Abstract
Food allergy imposes a severe global health burden, and thus, there is a dire need for safe and effective treatments. Allergen-specific immunotherapy (AIT) is currently the only approach to restore immune tolerance through administrating increasing doses of allergen extracts. Unfortunately, the development of AIT for food allergies has been impeded by the frequent anaphylactic side effects during the course of treatment. The emergence of component-resolved diagnosis has greatly improved our ability to identify causative allergens and revolutionized the design of AIT. Molecular features such as IgE-binding epitopes and T cell epitopes have been elucidated in most major food allergens, inspiring the use of multiple strategies to manipulate the allergens and design safer alternatives to AIT. Although these allergen-modifying approaches are currently restricted to preclinical characterization and animal studies, the employment of these strategies has certainly paved the way for improving the safety of existing AIT. A safe and effective AIT for food allergy is not far beyond reach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The prevalence of adverse reactions to food was reported to approach 8% in children and 5% in adults [1]. However, these figures are likely to be inaccurate as they depend on self-reported allergies and serological profiles. Accurate food prevalence is difficult to determine and often non-reproducible due to variations in definition of allergy, study population, methodology, age, and the food allergen studied. Nevertheless, it is certain that both food-related anaphylaxis and the rate of hospitalization for food-induced anaphylaxis are increasing globally [2]. Affecting an estimated 520 million people worldwide, food allergy is beyond question a severe global health burden (WAO White Book 2013).
Food allergy is categorized into IgE-mediated, non-IgE-mediated type, or an intermediate type showing features of both [3]. IgE-mediated reactions are the immediate type of hypersensitivity where allergic symptoms occur within 2 h of food ingestion. Clinical manifestations of IgE-mediated reactions include acute urticarial, nausea, and systemic anaphylaxis. Non-IgE-mediated type is delayed-type hypersensitivity and believed to be cell-mediated. Common symptoms associated with cell-mediated food allergic reactions include enterocolitis and proctocolitis. The third form of allergy is the combined IgE and cell-mediated hypersensitivity, which mainly involves infiltration of eosinophils and is considered as a type of delayed hypersensitivity reaction mediated by the type II T helper (Th2) cells and eosinophilic mediator IL-5 and eotaxins [4]. The chronic condition of eosinophilic infiltration of the gastrointestinal wall mainly at the stomach and small intestine is collectively known as eosinophilic gastroenteritis (EGE), which is one major form of eosinophilic gastrointestinal disorders (EGIDs). Elevated levels of total IgE and/or food-specific IgE could be detected in some EGE patients that is accompanied by positive skin prick test to certain food allergens without the development of immediate anaphylactic symptoms [5]. These thus impose difficulties in distinguishing patients from IgE-mediated food allergy and EGID by food-specific IgE and skin prick tests.
In this review, we will focus on the IgE-mediated food allergy, which is primarily responsible for fatal anaphylactic reactions. Currently, there are no preventive medications for IgE-mediated food allergy. The primary treatment option is strict avoidance of the causal food; however, accidental ingestion is often inevitable, especially in children [6]. Therefore, the need for a safe and effective treatment for food allergies is eminent. Allergen-specific immunotherapy (AIT) is a disease-modifying approach to restore immune tolerance to the allergens by administering increasing doses of sensitizing allergens until a maintenance dose is reached. Yet, despite advances in AIT for inhalant allergens, the development of AIT for food allergies has been at a stalemate due to frequently reported anaphylactic side effects during treatment [7,8,9,10,11,12]. Unlike inhalant allergens, food allergens are subject to digestion along the gastrointestinal tract and sometimes contain more than 20 IgE-binding epitopes [13]. This has undeniably increased the risk of food allergies treated with AIT using natural allergens.
Major Food Allergens
The molecular characterizations of the allergens, such as T cell or B cell epitopes, are a key to designing safer AIT. Advances in component-resolved diagnosis have greatly enhanced our knowledge of the allergenicity of individual allergens and facilitated the design of hypoallergenic derivatives. Here, we will briefly discuss the properties of the most common food allergens (Table 1).
Peanut Allergens
Peanut allergy is the most common cause for severe food-derived anaphylaxis [14]. The severity is likely due to the high protein content in a single peanut (~ 200 mg). Thirteen peanut allergens belonging to seven protein families have now been identified. Ara h 1 is a glycoprotein belonging to the vicilin seed storage protein family [15] and is believed to have modest allergenicity due to burial of epitopes [16, 17]. Ara h 2 belongs to the 2S albumin protein family and is the dominant allergen recognized by 90–100% of peanut-allergic patients [18]. Ara h 3 and Ara h 4 were originally thought to be distinct proteins but are now believed to be isoforms of the same allergen belonging to the legumin seed storage protein family [19, 20]. Ara h 5 belongs to the profiling family and is a minor peanut allergen in low levels in peanut extracts and recognized by a minority of peanut-allergic patients [19, 21]. Ara h 6 and Ara h 7 belong to the same 2S albumin protein family and share certain homology to Ara h 2 [21]. Ara h 8 is homologous to the birch pollen allergen Bet v 1 and contributes to the cross-reactivity between birch pollen and peanut allergy [22]. Ara h 9 belongs to the lipid transfer protein family and is the dominant peanut allergen in the Mediterranean population [23, 24]. Ara h 10 and Ara h 11 are members of the oleosin structural protein family and exist in purified peanut oil bodies [25]. Ara h 12 and Ara h 13 are cysteine-rich defensin peptides. IgE reactivity to these peptides is present in only a small portion of peanut-allergic patients [26].
Tree Nut Allergens
Tree nut allergens can be classified into different groups based on their structural and functional properties [27]. The first group is the seed storage proteins, comprising 11S legumin-like proteins, 7S vicilin-like proteins, and the 2S albumins. Both the 11S legumin-like proteins and 7S vicilin-like proteins belong to the cupin protein superfamily and exist as hexamers and trimers, respectively [20]. On the contrary, the 2S albumins are small heterodimers belonging to the prolamin superfamily [28]. These three allergens are the major tree nut allergens identified from most edible tree nuts. The second group is the pathogenesis-related (PR) proteins involved in the plant defense system, including chitinases [29], PR-10 [30], and lipid transfer proteins [31,32,33]. The third group comprises structural proteins that are highly conserved among plants, such as profilins [34, 35] and oleosins [36].
Cow’s Milk Allergens
About 20% of proteins in cow’s milk are whey and 80% are coagulum [37]. Whey contains globular protein including α-lactalbumin (Bos d 4), β-lactoglobulin (Bos d 5), bovine serum albumin (Bos d 6), bovine immunoglobulins (Bos d 7), and lactoferrin [38, 39]. The major allergens in the whey fraction are α-lactalbumin and β-lactoglobulin, which are involved in 80 and 76% of all allergic sensitization to cow’s milk, respectively [40]. The coagulum fraction consists mainly of four individual casein proteins coded by different genes on the same chromosome: αs1-casein, αs2-casein, β-casein, and κ-casein [38]. Due to the complexity of milk proteins and polysensitization, no single allergen or particular structure has been identified in the allergenicity of milk [41].
Egg Allergens
Six major allergens were identified in hen’s egg: ovomucoid (Gal d 1), ovalbumin (OVA; Gal d 2), ovotransferrin (Gal d 3), lysozyme (Gal d 4), albumin (Gal d 5), and YGP42 (Gal d 6) [42, 43]. The majority of egg allergens are found in the egg white (Gal d 1–4) except Gal d 5 and Gal d 6 which are found in the egg yolk. Two other egg proteins, lipocalin-type prostaglandin D synthase and egg white cystatin, were recently found to have IgE reactivity in egg-allergic patients, but their significance in egg allergies remains unclear [44]. While ovalbumin is the most abundant protein present in egg white, the heat stable protein ovomucoid is believed to be the dominant allergen [45].
Fish Allergens
Parvalbumin, a protein regulating calcium switching in skeletal muscle cells, was identified as the first fish allergen in the Baltic cod during the early 1970s [46,47,48]. Parvalbumin is recognized by 90% of fish-allergic patients [49,50,51] and belongs to the biggest group of food-derived allergens, the EF-hand domain family. In addition to parvalbumin, other minor allergens such as vitellogenin [52], collagen [53, 54], aldehyde phosphate dehydrogenase [55], triosephosphate isomerase [56], muscle creatine kinase [57], enolase [57, 58], and aldolase [58] have been identified in different fish species or fish-derived products such as caviar.
Shellfish Allergens
The muscle protein tropomyosin was identified as the major allergen in shrimp by three groups independently in the early 1990s [59,60,61] and later revealed as a pan-allergen among shellfish and other invertebrates [62, 63]. Tropomyosin is a coiled-coil secondary structure protein that belongs to the highly conserved actin filament-binding protein family. Tropomyosins are heat-stable with limited digestibility so they persist even after thorough cooking [64]. Apart from tropomyosins, other allergens have been identified in several shrimp species, such as arginine kinase [65,66,67], sarcoplasmic calcium-binding proteins [67,68,69], and myosin light chain [67, 70]. The detailed molecular features and cross-reactivity of these shellfish allergens have been reviewed elsewhere [71, 72]
Pathogenesis of Food Allergy
While normal individuals develop oral tolerance towards food proteins, food-allergic subjects mount an inappropriate IgE response to the food antigens. In such cases, food allergens are taken up by the antigen-presenting cells (APCs) and the T cell epitopes are presented to naïve T cells through the major histocompatibility complex (MHC) class II molecule. The activated T cells differentiate into Th2 cells. Th2 cells then promote a class switch in the cognate allergen-bound B cells through cell-cell interaction or cytokines (IL-4, IL-5, and IL-13). Class-switched B cells produce IgE antibodies, which rest on effector cells such as mast cells or basophils. Upon subsequent re-exposure to the same allergen, IgE cross-links the allergen on effector cells (e.g., mast cells and basophils) through the high affinity receptor FcεRI, causing degranulation. The mediators released by these cells, such as histamine, prostaglandins, and leukotrienes, are the major causes of allergic responses and anaphylactic shock. These immediate allergic symptoms can occur within minutes after allergen contact, either directly at the site of allergen exposure (i.e., mouth and intestine) or extend to other organs (i.e., skin and respiratory tract) when the allergen passes through the mucosa to the circulatory system [64, 73, 74].
Additionally, a dysregulated Th2-skewed response can be linked to functional defects in Foxp3, the key transcription factor of regulatory T (Treg) cells [1], as well as alterations in genes such as STAT3, DOCK8, or PGM3 and genes involved in TCR signaling including LAT, ZAP70, or RAG [75]. A recent study also suggests a link between mutations in the Wiskott-Aldrich syndrome (WAS) gene and increased frequency of sensitization to food allergens in patients [76]. Mutations in the WAS gene result in WAS protein (WASP) deficiency. WASP-deficient Treg cells show increased levels of Th2 transcriptional factor GATA3, therefore resulting in hyper IgE phenotype and intestinal mast cell expansion in mice. It is essential to continue investigating the activation of Th2 effector responses to fully understand its mechanism and its role in food allergy.
Allergen-Specific Immunotherapy
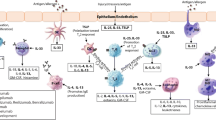
The aim of AIT is to initially achieve desensitization (i.e., temporary increase in the threshold of effector cell activation) and subsequently attain oral tolerance. Oral tolerance is the permanent state of unresponsiveness to the sensitizing allergen, indicating the absence of any allergen-specific response due to deletion or inactivation of T cells, or the presence of active IgG, IgA, Th1, and/or Treg responses (Fig. 1).
