Abstract
Mesenchymal stem cells (MSCs) are an important population of multipotent stem cells that differentiate into multiple lineages and display great potential in bone regeneration and repair. Although the role of protein-coding genes in the osteogenic differentiation of MSCs has been extensively studied, the functions of noncoding RNAs in the osteogenic differentiation of MSCs are unclear. The recent application of next-generation sequencing to MSC transcriptomes has revealed that long noncoding RNAs (lncRNAs) are associated with the osteogenic differentiation of MSCs. LncRNAs are a class of non-coding transcripts of more than 200 nucleotides in length. Noncoding RNAs are thought to play a key role in osteoblast differentiation through various regulatory mechanisms including chromatin modification, transcription factor binding, competent endogenous mechanism, and other post-transcriptional mechanisms. Here, we review the roles of lncRNAs in the osteogenic differentiation of bone marrow- and adipose-derived stem cells and provide a theoretical foundation for future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to genome analyses, 50–70% of genomic DNA is transcribed, but < 2% encodes proteins [1]. Accumulating evidence presented in recent years has revealed that noncoding RNAs (ncRNAs) constitute a major portion of transcripts and play an essential role in biological processes [2]. As a family of RNAs with no protein coding potential, ncRNAs can be divided into two major groups: housekeeper and regulatory ncRNAs [3]. In general, regulatory ncRNAs are further categorised into several groups: small noncoding RNAs of less than 200 nucleotides, long noncoding RNAs (lncRNAs) of greater than 200 nucleotides, and circular RNAs with a closed continuous loop [1, 4]. Although lncRNAs were previously considered “transcriptional noise”, they are now emerging as important regulators of biological processes due to recent developments in genomic technology [5]. Accumulating evidence has indicated that lncRNAs are involved in regulating the growth and differentiation of various types of cells [6, 7].
lncRNAs are classified into five groups, according to their position relative to protein-coding genes: sense, antisense, bidirectional, intronic, and intergenic lncRNAs [8]. The majority of lncRNA classes share similar characteristics with mRNAs including 5′ capping, splicing, and polyadenylation [1, 4]. In contrast to mRNAs, they generally do not possess protein-coding open reading frames and exhibit relatively lower levels of expression [9]. However, lncRNAs exhibit a higher degree of conservation of promoters and exons, which suggests that they have essential functions in biological processes [10]. Furthermore, lncRNAs are expressed in a tissue-, cell-, and developmental stage-specific manner [1, 9]. Researchers have confirmed the vital roles of lncRNAs in epigenetic, transcriptional, and post-transcriptional gene regulation such as X-chromosome inactivation, histone modification, imprinting, transcriptional interference, and nuclear and cytoplasmic trafficking [11,12,13,14,15]. Moreover, lncRNAs are closely linked with human diseases ranging from heart disease to cancer [2, 16].
Bone marrow and adipose tissue are two of the most frequently applied mesenchymal stem cell (MSC) sources in research because of their high proliferative capacity and potential regenerative properties [17, 18]. In this review, we highlight the role of lncRNAs in osteogenic differentiation of bone marrow-derived stem cells (BMSCs) and adipose-derived stem cells (ADSCs), and describe their modulatory mechanisms, which may be important for the therapeutic application of MSCs in bone regeneration and repair.
