biochim Biophys Acta 1802(2):292–300. https://doi.org/10.1016/j.bbadis.2009.10.015
Adamaki M, Lambrou GI, Athanasiadou A, Tzanoudaki M, Vlahopoulos S et al (2013) Implication of IRF4 aberrant gene expression in the acute leukemias of childhood. PLoS One 8(8):e72326. https://doi.org/10.1371/journal.pone.0072326
Yuan Y, Liu L, Chen H, Wang Y, Xu Y et al (2016) Comprehensive characterization of molecular differences in cancer between male and female patients. Cancer Cell 29(5):711–722. https://doi.org/10.1016/j.ccell.2016.04.001
Kreuzer M, Boffetta P, Whitley E, Ahrens W, Gaborieau V et al (2000) Gender differences in lung cancer risk by smoking: a multicentre case-control study in Germany and Italy. Br J Cancer 82(1):227–233. https://doi.org/10.1054/bjoc.1999.0904
Stabile LP, Davis AL, Gubish CT, Hopkins TM, Luketich JD et al (2002) Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res 62(7):2141–2150
Weige CC, Allred KF, Allred CD (2009) Estradiol alters cell growth in nonmalignant colonocytes and reduces the formation of preneoplastic lesions in the colon. Cancer Res 69(23):9118–9124. https://doi.org/10.1158/0008-5472.CAN-09-2348
Naugler WE, Sakurai T, Kim S, Maeda S, Kim K et al (2007) Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 317(5834):121–124. https://doi.org/10.1126/science.1140485
Hartwell HJ, Petrosky KY, Fox JG, Horseman ND, Rogers AB (2014) Prolactin prevents hepatocellular carcinoma by restricting innate immune activation of c-Myc in mice. Proc Natl Acad Sci U S A 111(31):11455–11460. https://doi.org/10.1073/pnas.1404267111
Yan C, Yang Q, Gong Z (2017) Tumor-associated neutrophils and macrophages promote gender disparity in hepatocellular carcinoma in zebrafish. Cancer Res 77(6):1395–1407. https://doi.org/10.1158/0008-5472.CAN-16-2200
Funding
The contribution of Ministero della Salute (RF-2013-02355470), Ministry of Education, University and Research (PRIN 2015YYKPNN), and the Associazione Italiana Ricerca sul Cancro (MFAG 2016 ID 18475) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethical Approval and Informed Consent
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Jaillon, S., Berthenet, K. & Garlanda, C. Sexual Dimorphism in Innate Immunity. Clinic Rev Allerg Immunol 56, 308–321 (2019). https://doi.org/10.1007/s12016-017-8648-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-017-8648-x