Possible immunological effects of AIT. Decrease in the activity of effector cells such as mast cells, eosinophils, and basophils occur at the early stage of AIT. Level of specific IgE usually shows an early increase followed by a late decrease. Increases in frequency of Th1 cells, levels of Th1 cytokines, the generation of allergen-specific Foxp3+ Treg cells, TGF-β-producing Th3 cells, and Breg cells follow subsequently and possibility antagonize the differentiation and activation of Th2 cells. The induction of CD103+ tolerogenic DCs happens in parallel that could also lead to the activation of Treg cells and IgA-secreting B cells. Increase of IgA and IgG inhibitory antibody continues throughout the treatment
The initial immunological changes generated through AIT may involve decreased activity and responsiveness of effector cells, such as mast cells and basophils [77, 78]. An increase in allergen-specific IgG4 can also be seen as early as within 1 week after the onset of AIT. This subclass of IgG antibody is generally regarded as protective as it can effectively capture the allergen before reaching the cell-bound IgE. Studies also suggest that it can downregulate IgE receptor FcεRI signaling and promote internalization of IgE in mast cells without triggering mast cell degranulation [79, 80]. Yet, the level of specific IgE typically increases in the first few months of AIT and a late decrease could only be seen when AIT is continued for extended periods of time.
Changes involving the modulation of T cell responses occur at a later stage in the course of AIT, which include a decrease in Th2 cells and release of their linked cytokines, leading ultimately to oral tolerance. These can be a result of the deletion of antigen-specific CD4+ T cells when a high dosage of the antigen is administered [81]. Additionally, oral tolerance can also be achieved through the development of suppressor T cells in the gastrointestinal lymphoid tissues. This type of tolerance is independent of the naturally occurring Treg (nTreg) cells derived from the thymus. It is mediated by two different subsets of Treg cells [82, 83]. Th3 cells suppress directly through a TGF-β-dependent manner. This cytokine blocks differentiation of Th1 and Th2 cells by modifying the expression of their respective transcription factors T-bet and GATA-3 [84, 85]. It also promotes the synthesis of IgA, as well as the expansion of suppressive CD4+CD25+Foxp3+ inducible Treg (iTreg) cells [86, 87]. iTreg cells may suppress using IL-10 which directly inhibits IgE synthesis, proliferation of Th2 cells, and cytokine production by blocking the CD2/CD28/ICOS costimulatory signaling pathway [88]. The development of these iTreg cells also depends on CD103+ dendritic cells (DCs) present in the lamina propria of the intestinal tract through TGF-β, retinoic acid, and other factors [89]. CD103+ DCs also promote IgA-secreting B cells, [90] which together with iTreg cells contribute to oral tolerance in food allergy.
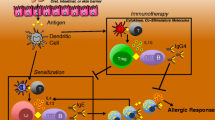
In the following sections, we will discuss different AIT strategies in detail, including the use of unmodified allergens delivered via different routes (Table 2); the identification and construction of hypoallergens, T cell epitopes, mimotopes, and conjugated molecules based on immunomolecular features of the native allergen (Fig. 2); as well as their efficacies in preventing and treating food allergies in animal models and clinical trials (Table 3).
Strategies for AIT modulator preparation. IgE reactivity and allergenicity of the native allergens can be abolished by manipulating or deleting the epitopes, altering the conformation by denaturation or chemical modification to construct hypoallergens. T cell epitopes that trigger CD4+ T cell responses and possess T cell modulatory functions can be synthesized and delivered as a single peptide or peptide cocktail to enhance Th1 and Treg cell activities. Mimotopes that mimic the structure and/or sequence of the IgE-binding epitopes are shown to induce allergen-recognizing antibodies that might serve inhibitory functions. Conjugation of allergens with molecules with immunomodulatory functions can promote the induction of tolerogenic DCs, Treg cells, and/or Th1 cells
Unmodified Allergens
The first AIT was recorded in 1911 when Noon reported the use of subcutaneous injection of grass pollen extracts to treat hay fever [91]. Since then, allergen extracts have been widely used in AIT, including those targeting food allergies. Recent advances in recombinant DNA technology have prompted the use of recombinant allergens in place of whole allergen extracts. Although AIT using unmodified allergens appears to be effective for treating inhalant allergies, pioneer studies of subcutaneous immunotherapy for food allergies were considered unsafe as anaphylactic side effects were frequently observed in clinical trials of peanut AIT [92, 8]. To improve the safety of AIT for food allergies, novel methods to deliver the allergen are under investigation. The oral, sublingual, and epicutaneous routes are three major delivery routes that have been extensively explored.
Oral immunotherapy (OIT) involves daily consumption of the sensitizing allergen, typically using a start-off dose below the threshold dose, which is then followed by a gradual increase in dosage to a maintenance dose until achieving desensitization and/or tolerance. Clinical trials of OIT targeting cow’s milk [93, 12, 94, 95, 9], hen’s egg [96,97,98], and peanut [99,100,101] allergies demonstrated positive immunological improvements. Reduced sensitivity in skin prick test (SPT), decreased level of specific IgE, and increased level of IgG4 and Treg cell population were consistently reported in these independent studies. Generally, OIT using native allergens has a high success rate to achieve at least partial desensitization in > 80% of subjects [102]. However, OIT with these unmodified allergens only leads to 25–40% specific oral tolerance and can also lead to adverse allergenic reactions. In a peanut OIT clinical trial, 92% of patients experienced respiratory or mild itching symptoms during the initial day of escalation [100]. All subjects experienced minor respiratory or skin reactions during home dosing, with two subjects requiring epinephrine treatment. A safe and successful establishment of permanent tolerance is yet to be accomplished in OIT.
Sublingual immunotherapy (SLIT) is considered an improvement of OIT, in that it uses allergen extracts that are usually 1000-fold less concentrated than OIT and kept under the tongue for a few minutes before spitting out or swallowing [102]. Although the exact immunological mechanisms are unclear, SLIT is believed to target the Langerhans-like dendritic cells in the sublingual mucosa that promote tolerance to allergens [103,104,105]. SLIT has been tested for allergen extracts of food items such as kiwi fruit [106, 107], hazelnut [108, 109], cow’s milk [110, 111], peach [112, 113], and peanut [114, 115]. The clinical improvements reported from the above studies indicate that SLIT could reduce sensitivity in SPT and increase specific IgG4 level as in OIT. However, Keet et al. suggested that OIT is more efficacious for desensitization than SLIT [111]. Only 1 of 10 subjects from the SLIT group passed the 8-g cow’s milk challenge, compared to 8 of 10 subjects from the OIT group passing the same challenge. However, the symptoms triggered in SLIT are confined to local reactions, with the frequency of reaction varying greatly between studies, from 0.2% in hazelnut to 89% in peach. In spite of the seemingly positive clinical improvements in both OIT and SLIT, there are uncertainties concerning their safety and long-term effects. In addition, the use of OIT and SLIT is restricted in patients with more severe food-induced anaphylaxis. Strategies for improving the current protocol are needed, which might include the use of modified allergens and other relevant immunomodulatory agents, or even alternative delivery schemes.
Epicutaneous immunotherapy (EPIT) has recently emerged as an alternative allergen delivery method. Allergens enclosed in a container are delivered onto intact [116] or tape-stripped skin [117]. As the epidermis is not vascularized, EPIT prevents systemic reactions caused by circulation of allergens. Similar to SLIT, the preventive effects of EPIT are thought to be mediated through the tolerogenic Langerhans cells in the epidermis [118,119,120]. Preliminary studies in mouse model suggest the induction of Treg cells and a shift to Th1 milieu by EPIT [121, 122]. A recent study reported the recruitment of gastrointestinal-homing latency-associated protein (LAP) + Foxp3-Treg cells by epicutaneous application of OVA-Viaskin patches in mice sensitized and challenged intragastrically by OVA. This subset of Treg cells protects against food allergy by suppressing mast cell activation via a TGF-β-dependent manner but not IgE antibodies [123]. However, in a pilot study of EPIT in children with cow’s milk allergy, only 50% of patients demonstrated an increase in cumulative tolerated dose in the oral food challenge. There was no significant increase in serum-specific IgG and IgE levels, but 25% of the recipients experienced local adverse reactions [124]. Further clinical studies are needed to monitor the safety and therapeutic efficacy of EPIT.
One main challenge towards the use of unmodified allergens in AIT lies within the optimum dosage being employed. We have recently investigated the dose-dependent safety and efficacy of AIT in a mouse model of shrimp allergy [125]. BALB/c mice were first sensitized and challenged with recombinant Met e 1 (rMet e 1) through the intragastric route. Subsequently, they were treated with low (0.01 mg), medium (0.05 mg), or high (0.1 mg) intraperitoneal injections of rMet e 1 for three times at a weekly interval. We found that despite all mice being successfully desensitized regardless of the dosage, Treg-associated regulatory mechanisms were only observed in the low or medium dosage groups. This is evident through the upregulation of Treg-associated genes and the infiltration of Foxp3+ cells in the gut lymphoid tissues exclusively in these two groups of mice. These findings suggest that low-dosage immunotherapy favors the induction of local Foxp3+ Treg cells and more likely to sustain long-term efficacy of AIT.
The frequent occurrence of adverse events during AIT with unmodified allergens, especially OIT, represents another major challenge. This thus leads to the investigations of adjunctive therapies, such as using the anti-IgE recombinant humanized monoclonal antibody omalizumab that can prevent the interaction between circulating IgE and FcεRI, to improve safety. The early off-label use of omalizumab was shown to reduce adverse reactions and enable more patients to achieve the maintenance dose in SCIT and SLIT and, more recently, in OIT [126]. In a double-blinded, placebo-controlled trial of cow’s milk-specific OIT, a comparable percentage of patients passed the 10-g oral cow’s milk challenge and achieved sustained unresponsiveness at 8-week after discontinuation of OIT for both omalizumab-treated and placebo-treated groups [127]. Yet, the overall percentage of symptom-free doses during the escalation phase of OIT was 91.5% in the omalizumab-treated group, comparing to 73.9% in the placebo-treated group. In a similar study adopting a rapid oral desensitization protocol for peanut allergy, omalizumab greatly increased the median tolerated dose on the first day of rush desensitization (250 mg peanut protein for the omalizumab group versus 22.5 mg for the placebo group) [128]. Significantly more omalizumab-treated subjects could tolerate a 2000-mg maintenance dose and passed a 4000-mg cumulative dose in an open peanut oral challenge compared to the placebo-treated group. Similarly, the immunological changes were similar between the two groups. These suggest that adjunctive treatment with omalizumab has no significant effects on the outcomes of efficacy, but improvements in safety and facilitation of rapid desensitization are remarkable. It is yet noteworthy that a subset of patients could display stronger responsiveness to IgE receptor cross-linking with omalizumab treatment [129]. The identification of baseline biomarkers, such as their pretreatment basophil reactivity and/or allergen-specific to total IgE ratio, is thus essential for selection of suitable patients who will benefit from such adjunctive treatment [130].
Hypoallergens
The major obstacles in AIT, especially OIT, for food allergies are the frequent adverse side effects and prolonged treatment duration due to the low dosage restricted by the use of unmodified allergens. Alternative approaches to reduce the allergenicity while retaining the immunogenicity of allergens, such as the use of hypoallergens, are therefore promising. General strategies deployed to construct hypoallergenic food allergens involve site-directed mutagenesis within the IgE-binding epitopes, sequence restructuring, chemical modifications, disruption of allergen conformation, allergen unfolding, and more [131,132,133,134,135].
Since previous studies have shown that subjects allergic to raw foods can tolerate cooked foods, the use of heated allergens in AIT can promote desensitization to the native allergen in patients [136]. Heating allergens causes aggregation, which reduces their absorption and transport through the mucosal layer. It also leads to conformational changes and/or changes to a significant proportion of the IgE-binding epitopes, reducing their allergenicity sufficiently for safe use in AIT [137, 138]. It was shown that mice sensitized with heated ovalbumin (h-OVA, heated to 70 °C for 10 min) have significantly lower levels of OVA-specific IgE and mouse mast cell protease-1 (mMCP-1) upon OVA challenges compared to control mice sensitized with OVA [139]. They also displayed higher levels of specific IgG2a and a prominent Th1-type immune response. The gain of desired antigenic properties of h-OVA was probably due to not only the irreversible structural changes but also the formation of a different panel of peptide fragments upon digestion compared to raw OVA. Although results from animal studies seem promising [140, 138], an open food challenge to baked egg in 236 egg-allergic children resulted in 36% reaction to the challenge, with 14% of them experiencing anaphylaxis [141]. In another baked-milk OIT therapy, only 3/14 OIT subjects reached their tolerance goal at a maintenance dose of 1.3 g per day [142]. Eight patients could not complete the therapy due to IgE-mediated side effects including anaphylaxis. It is apparent from these reports that treatments with heated molecules usually only generate desensitization but not tolerance, and heating allergens does not necessarily produce hypoallergenic proteins in all allergic patients.