Regulation of Osteogenic Differentiation in MSCs
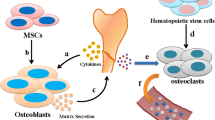
MSCs are an important group of multipotent cells that can differentiate into a broad range of cell types including adipocytes, osteoblasts, chondrocytes, tendon cells, enocytes, and myocytes [19]. Originally identified in the bone marrow, MSCs have been obtained from numerous types of mesenchymal tissue such as the bone marrow, fat, umbilical cord, placenta, lung, liver, and skin [20]. In particular, bone marrow and adipose tissue are attractive sources for the isolation of MSCs, which differentiate into osteoblasts in vitro under certain conditions, offering new therapeutic options for bone defects and metabolic diseases [21, 22]. Thus, it is important to investigate the signalling that regulates the osteogenic differentiation of MSCs. The process by which MSCs undergo osteogenic differentiation is not completely understood. Multiple signalling pathways are involved including the transforming growth factor (TGF)-β/bone morphogenetic protein (BMP), Wnt, Hedgehog, Notch, and fibroblast growth factor signalling pathways [23, 24]. Runt-related transcription factor 2 (Runx2) is a target of β-catenin/T cell factor 1 in the canonical Wnt signalling pathway in the control of osteoblast differentiation and skeletal development [25]. BMP binds heterodimeric receptors to phosphorylate and activate Smad proteins, which then activate osteogenesis-related genes either directly or via Runx2 [26]. TAZ promotes MSC differentiation into osteoblasts via direct interaction with Runx2 [27]. In addition, biological, chemical, and physical factors influence the differentiation of MSCs [28]. These events ultimately end in a series of elaborate transcriptional cascades dominated by two important transcription factors: peroxisome proliferator-activated receptor γ in adipogenesis and Runx2 in osteogenesis [28]. Although these processes are mainly controlled by these crucial transcription factors, a growing number of studies have reported that ncRNAs function as significant regulators of osteogenic differentiation. For instance, microRNA (miR)-216a ameliorates the dexamethasone-mediated inhibition of osteoblast differentiation in vitro and enhances bone formation in vivo via the c-Cbl-mediated phosphatidylinositol 3 kinase (PI3K)/Akt pathway [29]. MiR-20a promotes the osteoblast differentiation of human mesenchymal stem cells (hMSCs) by targeting negative regulators of BMP signalling [30]. Overexpression of miR-22 enhances osteoblast differentiation and suppresses adipogenic differentiation of human adipose-derived stem cells (hADSCs) by repressing histone deacetylase (HDAC) 6 [31], whereas miR-30e represses osteoblast differentiation by directly modulating the canonical Wnt/LDL receptor related protein 6 /β-catenin/TCF signalling pathway [32]. Moreover, miR-133 and miR-135 suppress the osteoblast differentiation of C2C12 cells by targeting the Runx2 and Smad5 genes, respectively [33]. Recently, lncRNAs have been shown to be differentially expressed in undifferentiated and osteogenically differentiated MSCs, and play vital regulatory roles in the differentiation process [34].
lncRNAs Are Involved in the Osteogenic Differentiation of BMSCs and ADSCs
The growing number of studies on the roles of lncRNAs in regulating the osteogenic differentiation of BMSCs and ADSCs has attracted widespread attention [35, 36]. Zou et al. in 2013 identified 116 aberrantly expressed lncRNAs during BMP2-stimulated osteogenesis of C3H10T1/2 MSCs compared to the untreated control. Meanwhile, 24 cooperatively expressed lncRNA and mRNA pairs were identified, which indicates that lncRNAs may function by regulating nearby protein-coding genes [37]. Subsequent studies have established the expression profiles of lncRNAs during osteogenic differentiation of human bone marrow-derived stem cells (hBMSCs) using lncRNA microarrays. In total, 1206 differentially expressed lncRNAs were identified during osteogenesis [36]. In a recent study by Song et al. using high-throughput RNA sequencing (RNA-Seq), 574 differentially expressed lncRNAs associated with the osteogenic differentiation of MSCs were identified [38]. In addition, increasing attention has been paid to the osteogenic differentiation of MSCs in the inflammatory microenvironment. More than 2000 lncRNAs were differentially expressed in SpA-treated hBMSCs, among which 641 were downregulated and 1392 were upregulated [39] (Table 1).
Key lncRNAs Regulating the Osteogenic Differentiation of BMSCs and ADSCs
H19
H19 is a maternally expressed and paternally imprinted 2.7 kb gene located on human chromosome 11 [40]. Because the exons of H19 have been highly conserved during evolution, the role of H19 in cell growth and differentiation has been extensively studied. In recent years, striking findings have revealed its role in the osteogenic differentiation of MSCs. Using lncRNA microarray technology, 1206 differentially expressed lncRNAs were identified, with 687 upregulated and 519 downregulated more than two-fold during osteogenic differentiation of MSCs. Two significantly changed lncRNAs (H19 and uc022axw.1) were selected for further analysis. The expression of H19 and uc022axw.1 increased from day 7 and was maintained at a high level from days 14 to 28, which indicates crucial functions in the early stages of osteogenic differentiation [36]. Subsequently, our group found that lncRNA H19 was significantly upregulated following the induction of osteoblast differentiation. Overexpression of H19 significantly stimulated osteoblast differentiation in vivo and in vitro, whereas H19 knockdown suppressed these effects [41]. Another study confirmed that H19 significantly accelerated osteoblast differentiation in vivo and in vitro [42].