While “natural” manipulation might not be sufficient in yielding hypoallergenic proteins, variants with reduced allergenicity can be generated by means of chemical modification or genetic engineering. Native allergens can be reduced and alkylated to disrupt the disulfide bonds, such as in the major peach allergen Pru p 3 [143], as well as in the peanut allergens Ara h 2 and Ara h 6, or additionally linked to glutaraldehyde to achieve structural changes in Ara h 2 and Ara h 6 [144]. These strategies could effectively produce hypoallergenic variants that have diminished IgE reactivity and allergenicity in immuno-blotting, rat basophilic leukemia (RBL) release assays, and immunization experiments. However, further studies are needed to confirm their antigenic characteristics as such modifications may cause drastic conformational changes resulting in the loss of T cell epitopes and increased risk of sensitization [145].
By inhibiting IgE/allergen cross-linking, anaphylactic side effects in AIT could be greatly reduced. In this context, the IgE-binding epitopes contain important information for constructing the relevant hypoallergens. IgE-binding epitopes of the majority of food allergens have been mapped, including α-lactalbumin (cow’s milk) [146], ovalbumin (egg white) [147], Ara h 2 (peanut) [148], Pen a 1 and Met e 1 (shrimp) [149, 150], as well as Pru p 3 (peach) [151]. Modifications of the amino acid sequences within these IgE-binding epitopes to abolish IgE reactivity can therefore be achieved simply by site-directed mutagenesis. The introduction of mutations into the 10 linear epitopes of Ara h 2 at positions 20, 33, 39, 51, 58, 64, 117, 127, and 144 resulted in a hypoallergen (mAra h 2) that displayed only 29.6% IgE binding reactivity relative to wild-type Ara h 2 (wAra h 2) [152]. mAra h 2 still maintained intact T cell epitopes as it had the same peripheral blood mononuclear cell (PBMC)-stimulating ability compared to wAra h 2. On the other hand, our laboratory identified nine major IgE-binding epitopes of the major shrimp allergen Met e 1 [149]. By comparing these epitope sequences to the homologous tropomyosin sequences of four edible fish species, 49 point mutations were introduced into the nine epitopes to construct the tropomyosin mutant MEM49. Another mutant, MED171, was constructed by deleting all the nine IgE-binding epitopes, resulting in a smaller truncated molecule of 171 amino acid residues. Both MEM49 and MED171 displayed > 70% reduction in their in vitro reactivity towards IgE of shrimp-allergic subjects compared to Met e 1. These two hypoallergens also had markedly reduced in vivo allergenicity in passive cutaneous anaphylaxis assay and immunization experiment. More importantly, mice immunized with either of the hypoallergens produced Met e 1-specific IgG2a antibody that inhibited IgE of shrimp-allergic subjects and sensitized mice from binding to Met e 1. Such phenomenon is considered beneficial as these antibodies can rapidly modulate allergic reactions. The prophylactic and therapeutic efficacies of the hypoallergens in the form of DNA vaccines are now under investigation using a mouse model of shrimp hypersensitivity [153, 154].
Instead of manipulating the IgE-binding epitopes through “tailored” modifications for each food allergen, a general approach to construct hypoallergens adaptable to multiple food allergens is highly desirable. Reports have shown that polyphenols from fruits and vegetables might modulate the immune cells and pathways involved in allergic responses, thus alleviating inflammatory symptoms [155]. A recent study described a polyphenol-containing cranberry juice mixed with peanut flour that allowed sorption of polyphenols to peanut proteins to form a cranberry polyphenol-fortified peanut matrix [156]. Using basophil degranulation assay, it was found that this matrix triggered significantly less degranulation (median = 37.2%) compared to unmodified peanut flour (median = 66.1%). Peanut-allergic mice challenged with the matrix also showed significantly reduced levels of mMCP-1. These data suggest that the matrix is hypoallergenic and potentially useful for AIT targeting peanut allergy. Similar technology of food-grade quality that is economical, simple in the preparation of hypoallergens, and readily adaptable to multiple food allergens should be explored to further advance food allergy management.
Currently, most hypoallergenic food allergens are largely restricted to the early stages of construction and preclinical characterization. One major step forward in the management of food allergy is an EU-funded collaborative project initiated in 2008 Food Allergy Specific Immunotherapy (FAST) [157]. This project aims to develop safe and effective subcutaneous AIT towards fish and peach allergies using hypoallergenic proteins. Two hypoallergens of fish parvalbumin Cyp c 1 have been developed in this project. The first one is a chemically modified mutant constructed by glutaraldehyde treatment, and the second one is a calcium binding site double mutant described by Swoboda et al. in 2007 [158]. The double mutant has a ~ 100-fold reduced allergenicity. Immunization with this mutant induced IgG antibody, leading to 67–76% inhibition towards IgE of fish-allergic patients. On the other hand, five hypoallergenic variants of peach Pru p 3 are constructed in the FAST project. These include a “natural hypoallergen” rFra a 3, rPru p 3 sur (surface mutant with three amino acids mutated to alanine), rPru p 3 cys (four cysteines mutated to serine), rPru p 3 RA (reduced and alkylated Pru p 3), and glutaraldehyde-treated rPru p 3.
The first stage of the FAST project involves production of the hypoallergens under good manufacturing practice for clinical trials. The preclinical development of the double mutant of fish parvalbumin Cyp c 1 and wild-type Cyp c 1 was recently reported [159]. This fish parvalbumin mutant was found to be a stable molecule upon expression and exhibited no toxic effects when adsorbed to aluminum hydroxide. This mutant also displayed ~ 1000-fold reduction in its allergenic activity in the ImmunoCAP inhibition experiment and RBL release assay while its immunogenicity in inducing PBMC proliferation was retained. The subsequent stage of the project will involve animal studies on the efficacies of subcutaneous treatment with the selected hypoallergens through a panel of in vitro and in vivo experiments. The group recently reported that the antisera generated by immunizing mice with hypoallergenic Cyp c 1 contained IgG antibodies that block fish-allergic patients’ IgE from binding to wild-type Cyp c 1 [160]. The antisera were also capable of reducing allergic responses in a murine model of Cyp c 1-induced hypersensitivity, probably by inhibiting IgE from binding to Cyp c 1 and basophil degranulation. The final stages of the project will include phase I/IIa and phase IIb clinical trials to determine safe dosages, as well as the tolerability and clinical outcome of the therapy in allergic patients through double-blind, placebo-controlled food challenge after treatment. Future reports from the FAST project are expected to provide novel strategies to replace avoidance, the standard “therapy” to food allergies, and bring our understanding of food allergy management to the next level.
T Cell Epitopes
T cell epitopes are short peptide fragments of the allergen that activate naïve T cells through the MHC class II molecules expressed on APCs [73]. These fragments lack secondary or tertiary structures and do not cross-link IgE or activate effector cells. However, they possess modulatory potential in re-shaping the T cell environment from a Th2-type to a Th1- and/or Treg-dominating response. These properties make them a safe and effective therapeutic modulator ideal for AIT use as shown in successful clinical trials for cat and bee venom allergies [161, 162].
Nonetheless, there have only been a limited number of animal studies involving the investigation of T cell epitopes as immunotherapy for food allergies. Using T cell lines (TCLs) generated from cow’s milk allergy patients and with reference to previous reports, three regions of β-lactoglobulin, (regions 13–48, 91–120, and 139–162) were found to contain T cell-reactive sequences [163]. However, oral pretreatment with the peptide mixtures covering any one of the three reactive regions did not protect against the development of cow’s milk allergy in mice subsequently sensitized to whey. Yet, pretreatment with a single peptide (peptide 6, AA31–48) significantly reduced acute allergic skin response, as well as levels of whey-specific IgE, IgG1, and IgG2a. Percentage of CD4+CD25+Foxp3+ Treg cells was also significantly increased in mice pretreated with peptide 6. On the other hand, data reported by Thang and Zhao are less promising [164]. Oral delivery of any of the T cell-reactive peptides of β-lactoglobulin (P1: 22-mer, AA67–88; P2: 15-mer, AA139–153) could only reduce the severity of hypersensitivity responses. There were no changes in the levels of specific IgE, IgG1, IgG2a, and IgA; in the levels of splenic IL-4, IL-12, and IL-10; or in the percentage of Treg cells, even though the peptides were delivered frequently and for a long duration (three consecutive days per week for 4 weeks at 1 mg/peptide). The discrepancy in the degree of clinical improvement reported in the two studies might be due to the use of peptides spanning different “immunogenic” regions of β-lactoglobulin. It is also noteworthy that peptide 6 used by Meulenbroek et al. [163, 178] actually could not elicit proliferation in any of the TCLs, leaving the question of whether the observed effects are contributed by a “true” T cell determinant of β-lactoglobulin.
The T cell epitopes of two major egg allergens, OVA and ovumucoid (Ovm), were previously identified [165, 166] and applied in AIT studies in murine models. Subcutaneous injection with a single T cell peptide of OVA (15-mer, AI-15: AA39–53) or a mixture T cell epitopes (three 15-mer peptides, covering AA39–53, AA147–161, and AA329–343) effectively reduced the levels of histamine and OVA-specific IgE while increasing the level of fecal IgA [167]. A peptide cocktail could generate more pronounced effects by upregulating the expressions of Th1-linked cytokine IFN-γ and Treg-associated genes TGF-β and Foxp3 in the small intestine of treated mice. Similar effects could be observed when the T cell epitope of Ovm was delivered as treatment [168]. Two synthetic peptides containing five residues as single peptide (SP) (AA157–171) and 51 residues as a multiple peptide (MP) (three repeated units of SP linked by alanine residues) were used to treat Ovm-sensitized mice orally. Both SP and MP markedly reduced the levels of specific IgE and IL-4 while increasing the levels of fecal IgA and IFN-γ. However, only treatment with SP significantly reduced the histamine level and boosted levels of IgG2a, splenic IL-10 and IL-12, as well as CD4+Foxp3+ cells.
Our laboratory identified six major T cell epitopes (20-mer long; T1–T6, AA26–45, AA56–75, AA86–105, AA146–165, AA221–240, and AA251–270) of shrimp tropomyosin Met e 1 and evaluated their therapeutic efficacy in a murine model of tropomyosin hypersensitivity [169]. These epitopes were mapped based on the proliferation and cytokine responses of spleen cells from BALB/c mice orally sensitized to Met e 1. Their epitope sequences are also similar to the reactive regions mapped on Pen a 1 using TCLs generated from shrimp-allergic subjects [170]. Similar to mice treated with the T cell epitopes of OVA and Ovm, the oral delivery of a mixture of these six Met e 1 T cell peptides also significantly reduced severity of systemic allergic symptoms, level of specific IgE, and expression of Th2 cytokines (IL-5 and IL-13) in the ileum. The effects of the treatment are limited not only to the restoration of Th1/Th2 balance and induction of Treg-like responses but also to the synthesis of IgG2a antibodies that have both in vitro and in vivo inhibitory functions.
OIT using unmodified allergens represents a promising regimen in the treatment of food allergies via the induction of oral tolerance [171, 7, 102, 172, 173]. Yet, the use of intact allergens is not completely without risk [174]. From the abovementioned studies, it is clearly demonstrated that oral delivery of dominant T cell peptides, as both prophylactic and therapeutic agents, could effectively confer oral tolerance in murine models of various food allergies, making oral peptide immunotherapy (PIT) a promising strategy for treatment. While the major T cell determinants of most major food allergens, such as Ara h 1 and Ara h 2 (peanut) [175,176,177], αs1-casein and α-lactoglobulin (cow’s milk) [178, 179], and Pru p 3 (peach) [180, 181], have been mapped, the efficacy of using these epitopes in PIT targeting these allergies has not been explored. The major obstacles in translating these identified epitopes into PIT might involve a large portion of sequences that can elicit a T cell response. For example, among the 69 20-mer overlapping peptides that span the full length Ara h 1, only four did not trigger proliferation in any of the TCLs generated from peanut-allergic patients [177]. The recognition was only 22–33% among the major respondents. A similar pattern was also found in the mapping of T cell epitopes of Pru p 3 [182]. Among the four T cell-activating regions, only Pru p 361–75 was recognized by 50% of patients while Pru p 313–27, Pru p 334–48, and Pru p 343–57 were only recognized by ~ 30% of patients. We suspect that a highly diverse set of HLA alleles and APCs along the digestive tract and diversity in the degree of allergen digestion in the allergic population might be the major confounding factors leading to the high diversity of T cell-activating sequences found among most food allergens [183, 64]. This therefore poses difficulties in pinpointing specific set of peptides as major immunodominant T cell epitopes for clinical use.
The use of peptide fragments of the allergens may be an alternative to the use of specific T cell epitopes for AIT. These fragments can be simply prepared by digesting the allergen extract using food-grade enzymes or pepsin. Yang et al. demonstrated that over 85% of the egg white fragments yielded by enzymatic hydrolysis only have molecular masses of < 1.3 kDa [184]. Oral treatment with the hydrolyzed extract could effectively reduce the levels of histamine, specific IgE, and the expression of both Th2 (IL-4 and IL-13) and Th1 (IFN-γ and IL-12) cytokines in the ileum. TGF-β and Foxp3, a cytokine and transcription factor of Treg cells, respectively, were significantly upregulated. In another similar study, Kulis et al. digested the cashew proteins by pepsin, which yielded peptide fragments of 3–6 kDa in size [185]. Intraperitoneal treatment with these pepsinized extracts also helped to reduce allergic responses and Th2 cytokines, as well as induce IgG production in a mouse model with established hypersensitivity to cashew. These studies demonstrate that the use of digested allergen extract might provide enough T cell-stimulating peptides to a heterogeneous pool of patients with food allergies, therefore generating positive immunological changes in AIT.
Mimotopes
Mimotopes are peptides mimicking the IgE-binding epitopes of an antigen. They have been proposed for use in the treatment of allergic diseases as they can induce blocking antibodies against the native allergen [186,187,188,189]. Since then, mimotopes specific to several allergens have been identified through biopanning of phage-displayed libraries, but their applications were mostly limited to epitope mapping [190,191,192,193,194]. Wallmann et al. reported the use of a single mimotope specific to the timothy grass pollen allergen Phl p 5 for treating allergic asthma in a mouse model [195]. Mimotope-treated mice showed a decrease in eosinophil infiltration and Th2-associated cytokines IL-4 and IL-5 in the bronchoalveolar fluid. No adverse effects or changes in Phl p 5-specific T cell reactivity were observed in the treated mice. The lack of T cell epitope could be a major advantage of mimotope treatment as this can avoid T cell-mediated side effects in conventional AIT.
Nevertheless, the therapeutic effects of mimotopes in food allergies remain unclear. This is partly because a mimotope identified by the biopanning method is restricted to a single consensus epitope while most food allergens possess multiple linear epitopes [13]. Our laboratory recently reported the use of a one-bead-one-compound (OBOC) combinatorial peptide library in identifying 25 mimotopes corresponding to six immunodominant regions of the major shrimp allergen tropomyosin [196]. The therapeutic efficacy of a mimotope cocktail consisting of a mixture of these mimotopes is currently under investigation using a mouse model of shrimp hypersensitivity [153, 154].
Conjugated Molecules
One therapeutic approach in food allergy is to design fusion allergens that are directed to specific regulatory signaling pathways. For example, a mannoside-bearing bovine serum albumin (BSA) fusion protein substantially reduces anaphylactic response in a food-induced anaphylaxis mouse model [197]. The mannoside-BSA selectively targeted the C-type lectin receptor on lamina propria DCs and helped to induce oral tolerance through the expression of IL-10 and promotion of CD4+ type I regulatory T cells. Another similar approach is to target toll-like receptor 5 (TLR5) on myeloid DCs with flagellin, which is a major constituent protein of bacteria flagella [198]. The flagellin-ovalbumin fusion protein could induce the expression of IL-10 by myeloid DCs and suppress IL-4 and IFN-γ secretion by ovalbumin-specific T cells.
Besides pattern recognition receptors like C-type lectin receptors or TLRs, it is also possible to target the low-affinity FcγRIIB receptor on mast cells or basophils. A peanut allergen (Ara h 2) fused to the Fc portion of human IgG1 could ameliorate anaphylactic response in a mouse model of peanut allergy induced by whole peanut extract [199]. The aggregation and cross-linking of FcγRIIB and FcεRI by the allergen prevented degranulation and resulted in a tolerogenic signaling pathway.
Another strategy is to coat the food allergens or extract in CpG oligonucleotides and/or poly(lactic-co-glycolic acid) (PLGA) nanoparticles. CpG provides an adjuvant effect to favor Th1 responses that antagonize the Th2 allergic responses. PLGA nanoparticles are biodegradable particles for oral drug delivery and stable in the gastrointestinal environment [200]. OIT with CpG-coated PLGA nanoparticles containing peanut extract was proven safe and effective in a murine model of peanut allergy [201]. Treated mice had their peanut-specific IgE and Th2 cytokine levels significantly reduced, and were protected from anaphylaxis. The treatment also boosted IgG2a antibody and IFN-γ levels. Most importantly, 16 of 22 treated mice remained protected for over 4 months after therapy even peanut extracts were given periodically.
The major advantage of using the fusion allergen strategy is that the molecular characteristics such as T cell or B cell epitopes of the allergens are not required to create the fusion protein. However, it also means that the allergen would be expressed in its native form. The safety of such fusion proteins must be thoroughly investigated before clinical application.
Concluding Remarks
The fact that patients can outgrow certain food allergies suggests that oral tolerance can be acquired through effective AIT. Despite some mild anaphylactic side effects during unmodified allergen-based OIT and SLIT, these forms of treatment do show promising results and a major step forward in the management of food allergies. Further understanding of their long-term efficacies and mechanisms will help to polish these regimens. Although the hypoallergens, T cell epitopes, mimotopes, and conjugated molecules discussed in this review are still currently at the level of preclinical characterization and animal studies, the use of these modulators in OIT and/or SLIT would further advance the safety of existing strategies. Large-scale clinical trials are much anticipated in the coming years, and effective AIT for food allergies will also soon be within reach.
Abbreviations
- AIT:
-
Allergen-specific immunotherapy
- APCs:
-
Antigen-presenting cells
- BSA:
-
Bovine serum albumin
- DCs:
-
Dendritic cells
- EPIT:
-
Epicutaneous immunotherapy
- FAST:
-
Food Allergy Specific Immunotherapy
- iTreg:
-
Inducible Treg
- LAP:
-
Latency-associated protein
- MHC:
-
Major histocompatibility complex
- nTreg:
-
Naturally occurring Treg
- OBOC:
-
One-bead-one-compound
- OIT:
-
Oral immunotherapy
- OVA:
-
Ovalbumin
- Ovm:
-
Ovumucoid
- PLGA:
-
Poly(lactic-co-glycolic-acid)
- rMet e 1:
-
Recombinant Met e 1
- SLIT:
-
Sublingual immunotherapy
- SPT:
-
Skin prick test
- TCLs:
-
T cell lines
- Th2:
-
Type II T helper
- WAS:
-
Wiskott-Aldrich syndrome
References
Sicherer SH, Sampson HA (2014) Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol 133:291–307. https://doi.org/10.1016/j.jaci.2013.11.020
Wawrzyniak P, Akdis CA, Finkelman FD, Rothenberg ME (2016) Advances and highlights in mechanisms of allergic disease in 2015. J Allergy Clin Immunol 137:1681–1696. https://doi.org/10.1016/j.jaci.2016.02.010
Ho MH, Wong WH, Chang C (2014) Clinical spectrum of food allergies: a comprehensive review. Clin Rev Allergy Immunol 46:225–240. https://doi.org/10.1007/s12016-012-8339-6
Uppal V, Kreiger P, Kutsch E (2016) Eosinophilic gastroenteritis and colitis: a comprehensive review. Clin Rev Allergy Immunol 50:175–188. https://doi.org/10.1007/s12016-015-8489-4
Kumar A, Teuber SS, Naguwa S, Prindiville T, Gershwin ME (2006) Eosinophilic gastroenteritis and citrus-induced urticaria. Clin Rev Allergy Immunol 30:61–70. https://doi.org/10.1385/CRIAI:30:1:061
Bock SA, Atkins FM (1989) The natural history of peanut allergy. J Allergy Clin Immunol 83:900–904
Longo G, Barbi E, Berti I, Meneghetti R, Pittalis A, Ronfani L, Ventura A (2008) Specific oral tolerance induction in children with very severe cow’s milk-induced reactions. J Allergy Clin Immunol 121:343–347. https://doi.org/10.1016/j.jaci.2007.10.029
Nelson HS, Lahr J, Rule R, Bock A, Leung D (1997) Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol 99:744–751
Pajno GB, Caminiti L, Ruggeri P, De Luca R, Vita D, La Rosa M, Passalacqua G (2010) Oral immunotherapy for cow’s milk allergy with a weekly up-dosing regimen: a randomized single-blind controlled study. Ann Allergy Asthma Immunol 105:376–381. https://doi.org/10.1016/j.anai.2010.03.015