Myocardial Infarction-Associated Transcript
First identified as a lncRNA in 2000, myocardial infarction-associated transcript (MIAT) is predominantly expressed in the heart and foetal brain tissue [43]. It has been implicated in a variety of diseases including myocardial infarction, microvascular dysfunction, and schizophrenia [44,45,46]. MIAT knockdown promotes the osteoblast differentiation of hADSCs both in vitro and in vivo and rescues the inhibition of osteogenic differentiation of hADSCs in the tumour necrosis factor α-induced inflammatory microenvironment [47]. MIAT was previously shown to act as a miR-150-5p sponge that regulates the expression of related genes upon exposure to inflammatory conditions [48]. However, it is unknown if MIAT interacts with miRNAs mechanistically during osteogenic differentiation.
Anti-differentiation Noncoding RNA
Anti-differentiation noncoding RNA (ANCR) is a newly identified lncRNA located on human chromosome 4. The expression of ANCR was lower in several terminally differentiated cell types (keratinocytes, osteoblasts, and adipocytes) than in stem cells, which indicates its possible role in regulating differentiation [49]. ANCR is required to maintain cells in the undifferentiated state within the epidermis [49]. However, its function in osteogenic differentiation has not been confirmed. Recently, the significantly decreased expression of ANCR was detected during differentiation of the human foetal osteoblast cell line (hFOB1.19). Knockdown of endogenous ANCR resulted in osteoblast differentiation, whereas its overexpression inhibited differentiation [50]. The inhibitory effects of ANCR on osteogenic differentiation were also observed in periodontal ligament stem cells and other dental tissue-derived stem cells [51, 52].
Hox Transcript Antisense Intergenic RNA
Hox transcript antisense intergenic RNA (HOTAIR) is encoded by the HOXC locus on chromosome 12q, and represses the expression of the more distal HOXD locus and genes on other chromosomes [13]. HOTAIR is involved in a variety of cancers such as breast, cancer, and liver cancer, among others [53,54,55]. Research on HOTAIR has mainly focused on cancer, but Xing et al. first detected HOTAIR in cartilage. The authors found that HOTAIR is overexpressed in osteoarthritis (OA) tissue compared to normal tissue, which indicates its potential function in the pathogenesis of OA. Furthermore, higher expression of HOTAIR was observed in samples of non-traumatic osteonecrosis of the femoral head compared to OA samples [56]. Downregulation of HOTAIR resulted in elevated mRNA expression of osteogenic differentiation markers, including Runx2 and collagen type 1 alpha 1 (COL1A1), as well as alkaline phosphatase (ALP) activity. Meanwhile, upregulation of HOTAIR produced the opposite results [57].
Maternally Expressed Gene 3
The maternally expressed gene 3 (MEG3) encodes a lncRNA that is expressed in many normal tissues. Downregulation of MEG3 expression has been demonstrated in various types of humans and tumour cell lines, which indicates that MEG3 may function as a tumour suppressor gene [58, 59]. Recently, Zhuang et al. observed a lower expression level of MEG3 in MSCs derived from patients with multiple myeloma (MM-MSCs) relative to that of normal donors (ND-MSCs) during osteogenic differentiation. The authors found that the overexpression of MEG3 could promote osteogenic differentiation of MM-MSCs and that MEG3 knockdown inhibits osteogenic differentiation of ND-MSCs. This suggested the possible regulatory role of MEG3 in osteogenic differentiation of MSCs, which is discussed in a later section [60]. Our previous study further confirmed the positive regulatory role of MEG3 in the osteogenic differentiation of hADSCs. We found that knockdown of MEG3 enhanced adipogenic differentiation of hADSCs, whereas it inhibited osteogenic differentiation [61]. However, in another study of postmenopausal osteoporosis (PMOP), MEG3 was significantly increased in BMSCs derived from mouse pathologic models and patients with PMOP. This study also showed that the overexpression of MEG3 caused a decrease, and the silencing of MEG3 caused an increase, in mineralised nodule formation, ALP activity, and Runx2, osteocalcin, and osteopontin protein levels [62]. These contradictory results might be partially attributed to the different characteristics and distinct post-transcriptional regulation in various tissue-specific MSCs.