Patriarca G, Nucera E, Roncallo C et al (2003) Oral desensitizing treatment in food allergy: clinical and immunological results. Aliment Pharmacol Ther 17:459–465
Salmivesi S, Korppi M, Makela MJ, Paassilta M (2013) Milk oral immunotherapy is effective in school-aged children. Acta Paediatr 102:172–176. https://doi.org/10.1111/j.1651-2227.2012.02815.x
Skripak JM, Nash SD, Rowley H, Brereton NH, Oh S, Hamilton RG, Matsui EC, Burks AW, Wood RA (2008) A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol 122:1154–1160. https://doi.org/10.1016/j.jaci.2008.09.030
Burks AW, Shin D, Cockrell G, Stanley JS, Helm RM, Bannon GA (1997) Mapping and mutational analysis of the IgE-binding epitopes on Ara h 1, a legume vicilin protein and a major allergen in peanut hypersensitivity. Eur J Biochem 245:334–339
Al-Muhsen S, Clarke AE, Kagan RS (2003) Peanut allergy: an overview. CMAJ 168:1279–1285
Burks AW, Cockrell G, Stanley JS, Helm RM, Bannon GA (1995) Recombinant peanut allergen Ara h I expression and IgE binding in patients with peanut hypersensitivity. J Clin Invest 96:1715–1721. https://doi.org/10.1172/JCI118216
Shin DS, Compadre CM, Maleki SJ, Kopper RA, Sampson H, Huang SK, Burks AW, Bannon GA (1998) Biochemical and structural analysis of the IgE binding sites on ara h1, an abundant and highly allergenic peanut protein. J Biol Chem 273:13753–13759
Palmer GW, Dibbern DA Jr, Burks AW, Bannon GA, Bock SA, Porterfield HS, McDermott RA, Dreskin SC (2005) Comparative potency of Ara h 1 and Ara h 2 in immunochemical and functional assays of allergenicity. Clin Immunol 115:302–312. https://doi.org/10.1016/j.clim.2005.02.011
Stanley JS, King N, Burks AW, Huang SK, Sampson H, Cockrell G, Helm RM, West CM, Bannon GA (1997) Identification and mutational analysis of the immunodominant IgE binding epitopes of the major peanut allergen Ara h 2. Arch Biochem Biophys 342:244–253. https://doi.org/10.1006/abbi.1997.9998
Koppelman SJ, Knol EF, Vlooswijk RA, Wensing M, Knulst AC, Hefle SL, Gruppen H, Piersma S (2003) Peanut allergen Ara h 3: isolation from peanuts and biochemical characterization. Allergy 58:1144–1151
Breiteneder H, Radauer C (2004) A classification of plant food allergens. J Allergy Clin Immunol 113:821–830. https://doi.org/10.1016/j.jaci.2004.01.779
Kleber-Janke T, Crameri R, Appenzeller U, Schlaak M, Becker WM (1999) Selective cloning of peanut allergens, including profilin and 2S albumins, by phage display technology. Int Arch Allergy Immunol 119:265-274. doi:24203
Mittag D, Akkerdaas J, Ballmer-Weber BK et al (2004) Ara h 8, a Bet v 1-homologous allergen from peanut, is a major allergen in patients with combined birch pollen and peanut allergy. J Allergy Clin Immunol 114:1410–1417. https://doi.org/10.1016/j.jaci.2004.09.014
Krause S, Reese G, Randow S et al (2009) Lipid transfer protein (Ara h 9) as a new peanut allergen relevant for a Mediterranean allergic population. J Allergy Clin Immunol 124:771–778. https://doi.org/10.1016/j.jaci.2009.06.008
Lauer I, Dueringer N, Pokoj S et al (2009) The non-specific lipid transfer protein, Ara h 9, is an important allergen in peanut. Clin Exp Allergy 39:1427–1437. https://doi.org/10.1111/j.1365-2222.2009.03312.x
Pons L, Chery C, Romano A, Namour F, Artesani MC, Gueant JL (2002) The 18 kDa peanut oleosin is a candidate allergen for IgE-mediated reactions to peanuts. Allergy 57(Suppl 72):88–93
Petersen A, Kull S, Rennert S et al (2015) Peanut defensins: novel allergens isolated from lipophilic peanut extract. J Allergy Clin Immunol 136:1295–1301. https://doi.org/10.1016/j.jaci.2015.04.010
Willison LN, Sathe SK, Roux KH (2014) Production and analysis of recombinant tree nut allergens. Methods 66:34–43. https://doi.org/10.1016/j.ymeth.2013.07.033
Shewry PR, Napier JA, Tatham AS (1995) Seed storage proteins: structures and biosynthesis. Plant Cell 7:945–956. https://doi.org/10.1105/tpc.7.7.945
Diaz-Perales A, Collada C, Blanco C, Sanchez-Monge R, Carrillo T, Aragoncillo C, Salcedo G (1998) Class I chitinases with hevein-like domain, but not class II enzymes, are relevant chestnut and avocado allergens. J Allergy Clin Immunol 102:127–133
Kos T, Hoffmann-Sommergruber K, Ferreira F et al (1993) Purification, characterization and N-terminal amino acid sequence of a new major allergen from European chestnut pollen—Cas s 1. Biochem Biophys Res Commun 196:1086–1092
Pastorello EA, Robino AM (2004) Clinical role of lipid transfer proteins in food allergy. Mol Nutr Food Res 48:356–362. https://doi.org/10.1002/mnfr.200400047
Schocker F, Luttkopf D, Scheurer S et al (2004) Recombinant lipid transfer protein Cor a 8 from hazelnut: a new tool for in vitro diagnosis of potentially severe hazelnut allergy. J Allergy Clin Immunol 113:141–147. https://doi.org/10.1016/j.jaci.2003.09.013
Diaz-Perales A, Lombardero M, Sanchez-Monge R, Garcia-Selles FJ, Pernas M, Fernandez-Rivas M, Barber D, Salcedo G (2000) Lipid-transfer proteins as potential plant panallergens: cross-reactivity among proteins of Artemisia pollen, Castanea nut and Rosaceae fruits, with different IgE-binding capacities. Clin Exp Allergy 30:1403–1410
Lauer I, Alessandri S, Pokoj S, Reuter A, Conti A, Vieths S, Scheurer S (2008) Expression and characterization of three important panallergens from hazelnut. Mol Nutr Food Res 52(Suppl 2):S262–S271. https://doi.org/10.1002/mnfr.200700426
Tawde P, Venkatesh YP, Wang F, Teuber SS, Sathe SK, Roux KH (2006) Cloning and characterization of profilin (Pru du 4), a cross-reactive almond (Prunus dulcis) allergen. J Allergy Clin Immunol 118:915–922. https://doi.org/10.1016/j.jaci.2006.05.028
Akkerdaas JH, Schocker F, Vieths S et al (2006) Cloning of oleosin, a putative new hazelnut allergen, using a hazelnut cDNA library. Mol Nutr Food Res 50:18–23. https://doi.org/10.1002/mnfr.200500147
Jost R (1988) Physicochemical treatment of food allergens: application to cow’s milk proteins. Nestle Nutr Workshop Ser 17:187–197
Wal JM (1998) Cow’s milk allergens. Allergy 53:1013–1022
Host A, Husby S, Gjesing B, Larsen JN, Lowenstein H (1992) Prospective estimation of IgG, IgG subclass and IgE antibodies to dietary proteins in infants with cow milk allergy. Levels of antibodies to whole milk protein, BLG and ovalbumin in relation to repeated milk challenge and clinical course of cow milk allergy. Allergy 47:218–229
Fiocchi A, Brozek J, Schunemann H et al (2010) World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) guidelines. World Allergy Organ J 3:57–161. https://doi.org/10.1097/WOX.0b013e3181defeb9
Hochwallner H, Schulmeister U, Swoboda I, Spitzauer S, Valenta R (2014) Cow’s milk allergy: from allergens to new forms of diagnosis, therapy and prevention. Methods 66:22–33. https://doi.org/10.1016/j.ymeth.2013.08.005
Chokshi NY, Sicherer SH (2015) Molecular diagnosis of egg allergy: an update. Expert Rev Mol Diagn 15:895–906. https://doi.org/10.1586/14737159.2015.1041927
De Silva C, Dhanapala P, Doran T, Tang ML, Suphioglu C (2016) Molecular and immunological analysis of hen’s egg yolk allergens with a focus on YGP42 (Gal d 6). Mol Immunol 71:152–160. https://doi.org/10.1016/j.molimm.2016.02.005
Suzuki M, Fujii H, Fujigaki H et al (2010) Lipocalin-type prostaglandin D synthase and egg white cystatin react with IgE antibodies from children with egg allergy. Allergol Int 59:175–183. https://doi.org/10.2332/allergolint.09-OA-0121
Bernhisel-Broadbent J, Dintzis HM, Dintzis RZ, Sampson HA (1994) Allergenicity and antigenicity of chicken egg ovomucoid (Gal d III) compared with ovalbumin (Gal d I) in children with egg allergy and in mice. J Allergy Clin Immunol 93:1047–1059
Aas K (1969) Antigens and allergens of fish. Int Arch Allergy Appl Immunol 36:152–155
Aas K, Lundkvist U (1973) The radioallergosorbent test with a purified allergen from codfish. Clin Allergy 3:255–261
Elsayed SM, Aas K (1970) Characterization of a major allergen (cod.) chemical composition and immunological properties. Int Arch Allergy Appl Immunol 38:536–548
Swoboda I, Bugajska-Schretter A, Verdino P, Keller W, Sperr WR, Valent P, Valenta R, Spitzauer S (2002) Recombinant carp parvalbumin, the major cross-reactive fish allergen: a tool for diagnosis and therapy of fish allergy. J Immunol 168:4576–4584
Bugajska-Schretter A, Elfman L, Fuchs T, Kapiotis S, Rumpold H, Valenta R, Spitzauer S (1998) Parvalbumin, a cross-reactive fish allergen, contains IgE-binding epitopes sensitive to periodate treatment and Ca2+ depletion. J Allergy Clin Immunol 101:67–74. https://doi.org/10.1016/S0091-6749(98)70195-2
Lim DL, Neo KH, Yi FC, Chua KY, Goh DL, Shek LP, Giam YC, Van Bever HP, Lee BW (2008) Parvalbumin—the major tropical fish allergen. Pediatr Allergy Immunol 19:399–407. https://doi.org/10.1111/j.1399-3038.2007.00674.x
Perez-Gordo M, Sanchez-Garcia S, Cases B, Pastor C, Vivanco F, Cuesta-Herranz J (2008) Identification of vitellogenin as an allergen in Beluga caviar allergy. Allergy 63:479–480. https://doi.org/10.1111/j.1398-9995.2007.01614.x
Hamada Y, Nagashima Y, Shiomi K (2001) Identification of collagen as a new fish allergen. Biosci Biotechnol Biochem 65:285–291. https://doi.org/10.1271/bbb.65.285
Sakaguchi M, Toda M, Ebihara T et al (2000) IgE antibody to fish gelatin (type I collagen) in patients with fish allergy. J Allergy Clin Immunol 106:579–584. https://doi.org/10.1067/mai.2000.108499
Das Dores S, Chopin C, Romano A, Galland-Irmouli AV, Quaratino D, Pascual C, Fleurence J, Gueant JL (2002) IgE-binding and cross-reactivity of a new 41 kDa allergen of codfish. Allergy 57(Suppl 72):84–87
Wang B, Li Z, Zheng L, Liu X, Lin H (2011) Identification and characterization of a new IgE-binding protein in mackerel (Scomber japonicus) by MALDI-TOF-MS. J Ocean Univ China 10:93–98
Liu R, Krishnan HB, Xue W, Liu C (2011) Characterization of allergens isolated from the freshwater fish blunt snout bream (Megalobrama amblycephala). J Agric Food Chem 59:458–463. https://doi.org/10.1021/jf103942p
Kuehn A, Hilger C, Lehners-Weber C et al (2013) Identification of enolases and aldolases as important fish allergens in cod, salmon and tuna: component resolved diagnosis using parvalbumin and the new allergens. Clin Exp Allergy 43:811–822. https://doi.org/10.1111/cea.12117
Shanti KN, Martin BM, Nagpal S, Metcalfe DD, Rao PVS (1993) Identification of tropomyosin as the major shrimp allergen and characterization of its IgE-binding epitopes. J Immunol 151:5354–5363
Daul CB, Slattery M, Reese G, Lehrer SB (1994) Identification of the major brown shrimp (Penaeus aztecus) allergen as the muscle protein tropomyosin. Int Arch Allergy Immunol 105:49–55
Leung PS, Chu KH, Chow WK, Ansari A, Bandea CI, Kwan HS, Nagy SM, Gershwin ME (1994) Cloning, expression, and primary structure of Metapenaeus ensis tropomyosin, the major heat-stable shrimp allergen. J Allergy Clin Immunol 94:882–890
Reese G, Ayuso R, Lehrer SB (1999) Tropomyosin: an invertebrate pan-allergen. Int Arch Allergy Immunol 119:247-258. doi:24201 [pii] 24201
Leung PS, Chow WK, Duffey S, Kwan HS, Gershwin ME, Chu KH (1996) IgE reactivity against a cross-reactive allergen in crustacea and mollusca: evidence for tropomyosin as the common allergen. J Allergy Clin Immunol 98:954–961
Leung NY, Wai CY, Shu S, Wang J, Kenny TP, Chu KH, Leung PS (2014) Current immunological and molecular biological perspectives on seafood allergy: a comprehensive review. Clin Rev Allergy Immunol 46:180–197. https://doi.org/10.1007/s12016-012-8336-9
Garcia-Orozco KD, Aispuro-Hernandez E, Yepiz-Plascencia G, Calderon-de-la-Barca AM, Sotelo-Mundo RR (2007) Molecular characterization of arginine kinase, an allergen from the shrimp Litopenaeus vannamei. Int Arch Allergy Immunol 144:23–28. https://doi.org/10.1159/000102610
Yu CJ, Lin YF, Chiang BL, Chow LP (2003) Proteomics and immunological analysis of a novel shrimp allergen, Pen m 2. J Immunol 170:445–453
Bauermeister K, Wangorsch A, Garoffo LP et al (2011) Generation of a comprehensive panel of crustacean allergens from the North Sea Shrimp Crangon crangon. Mol Immunol 48:1983–1992. https://doi.org/10.1016/j.molimm.2011.06.216
Ayuso R, Grishina G, Ibanez MD, Blanco C, Carrillo T, Bencharitiwong R, Sanchez S, Nowak-Wegrzyn A, Sampson HA (2009) Sarcoplasmic calcium-binding protein is an EF-hand-type protein identified as a new shrimp allergen. J Allergy Clin Immunol 124:114–120. https://doi.org/10.1016/j.jaci.2009.04.016
Shiomi K, Sato Y, Hamamoto S, Mita H, Shimakura K (2008) Sarcoplasmic calcium-binding protein: identification as a new allergen of the black tiger shrimp Penaeus monodon. Int Arch Allergy Immunol 146:91–98. https://doi.org/10.1159/000113512
Ayuso R, Grishina G, Bardina L, Carrillo T, Blanco C, Ibanez MD, Sampson HA, Beyer K (2008) Myosin light chain is a novel shrimp allergen, Lit v 3. J Allergy Clin Immunol 122:795–802. https://doi.org/10.1016/j.jaci.2008.07.023
Pedrosa M, Boyano-Martinez T, Garcia-Ara C, Quirce S (2015) Shellfish allergy: a comprehensive review. Clin Rev Allergy Immunol 49:203–216. https://doi.org/10.1007/s12016-014-8429-8
Leung NYH, Wai CYY, Shu S, Wang J, Kenny TP, Chu KH, Leung PSC (2014) Current immunological and molecular biological perspectives on seafood allergy: a comprehensive review. Clin Rev Allergy Immunol 46:180–197. https://doi.org/10.1007/s12016-012-8336-9
Wai CYY, Leung NYH, Chu KH, Leung PSC (2012) From molecule studies of allergens to development of immunotherapy of allergies. J Aller Ther 3:124. https://doi.org/10.4172/2155-6121.1000124
Valenta R, Hochwallner H, Linhart B, Pahr S (2015) Food allergies: the basics. Gastroenterology 148:1120–1123. https://doi.org/10.1053/j.gastro.2015.02.006
Yong PF, Freeman AF, Engelhardt KR, Holland S, Puck JM, Grimbacher B (2012) An update on the hyper-IgE syndromes. Arthritis Res Ther 14:228. https://doi.org/10.1186/ar4069
Lexmond WS, Goettel JA, Lyons JJ et al (2016) FOXP3+ Tregs require WASP to restrain Th2-mediated food allergy. J Clin Invest 126:4030–4044. https://doi.org/10.1172/JCI85129
Kulis M, Wright BL, Jones SM, Burks AW (2015) Diagnosis, management, and investigational therapies for food allergies. Gastroenterology 148:1132–1142. https://doi.org/10.1053/j.gastro.2015.01.034
Akdis CA, Akdis M (2011) Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol 127:18–27. https://doi.org/10.1016/j.jaci.2010.11.030
Strait RT, Morris SC, Finkelman FD (2006) IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking. J Clin Invest 116:833–841. https://doi.org/10.1172/JCI25575
Uermösi C, Zabel F, Manolova V, Bauer M, Beerli RR, Senti G, Kündig TM, Saudan P, Bachmann MF (2014) IgG-mediated down-regulation of IgE bound to mast cells: a potential novel mechanism of allergen-specific desensitization. Allergy 69:338–347. https://doi.org/10.1111/all.12327
Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL (1995) Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature 376:177–180. https://doi.org/10.1038/376177a0
Akdis CA, Akdis M (2009) Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol 123:735–746. https://doi.org/10.1016/j.jaci.2009.02.030
Berin MC, Mayer L (2013) Can we produce true tolerance in patients with food allergy? J Allergy Clin Immunol 131:14–22. https://doi.org/10.1016/j.jaci.2012.10.058
Gorelik L, Fields PE, Flavell RA (2000) Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J Immunol 165:4773–4777
Gorelik L, Constant S, Flavell RA (2002) Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med 195:1499–1505
Jutel M, Akdis M, Budak F, Aebischer-Casaulta C, Wrzyszcz M, Blaser K, Akdis CA (2003) IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol 33:1205–1214. https://doi.org/10.1002/eji.200322919
Weiner HL (2001) Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev 182:207–214
Taylor A, Akdis M, Joss A et al (2007) IL-10 inhibits CD28 and ICOS costimulations of T cells via src homology 2 domain-containing protein tyrosine phosphatase 1. J Allergy Clin Immunol 120:76–83. https://doi.org/10.1016/j.jaci.2007.04.004
Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F (2007) A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 204:1757–1764. https://doi.org/10.1084/jem.20070590
Mora JR, Iwata M, Eksteen B et al (2006) Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314:1157–1160. https://doi.org/10.1126/science.1132742
Noon L (1911) Prophylactic inoculation against hay fever. Lancet 177:1572-1573. doi:https://doi.org/10.1016/S0140-6736(00)78276-6
Oppenheimer JJ, Nelson HS, Bock SA, Christensen F, Leung DY (1992) Treatment of peanut allergy with rush immunotherapy. J Allergy Clin Immunol 90:256–262
Meglio P, Bartone E, Plantamura M, Arabito E, Giampietro PG (2004) A protocol for oral desensitization in children with IgE-mediated cow’s milk allergy. Allergy 59:980–987. https://doi.org/10.1111/j.1398-9995.2004.00542.x
Narisety SD, Skripak JM, Steele P, Hamilton RG, Matsui EC, Burks AW, Wood RA (2009) Open-label maintenance after milk oral immunotherapy for IgE-mediated cow’s milk allergy. J Allergy Clin Immunol 124:610–612. https://doi.org/10.1016/j.jaci.2009.06.025
Keet CA, Seopaul S, Knorr S, Narisety S, Skripak J, Wood RA (2013) Long-term follow-up of oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol 132:737–739. https://doi.org/10.1016/j.jaci.2013.05.006
Buchanan AD, Green TD, Jones SM et al (2007) Egg oral immunotherapy in nonanaphylactic children with egg allergy. J Allergy Clin Immunol 119:199–205. https://doi.org/10.1016/j.jaci.2006.09.016
Burks AW, Jones SM, Wood RA et al (2012) Oral immunotherapy for treatment of egg allergy in children. N Engl J Med 367:233–243. https://doi.org/10.1056/NEJMoa1200435
Caminiti L, Pajno GB, Crisafulli G, Chiera F, Collura M, Panasci G, Ruggeri P, Guglielmo F, Passalacqua G (2015) Oral immunotherapy for egg allergy: a double-blind placebo-controlled study, with postdesensitization follow-up. J Allergy Clin Immunol Pract 3:532–539. https://doi.org/10.1016/j.jaip.2015.01.017
Anagnostou K, Islam S, King Y et al (2014) Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet 383:1297–1304. https://doi.org/10.1016/S0140-6736(13)62301-6
Jones SM, Pons L, Roberts JL et al (2009) Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol 124:292–300. https://doi.org/10.1016/j.jaci.2009.05.022
Varshney P, Jones SM, Scurlock AM et al (2011) A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol 127:654–660. https://doi.org/10.1016/j.jaci.2010.12.1111
Moran TP, Vickery BP, Burks AW (2013) Oral and sublingual immunotherapy for food allergy: current progress and future directions. Curr Opin Immunol 25:781–787. https://doi.org/10.1016/j.coi.2013.07.011
Moingeon P, Mascarell L (2012) Induction of tolerance via the sublingual route: mechanisms and applications. Clin Dev Immunol 2012:623474. https://doi.org/10.1155/2012/623474
Allam JP, Novak N, Fuchs C, Asen S, Berge S, Appel T, Geiger E, Kochan JP, Bieber T (2003) Characterization of dendritic cells from human oral mucosa: a new Langerhans’ cell type with high constitutive FcepsilonRI expression. J Allergy Clin Immunol 112:141–148
Allam JP, Peng WM, Appel T, Wenghoefer M, Niederhagen B, Bieber T, Berge S, Novak N (2008) Toll-like receptor 4 ligation enforces tolerogenic properties of oral mucosal Langerhans cells. J Allergy Clin Immunol 121(368-374):e361. https://doi.org/10.1016/j.jaci.2007.09.045
Mempel M, Rakoski J, Ring J, Ollert M (2003) Severe anaphylaxis to kiwi fruit: Immunologic changes related to successful sublingual allergen immunotherapy. J Allergy Clin Immunol 111:1406–1409
Kerzl R, Simonowa A, Ring J, Ollert M, Mempel M (2007) Life-threatening anaphylaxis to kiwi fruit: protective sublingual allergen immunotherapy effect persists even after discontinuation. J Allergy Clin Immunol 119:507–508. https://doi.org/10.1016/j.jaci.2006.09.041
Enrique E, Pineda F, Malek T et al (2005) Sublingual immunotherapy for hazelnut food allergy: a randomized, double-blind, placebo-controlled study with a standardized hazelnut extract. J Allergy Clin Immunol 116:1073–1079. https://doi.org/10.1016/j.jaci.2005.08.027
Enrique E, Malek T, Pineda F, Palacios R, Bartra J, Tella R, Basagana M, Alonso R, Cistero-Bahima A (2008) Sublingual immunotherapy for hazelnut food allergy: a follow-up study. Ann Allergy Asthma Immunol 100:283–284. https://doi.org/10.1016/S1081-1206(10)60456-5
de Boissieu D, Dupont C (2006) Sublingual immunotherapy for cow’s milk protein allergy: a preliminary report. Allergy 61:1238–1239. https://doi.org/10.1111/j.1398-9995.2006.01196.x
Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A et al (2012) The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol 129:448–455. https://doi.org/10.1016/j.jaci.2011.10.023
Fernandez-Rivas M, Garrido Fernandez S, Nadal JA et al (2009) Randomized double-blind, placebo-controlled trial of sublingual immunotherapy with a Pru p 3 quantified peach extract. Allergy 64:876–883. https://doi.org/10.1111/j.1398-9995.2008.01921.x
Garcia BE, Gonzalez-Mancebo E, Barber D et al (2010) Sublingual immunotherapy in peach allergy: monitoring molecular sensitizations and reactivity to apple fruit and Platanus pollen. J Investig Allergol Clin Immunol 20:514–520
Kim EH, Bird JA, Kulis M et al (2011) Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol 127:640–646. https://doi.org/10.1016/j.jaci.2010.12.1083