Other lncRNAs Regulating Osteogenic Differentiation of BMSCs and ADSCs
Hypoxia-Inducible Factor 1α-antisense 1
The lncRNA hypoxia-inducible factor 1α-antisense 1 (HIF1α-AS1) plays a vital role in the pathogenesis of cardiovascular disease [32, 63]. Suppression of HIF1α-AS1 inhibits apoptosis and promotes the proliferation of vascular smooth muscle cells in vitro [64]. Furthermore, overexpression of HIF1α-AS1 upregulates the expression of homeobox D10 (HOXD10), ultimately resulting in the osteogenic differentiation of BMSCs [65].
HOXA Cluster Antisense RNA 3
HOXA cluster antisense RNA 3 (HOXA-AS3) is located on the antisense strand of the HOXA gene cluster on chromosome 7. A large number of HOX genes act as transcriptional regulators to affect cell proliferation and differentiation [66]. HOXA-AS3 affects both adipogenic and osteogenic lineages, and knockdown of HOXA-AS3 in MSCs increases osteogenesis and the expression of osteogenic markers, including Runx2, SP7, and COL1A1 [67].
MIR31HG
Located on chromosome 9, MIR31 host gene (MIR31HG) plays an important role in cell growth and invasion in cancer [68, 69]. It is the host gene of miR-31, which maps to the first intron of MIR31HG. We previously showed that MIR31HG positively regulates the osteogenic differentiation of hADSCs and rescues the inflammation-induced inhibition of osteogenesis and bone formation [70].
AK141205/AK028326
AK141205 and AK028326 were identified as direct targets of key pluripotency transcription factors, Nanog and Oct4 [71]. Expression of AK141205 and CXC chemokine ligand-13 (CXCL13) is upregulated in osteogenic growth peptide (OGP)-triggered osteogenic differentiation of MSCs. Moreover, AK141205 promotes the osteogenic differentiation of MSCs mediated by increased expression of CXCL13 [72]. High glucose inhibits the osteogenic differentiation of MSCs by inhibiting CXCL13 expression through the lncRNA AK028326 [73].
TCONS_00046478/TCONS_00027225/TCONS_00007697
In a recent study, Song et al. identified 574 differentially expressed lncRNAs associated with the osteogenic differentiation of MSCs using high-throughput RNA-Seq data. Among them, three novel lncRNAs (TCONS_00046478, TCONS_00027225, and TCONS_00007697) were precursors of miR-689, miR-544, and miR-640 and regulated the expression of co-expressed genes (COL4A4, COL21A1, and WNT2) [38]. Their roles in osteogenic differentiation remain to be determined (Table 2).
lncRNA Regulation of Osteogenic Differentiation of BMSCs and ADSCs
In addition to the identification of lncRNAs differentially expressed during osteogenesis, several studies have revealed the mechanisms by which lncRNAs regulate osteogenic differentiation of BMSCs and ADSCs. Unlike miRNAs that are found primarily in the cytoplasm and typically repress gene expression through partial or complete complementary binding with the 3′UTRs of mRNA transcripts [74], lncRNAs, which are localised both in the nucleus and on chromatin, function through a diverse set of interactions with numerous components of the gene regulatory machinery [75]. In the next section, we will illuminate the regulatory functions of lncRNAs in osteoblast differentiation of BMSCs and ADSCs including chromatin modification, transcription factor binding, and miRNA regulation.