Fleischer DM, Burks AW, Vickery BP et al (2013) Sublingual immunotherapy for peanut allergy: a randomized, double-blind, placebo-controlled multicenter trial. J Allergy Clin Immunol 131:119–127. https://doi.org/10.1016/j.jaci.2012.11.011
Mondoulet L, Dioszeghy V, Puteaux E, Ligouis M, Dhelft V, Letourneur F, Dupont C, Benhamou PH (2012) Intact skin and not stripped skin is crucial for the safety and efficacy of peanut epicutaneous immunotherapy (EPIT) in mice. Clin Transl Allergy 2:22. https://doi.org/10.1186/2045-7022-2-22
Senti G, Graf N, Haug S, Ruedi N, von Moos S, Sonderegger T, Johansen P, Kundig TM (2009) Epicutaneous allergen administration as a novel method of allergen-specific immunotherapy. J Allergy Clin Immunol 124:997–1002. https://doi.org/10.1016/j.jaci.2009.07.019
Stoitzner P, Tripp CH, Eberhart A, Price KM, Jung JY, Bursch L, Ronchese F, Romani N (2006) Langerhans cells cross-present antigen derived from skin. Proc Natl Acad Sci U S A 103:7783–7788. https://doi.org/10.1073/pnas.0509307103
Shklovskaya E, O’Sullivan BJ, Ng LG, Roediger B, Thomas R, Weninger W, Fazekas de St Groth B (2011) Langerhans cells are precommitted to immune tolerance induction. Proc Natl Acad Sci U S A 108:18049–18054. https://doi.org/10.1073/pnas.1110076108
Gomez de Aguero M, Vocanson M, Hacini-Rachinel F, Taillardet M, Sparwasser T, Kissenpfennig A, Malissen B, Kaiserlian D, Dubois B (2012) Langerhans cells protect from allergic contact dermatitis in mice by tolerizing CD8(+) T cells and activating Foxp3(+) regulatory T cells. J Clin Invest 122:1700–1711. https://doi.org/10.1172/JCI59725
Dioszeghy V, Mondoulet L, Dhelft V, Ligouis M, Puteaux E, Benhamou PH, Dupont C (2011) Epicutaneous immunotherapy results in rapid allergen uptake by dendritic cells through intact skin and downregulates the allergen-specific response in sensitized mice. J Immunol 186:5629–5637. https://doi.org/10.4049/jimmunol.1003134
Dioszeghy V, Mondoulet L, Dhelft V, Ligouis M, Puteaux E, Dupont C, Benhamou PH (2014) The regulatory T cells induction by epicutaneous immunotherapy is sustained and mediates long-term protection from eosinophilic disorders in peanut-sensitized mice. Clin Exp Allergy 44:867–881. https://doi.org/10.1111/cea.12312
Tordesillas L, Mondoulet L, Blazquez AB, Benhamou PH, Sampson HA, Berin MC (2017) Epicutaneous immunotherapy induces gastrointestinal LAP+ regulatory T cells and prevents food-induced anaphylaxis. J Allergy Clin Immunol 139:189–201. https://doi.org/10.1016/j.jaci.2016.03.057
Dupont C, Kalach N, Soulaines P, Legoue-Morillon S, Piloquet H, Benhamou PH (2010) Cow’s milk epicutaneous immunotherapy in children: a pilot trial of safety, acceptability, and impact on allergic reactivity. J Allergy Clin Immunol 125:1165–1167. https://doi.org/10.1016/j.jaci.2010.02.029
Leung NYH, Wai CYY, Shu SA, Chang CC, Chu KH, Leung PSC (2017) Low dose allergen-specific immunotherapy induces tolerance in a murine model of shrimp allergy. Int Arch Allergy Immunol:(in press)
El-Qutob D (2016) Off-label uses of omalizumab. Clin Rev Allergy Immunol 50:84–96. https://doi.org/10.1007/s12016-015-8490-y
Wood RA, Kim JS, Lindblad R, Nadeau K, Henning AK, Dawson P, Plaut M, Sampson HA (2016) A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow’s milk allergy. J Allergy Clin Immunol 137:1103–1110. https://doi.org/10.1016/j.jaci.2015.10.005
MacGinnitie AJ, Rachid R, Gragg H et al (2017) Omalizumab facilitates rapid oral desensitization for peanut allergy. J Allergy Clin Immunol 139:873–881. https://doi.org/10.1016/j.jaci.2016.08.010
Macglashan DW Jr, Saini SS (2013) Omalizumab increases the intrinsic sensitivity of human basophils to IgE-mediated stimulation. J Allergy Clin Immunol 132(906-911):e901–e904. https://doi.org/10.1016/j.jaci.2013.04.056
Frischmeyer-Guerrerio PA, Masilamani M, Gu W et al (2017) Mechanistic correlates of clinical responses to omalizumab in the setting of oral immunotherapy for milk allergy. J Allergy Clin Immunol. https://doi.org/10.1016/j.jaci.2017.03.028
Rupa P, Nakamura S, Katayama S, Mine Y (2014) Effects of ovalbumin glycoconjugates on alleviation of orally induced egg allergy in mice via dendritic-cell maturation and T-cell activation. Mol Nutr Food Res 58:405–417. https://doi.org/10.1002/mnfr.201300067
Yang Z, Li Y, Li C, Wang Z (2012) Synthesis of hypoallergenic derivatives of the major allergen Fag t 1 from tartary buckwheat via sequence restructuring. Food Chem Toxicol 50:2675–2680. https://doi.org/10.1016/j.fct.2012.03.039
Reese G, Ballmer-Weber BK, Wangorsch A, Randow S, Vieths S (2007) Allergenicity and antigenicity of wild-type and mutant, monomeric, and dimeric carrot major allergen Dau c 1: destruction of conformation, not oligomerization, is the roadmap to save allergen vaccines. J Allergy Clin Immunol 119:944–951. https://doi.org/10.1016/j.jaci.2006.11.699
Bannon GA, Cockrell G, Connaughton C et al (2001) Engineering, characterization and in vitro efficacy of the major peanut allergens for use in immunotherapy. Int Arch Allergy Immunol 124:70–72. https://doi.org/10.1159/000053672
Starkl P, Felix F, Krishnamurthy D et al (2012) An unfolded variant of the major peanut allergen Ara h 2 with decreased anaphylactic potential. Clin Exp Allergy 42:1801–1812. https://doi.org/10.1111/cea.12031
Lemon-Mule H, Sampson HA, Sicherer SH, Shreffler WG, Noone S, Nowak-Wegrzyn A (2008) Immunologic changes in children with egg allergy ingesting extensively heated egg. J Allergy Clin Immunol 122:977–983. https://doi.org/10.1016/j.jaci.2008.09.007
Martos G, Lopez-Exposito I, Bencharitiwong R, Berin MC, Nowak-Wegrzyn A (2011) Mechanisms underlying differential food allergy response to heated egg. J Allergy Clin Immunol 127:990–997. https://doi.org/10.1016/j.jaci.2011.01.057
Watanabe H, Toda M, Sekido H, Wellner A, Fujii T, Henle T, Hachimura S, Nakajima-Adachi H (2014) Heat treatment of egg white controls allergic symptoms and induces oral tolerance to ovalbumin in a murine model of food allergy. Mol Nutr Food Res 58:394–404. https://doi.org/10.1002/mnfr.201300205
Golias J, Schwarzer M, Wallner M et al (2012) Heat-induced structural changes affect OVA-antigen processing and reduce allergic response in mouse model of food allergy. PLoS One 7:e37156. https://doi.org/10.1371/journal.pone.0037156
Jimenez-Saiz R, Rupa P, Mine Y (2011) Immunomodulatory effects of heated ovomucoid-depleted egg white in a BALB/c mouse model of egg allergy. J Agric Food Chem 59:13195–13202. https://doi.org/10.1021/jf202963r
Turner PJ, Mehr S, Joshi P, Tan J, Wong M, Kakakios A, Campbell DE (2013) Safety of food challenges to extensively heated egg in egg-allergic children: a prospective cohort study. Pediatr Allergy Immunol 24:450–455. https://doi.org/10.1111/pai.12093
Goldberg MR, Nachshon L, Appel MY, Elizur A, Levy MB, Eisenberg E, Sampson HA, Katz Y (2015) Efficacy of baked milk oral immunotherapy in baked milk-reactive allergic patients. J Allergy Clin Immunol. https://doi.org/10.1016/j.jaci.2015.05.040
Toda M, Reese G, Gadermaier G et al (2011) Protein unfolding strongly modulates the allergenicity and immunogenicity of Pru p 3, the major peach allergen. J Allergy Clin Immunol 128:1022–1030. https://doi.org/10.1016/j.jaci.2011.04.020
Bencharitiwong R, van der Kleij HP, Koppelman SJ, Nowak-Wegrzyn A (2015) Effect of chemical modifications on allergenic potency of peanut proteins. Allergy Asthma Proc 36:185–191. https://doi.org/10.2500/aap.2015.36.3840
Pree I, Reisinger J, Focke M et al (2007) Analysis of epitope-specific immune responses induced by vaccination with structurally folded and unfolded recombinant Bet v 1 allergen derivatives in man. J Immunol 179:5309–5316
Hochwallner H, Schulmeister U, Swoboda I et al (2010) Visualization of clustered IgE epitopes on alpha-lactalbumin. J Allergy Clin Immunol 125:1279–1285. https://doi.org/10.1016/j.jaci.2010.03.007
Mine Y, Rupa P (2003) Fine mapping and structural analysis of immunodominant IgE allergenic epitopes in chicken egg ovalbumin. Protein Eng 16:747–752
Shreffler WG, Lencer DA, Bardina L, Sampson HA (2005) IgE and IgG4 epitope mapping by microarray immunoassay reveals the diversity of immune response to the peanut allergen, Ara h 2. J Allergy Clin Immunol 116:893–899. https://doi.org/10.1016/j.jaci.2005.06.033
Wai CYY, Leung NYH, Ho MH, Gershwin LJ, Shu SA, Leung PSC, Chu KH (2014) Immunization with hypoallergens of shrimp allergen tropomyosin inhibits shrimp tropomyosin specific IgE reactivity. PLoS One 9:e111649. https://doi.org/10.1371/journal.pone.0111649
Ayuso R, Lehrer SB, Reese G (2002) Identification of continuous, allergenic regions of the major shrimp allergen Pen a 1 (tropomyosin). Int Arch Allergy Immunol 127:27-37. doi:48166 [pii] 48166
Garcia-Casado G, Pacios LF, Diaz-Perales A, Sanchez-Monge R, Lombardero M, Garcia-Selles FJ, Polo F, Barber D, Salcedo G (2003) Identification of IgE-binding epitopes of the major peach allergen Pru p 3. J Allergy Clin Immunol 112:599–605
King N, Helm R, Stanley JS et al (2005) Allergenic characteristics of a modified peanut allergen. Mol Nutr Food Res 49:963–971. https://doi.org/10.1002/mnfr.200500073
Lam YF, Tong KK, Kwan KM, Tsuneyama K, Shu SA, Leung PS, Chu KH (2015) Gastrointestinal immune response to the shrimp allergen tropomyosin: histological and immunological analysis in an animal model of shrimp tropomyosin hypersensitivity. Int Arch Allergy Immunol 167:29–40. https://doi.org/10.1159/000431228
Leung PSC, Lee YS, Tang CY, Kung WY, Chuang YH, Chiang BL, Fung MC, Chu KH (2008) Induction of shrimp tropomyosin-specific hypersensitivity in mice. Int Arch Allergy Immunol 147:305–314. https://doi.org/10.1159/000144038
Singh A, Holvoet S, Mercenier A (2011) Dietary polyphenols in the prevention and treatment of allergic diseases. Clin Exp Allergy 41:1346–1359. https://doi.org/10.1111/j.1365-2222.2011.03773.x
Plundrich NJ, Kulis M, White BL, Grace MH, Guo R, Burks AW, Davis JP, Lila MA (2014) Novel strategy to create hypoallergenic peanut protein-polyphenol edible matrices for oral immunotherapy. J Agric Food Chem 62:7010–7021. https://doi.org/10.1021/jf405773b
Zuidmeer-Jongejan L, Fernandez-Rivas M, Poulsen LK et al (2012) FAST: towards safe and effective subcutaneous immunotherapy of persistent life-threatening food allergies. Clin Transl Allergy 2:5. https://doi.org/10.1186/2045-7022-2-5
Swoboda I, Bugajska-Schretter A, Linhart B et al. (2007) A recombinant hypoallergenic parvalbumin mutant for immunotherapy of IgE-mediated fish allergy. J Immunol 178:6290-6296. doi:178/10/6290 [pii]