Regulation of Transcription by Chromatin Modification
lncRNAs modulate epigenetic changes by recruiting chromatin remodelling complexes to specific genomic loci and inducing DNA methylation and histone modifications [76, 77]. Some well-known epigenetic molecules have been shown to interact with lncRNAs. Enhancer of zeste homology 2 (EZH2) is a component of the polycomb repressive complex 2 (PRC2). The histone methyltransferase EZH2 trimethylates lysine 27 of histone 3 (H3K27me3) in target gene promoters to silence gene expression. EZH2 has been implicated in the lineage commitment of MSCs and epigenetically regulates the switch to osteogenesis and adipogenesis [78]. Some lncRNAs have recently been shown to regulate gene expression via interactions with EZH2, which indicates that these lncRNAs may potentially regulate osteogenesis [79]. The newly identified lncRNA ANCR is downregulated during stem cell differentiation [52]. According to Zhu and Xu, downregulation of ANCR promoted osteogenic differentiation by targeting EZH2 and regulating Runx2 expression (Fig. 1a), which suggests that ANCR is an important lncRNA involved in osteoblast differentiation [50]. The lncRNA HOXA-AS3 interacts with EZH2 to repress expression of the transcription factor Runx2 (Fig. 1a). Silencing of HOXA-AS3 in MSCs enhances osteogenesis and inhibits adipogenesis [67], which indicates that HOXA-AS3 may act as an epigenetic switch that determines the lineage specification of MSCs, in addition to its important role in the switch to osteogenesis and adipogenesis. Histone acetylation participates in a number of cellular processes including gene transcription, DNA replication, and chromatin condensation. The lncRNA HIF1α-AS1 increases HOXD10 expression by promoting histone acetylation, which enhances osteogenic differentiation of BMSCs. In addition, a regulatory network is established in response to the inhibition of TGF-β when sirtuin 1 expression is decreased in BMSCs [65], followed by upregulation of HIF1α-AS1, which then increases HOXD10 expression and promotes the osteogenesis of BMSCs (Fig. 1c). The lncRNA AK141205 was identified as a direct target of Nanog, a transcription factor important for pluripotency [80]. AK141205 in upregulated in OGP-triggered osteogenic differentiation of MSCs [72]. Mechanistically, AK141205 positively regulates the expression of CXCL13 by acetylating the promoter of histone H4, promoting osteoblast differentiation (Fig. 1b). A considerable proportion of lncRNAs expressed in a particular cell type are physically and functionally associated with chromatin-modifying proteins, such as PRC2 [81]. Based on the abovementioned research, it has been suggested that many lncRNAs collaborate with chromatin-modifying proteins to repress gene expression at specific loci, but further research is needed to confirm this hypothesis.
lncRNAs regulate osteogenic differentiation by chromatin modification. a Downregulation of ANCR/ HOXA-AS3 promotes osteogenic differentiation by targeting EZH2 and regulating Runx2 expression. b AK141205 positively regulates CXCL13 expression by acetylating histone H4 in the promoter region and promoting osteoblast differentiation. c Inhibition of TGF-β leads to decreased SIRT1 expression in BMSCs, followed by upregulation of HIF1α-AS1, which then increases HOXD10 expression by promoting histone acetylation and promoting the osteogenesis of BMSCs
Regulation by Binding to Transcription Factors
lncRNAs regulate gene transcription in several ways. For instance, lncRNAs bind transcription factor RNAs to modulate transcription factor activity and regulate transcription. MEG3 has been extensively studied as a tumour suppressor, which has an important regulatory role in various types of human tumours [59, 82]. Recently, its biological role and the mechanism by which it regulates the osteogenesis of MSCs have been revealed. Overexpression of MEG3 promoted the osteogenic differentiation of MSCs from patients with MM by targeting BMP4 transcription [60]. MEG3, which is located near the BMP4 gene, is thought to dissociate the transcription factor SRY-box 2 (SOX2) from the BMP4 promoter to increase expression of the BMP4 gene (Fig. 2a). Another lncRNA, MIR31HG, mediates osteogenic differentiation by binding to transcription factors upon exposure to an inflammatory microenvironment. MIR31HG knockdown enhances osteogenic differentiation and rescues the inflammation-induced inhibition of osteogenesis in hADSCs via a nuclear factor (NF)-κB regulatory feedback circuit [70]. NF-κB upregulates MIR31HG expression via the direct binding of p65 to the MIR31HG promoter. MIR31HG in turn directly binds to IκBα, contributing to IκBα phosphorylation and NF-κB activation and ultimately inhibiting osteogenic differentiation (Fig. 2b). In recent years, an increasing amount of attention has been paid to lncRNA–protein interactions. In particular, lncRNAs form lncRNA–protein complexes with transcription factors and modulate their activity. The diverse transcriptional regulatory mechanisms important in the osteogenic differentiation of MSCs remain to be explored.