Zuidmeer-Jongejan L, Huber H, Swoboda I et al (2015) Development of a hypoallergenic recombinant parvalbumin for first-in-man subcutaneous immunotherapy of fish allergy. Int Arch Allergy Immunol 166:41–51. https://doi.org/10.1159/000371657
Freidl R, Gstoettner A, Baranyi U et al (2017) Blocking antibodies induced by immunization with a hypoallergenic parvalbumin mutant reduce allergic symptoms in a mouse model of fish allergy. J Allergy Clin Immunol 139(1897-1905):e1891. https://doi.org/10.1016/j.jaci.2016.10.018
Larche M (2011) T cell epitope-based allergy vaccines. Curr Top Microbiol Immunol 352:107–119. https://doi.org/10.1007/82_2011_131
Prickett SR, Rolland JM, O’Hehir RE (2015) Immunoregulatory T cell epitope peptides: the new frontier in allergy therapy. Clin Exp Allergy 45:1015–1026. https://doi.org/10.1111/cea.12554
Meulenbroek LA, van Esch BC, Hofman GA et al (2013) Oral treatment with beta-lactoglobulin peptides prevents clinical symptoms in a mouse model for cow’s milk allergy. Pediatr Allergy Immunol 24:656–664. https://doi.org/10.1111/pai.12120
Thang CL, Zhao X (2015) Effects of orally administered immunodominant T-cell epitope peptides on cow’s milk protein allergy in a mouse model. Food Res Int 71:126–131
Yang M, Mine Y (2009) Novel T-cell epitopes of ovalbumin in BALB/c mouse: potential for peptide-immunotherapy. Biochem Biophys Res Commun 378:203–208. https://doi.org/10.1016/j.bbrc.2008.11.037
Mizumachi K, Kurisaki J (2003) Localization of T cell epitope regions of chicken ovomucoid recognized by mice. Biosci Biotechnol Biochem 67:712–719
Yang M, Yang C, Mine Y (2010) Multiple T cell epitope peptides suppress allergic responses in an egg allergy mouse model by the elicitation of forkhead box transcription factor 3- and transforming growth factor-beta-associated mechanisms. Clin Exp Allergy 40:668–678. https://doi.org/10.1111/j.1365-2222.2009.03442.x
Rupa P, Mine Y (2012) Oral immunotherapy with immunodominant T-cell epitope peptides alleviates allergic reactions in a Balb/c mouse model of egg allergy. Allergy 67:74–82. https://doi.org/10.1111/j.1398-9995.2011.02724.x
Wai CYY, Leung NYH, Leung PSC, Chu KH (2016) T cell epitope immunotherapy ameliorates allergic responses in a murine model of shrimp allergy. Clin Exp Allergy 46:491–503. https://doi.org/10.1111/cea.12684
Ravkov EV, Pavlov IY, Martins TB, Gleich GJ, Wagner LA, Hill HR, Delgado JC (2013) Identification and validation of shrimp-tropomyosin specific CD4 T cell epitopes. Hum Immunol 74:1542–1549. https://doi.org/10.1016/j.humimm.2013.08.276
Clark AT, Islam S, King Y, Deighton J, Anagnostou K, Ewan PW (2009) Successful oral tolerance induction in severe peanut allergy. Allergy 64:1218–1220. https://doi.org/10.1111/j.1398-9995.2009.01982.x
Patil SU, Ogunniyi AO, Calatroni A, Tadigotla VR, Ruiter B, Ma A, Moon J, Love JC, Shreffler WG (2015) Peanut oral immunotherapy transiently expands circulating Ara h 2-specific B cells with a homologous repertoire in unrelated subjects. J Allergy Clin Immunol 136:125–134. https://doi.org/10.1016/j.jaci.2015.03.026
Syed A, Garcia MA, Lyu SC et al (2014) Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). J Allergy Clin Immunol 133:500–510. https://doi.org/10.1016/j.jaci.2013.12.1037
Nowak-Wegrzyn A, Albin S (2015) Oral immunotherapy for food allergy: mechanisms and role in management. Clin Exp Allergy 45:368–383. https://doi.org/10.1111/cea.12382
Pascal M, Konstantinou GN, Masilamani M, Lieberman J, Sampson HA (2013) In silico prediction of Ara h 2 T cell epitopes in peanut-allergic children. Clin Exp Allergy 43:116–127. https://doi.org/10.1111/cea.12014
Prickett SR, Voskamp AL, Dacumos-Hill A, Symons K, Rolland JM, O’Hehir RE (2011) Ara h 2 peptides containing dominant CD4+ T-cell epitopes: candidates for a peanut allergy therapeutic. J Allergy Clin Immunol 127:608–615. https://doi.org/10.1016/j.jaci.2010.09.027
Prickett SR, Voskamp AL, Phan T, Dacumos-Hill A, Mannering SI, Rolland JM, O’Hehir RE (2013) Ara h 1 CD4+ T cell epitope-based peptides: candidates for a peanut allergy therapeutic. Clin Exp Allergy 43:684–697. https://doi.org/10.1111/cea.12113
Meulenbroek LA, den Hartog Jager CF, Lebens AF, Knulst AC, Bruijnzeel-Koomen CA, Garssen J, Knippels LM, van Hoffen E (2014) Characterization of T cell epitopes in bovine alpha-lactalbumin. Int Arch Allergy Immunol 163:292–296. https://doi.org/10.1159/000360733
Ruiter B, Tregoat V, M’Rabet L, Garssen J, Bruijnzeel-Koomen CA, Knol EF, Hoffen E (2006) Characterization of T cell epitopes in alphas1-casein in cow’s milk allergic, atopic and non-atopic children. Clin Exp Allergy 36:303–310. https://doi.org/10.1111/j.1365-2222.2006.02436.x
Pastorello EA, Monza M, Pravettoni V, Longhi R, Bonara P, Scibilia J, Primavesi L, Scorza R (2010) Characterization of the T-cell epitopes of the major peach allergen Pru p 3. Int Arch Allergy Immunol 153:1–12. https://doi.org/10.1159/000301573
Tordesillas L, Cuesta-Herranz J, Gonzalez-Munoz M, Pacios LF, Compes E, Garcia-Carrasco B, Sanchez-Monge R, Salcedo G, Diaz-Perales A (2009) T-cell epitopes of the major peach allergen, Pru p 3: identification and differential T-cell response of peach-allergic and non-allergic subjects. Mol Immunol 46:722–728. https://doi.org/10.1016/j.molimm.2008.10.018
Schulten V, Radakovics A, Hartz C et al (2009) Characterization of the allergic T-cell response to Pru p 3, the nonspecific lipid transfer protein in peach. J Allergy Clin Immunol 124:100–107. https://doi.org/10.1016/j.jaci.2009.02.010
Nielsen M, Lund O, Buus S, Lundegaard C (2010) MHC class II epitope predictive algorithms. Immunology 130:319–328. https://doi.org/10.1111/j.1365-2567.2010.03268.x
Yang M, Yang C, Nau F, Pasco M, Juneja LR, Okubo T, Mine Y (2009) Immunomodulatory effects of egg white enzymatic hydrolysates containing immunodominant epitopes in a BALB/c mouse model of egg allergy. J Agric Food Chem 57:2241–2248. https://doi.org/10.1021/jf803372b
Kulis M, Macqueen I, Li Y, Guo R, Zhong XP, Burks AW (2012) Pepsinized cashew proteins are hypoallergenic and immunogenic and provide effective immunotherapy in mice with cashew allergy. J Allergy Clin Immunol 130:716–723. https://doi.org/10.1016/j.jaci.2012.05.044
Suphioglu C, Schappi G, Kenrick J, Levy D, Davies JM, O’Hehir RE (2001) A novel grass pollen allergen mimotope identified by phage display peptide library inhibits allergen-human IgE antibody interaction. FEBS Lett 502:46–52
Ganglberger E, Grunberger K, Wiedermann U, Vermes M, Sponer B, Breiteneder H, Scheiner O, Boltz G, Jensen-Jarolim E (2001) IgE mimotopes of birch pollen allergen Bet v 1 induce blocking IgG in mice. Int Arch Allergy Immunol 124:395-397. doi:53768 [pii] 53768
Ganglberger E, Grunberger K, Sponer B, Radauer C, Breiteneder H, Boltz-Nitulescu G, Scheiner O, Jensen-Jarolim E (2000) Allergen mimotopes for 3-dimensional epitope search and induction of antibodies inhibiting human IgE. FASEB J 14:2177–2184. https://doi.org/10.1096/fj.99-1000com
Jensen-jarolim E, Leitner A, Kalchhauser H, Zurcher A, Ganglberger E, Bohle B, Scheiner O, Boltz-nitulescu G, Breiteneder H (1998) Peptide mimotopes displayed by phage inhibit antibody binding to bet v 1, the major birch pollen allergen, and induce specific IgG response in mice. FASEB J 12:1635–1642
Hantusch B, Krieger S, Untersmayr E et al (2004) Mapping of conformational IgE epitopes on Phl p 5a by using mimotopes from a phage display library. J Allergy Clin Immunol 114:1294–1300. https://doi.org/10.1016/j.jaci.2004.06.048
Pacios LF, Tordesillas L, Cuesta-Herranz J, Compes E, Sanchez-Monge R, Palacin A, Salcedo G, Diaz-Perales A (2008) Mimotope mapping as a complementary strategy to define allergen IgE-epitopes: peach Pru p 3 allergen as a model. Mol Immunol 45:2269–2276. https://doi.org/10.1016/j.molimm.2007.11.022
Untersmayr E, Szalai K, Riemer AB et al (2006) Mimotopes identify conformational epitopes on parvalbumin, the major fish allergen. Mol Immunol 43:1454–1461. https://doi.org/10.1016/j.molimm.2005.07.038
Szalai K, Jensen-Jarolim E, Pali-Scholl I (2008) Vaccination strategies based on the mimotope concept. G Ital Dermatol Venereol 143:95–104
Tiwari R, Negi SS, Braun B, Braun W, Pomes A, Chapman MD, Goldblum RM, Midoro-Horiuti T (2012) Validation of a phage display and computational algorithm by mapping a conformational epitope of Bla g 2. Int Arch Allergy Immunol 157:323–330. https://doi.org/10.1159/000330108
Wallmann J, Epstein MM, Singh P, Brunner R, Szalai K, El-Housseiny L, Pali-Scholl I, Jensen-Jarolim E (2010) Mimotope vaccination for therapy of allergic asthma: anti-inflammatory effects in a mouse model. Clin Exp Allergy 40:650–658. https://doi.org/10.1111/j.1365-2222.2009.03392.x
Leung NYH, Wai CYY, Ho MH, Liu R, Lam KS, Wang JJ, Shu SA, Chu KH, Leung PSC (2017) Screening and identification of mimotopes of the major shrimp allergen tropomyosin using one-bead-one-compound peptide libraries. Cell Mol Immunol 14:308–318. https://doi.org/10.1038/cmi.2015.83
Zhou Y, Kawasaki H, Hsu SC et al (2010) Oral tolerance to food-induced systemic anaphylaxis mediated by the C-type lectin SIGNR1. Nat Med 16:1128–1133. https://doi.org/10.1038/nm.2201
Schulke S, Burggraf M, Waibler Z, Wangorsch A, Wolfheimer S, Kalinke U, Vieths S, Toda M, Scheurer S (2011) A fusion protein of flagellin and ovalbumin suppresses the TH2 response and prevents murine intestinal allergy. J Allergy Clin Immunol 128:1340–1348. https://doi.org/10.1016/j.jaci.2011.07.036
Liu Y, Sun Y, Chang LJ et al (2013) Blockade of peanut allergy with a novel Ara h 2-Fcgamma fusion protein in mice. J Allergy Clin Immunol 131:213–221. https://doi.org/10.1016/j.jaci.2012.10.018
Hunter Z, McCarthy DP, Yap WT, Harp CT, Getts DR, Shea LD, Miller SD (2014) A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS Nano 8:2148–2160. https://doi.org/10.1021/nn405033r
Srivastava KD, Siefert A, Fahmy TM, Caplan MJ, Li XM, Sampson HA (2016) Investigation of peanut oral immunotherapy with CpG/peanut nanoparticles in a murine model of peanut allergy. J Allergy Clin Immunol 138:536–543. https://doi.org/10.1016/j.jaci.2016.01.047
Acknowledgements
The authors would like to thank Mr. Yaoyu Gong for his contribution to this review. The work on shellfish allergy of the authors is supported by a grant from the Health and Medical Research Fund (02130206), HKSAR, China. CYY Wai is currently funded by an AXA Postdoctoral Fellowship (AXA Research Fund).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wai, C.Y.Y., Leung, N.Y.H., Leung, P.S.C. et al. Immunotherapy of Food Allergy: a Comprehensive Review. Clinic Rev Allerg Immunol 57, 55–73 (2019). https://doi.org/10.1007/s12016-017-8647-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-017-8647-y