lncRNAs regulate osteogenic differentiation by binding to transcription factors. a MEG3 dissociates the transcription factor SOX2 from the BMP4 promoter to increase expression of BMP4 and promote osteogenic differentiation of MSCs. b MIR31HG directly binds to IκBα, contributing to IκBα phosphorylation and NF-κB activation and ultimately inhibiting osteogenic differentiation. In turn, NF-κB directly binds p65 at the MIR31HG promoter and upregulates MIR31HG expression
Mirna-Related lncRNAs
A widespread network of crosstalk between coding and non-coding RNAs that regulate one another via competition for miRNA binding sites has been identified. Based on this network, the competing endogenous RNA hypothesis has been proposed [83]. Certain lncRNAs base pair with small RNAs and effectively deplete miRNAs by acting as sponges or decoys [84, 85]. In addition, some lncRNAs act as precursors for miRNAs to regulate gene expression [86]. The maternally expressed and paternally imprinted lncRNA H19 (2.7 kb) is a primary miRNA precursor of miR-675. We previously showed that H19/miR-675/TGF-β1/Smad3/HDAC signalling pathway plays a significant role in the osteogenic differentiation of human MSCs [41]. The expression of H19 and miR-675 was significantly increased during the osteogenic differentiation of hMSCs, which indicates that H19 promotes osteogenic differentiation at least partially through miR-675. Moreover, miR-675 downregulated expression of TGF-β1 and subsequently suppressed phosphorylation of Smad3. Meanwhile, miR-675 negatively regulated HDAC 4/5, inhibiting the recruitment of HDAC(s) to the Runx2-bound DNA sequence and subsequently enhancing osteogenic differentiation (Fig. 3a). Consistent with this, Liang et al. revealed that H19 acts as a miRNA sponge to inhibit the endogenous functions of miR-141 and miR-22 [42], both of which downregulate β-catenin expression. As a result, H19 enhances osteogenesis through direct activation of the Wnt/β-catenin pathway (Fig. 3b). In addition, a novel regulatory feedback loop between H19 and its encoded miRNA has been proposed in which miR-675-5p directly targets H19 and inhibits osteoblast differentiation. These two studies have improved our understanding of the key role of H19 in promoting osteogenesis, whereas analysis of the expression of miRNA-675 yielded contrasting results. The second study identified uncoordinated expression patterns of miRNA-675 and H19. The authors concluded that the excision of miR-675 from exon 1 of H19 is dynamically regulated by the RNA binding protein HuR, although little is known about the expression patterns of RNA binding proteins during osteogenesis [42]. Therefore, the functions of H19 have been divided into two major categories: a reservoir for miR-675 that suppresses its target genes, and a regulator of miRNAs or proteins through binding. A recent study showed that the expression of miR-133a-3p was positively correlated with MEG3 expression in PMOP-derived BMSCs [62]. After further investigation, the researchers found that overexpression of MEG3 downregulated SLC39A1 expression by directly binding to miR-133a-3p and elevating its expression, leading to inhibition of osteogenic differentiation of BMSCs and inducing PMOP (Fig. 3c). Moreover, our group found that the expression of miR-140-5p was inversely correlated with MEG3 expression and elucidated that MEG3 may participate in the osteogenic differentiation of hADSCs at least partially via miR-140-5p [61]. HOTAIR, the first trans-acting lncRNA discovered, has been extensively studied in a range of human diseases [87]. Recently, it was confirmed to play a novel role in osteogenic differentiation and proliferation by modulating the expression of miR-17-5p and its target gene SMAD7 in non-traumatic osteonecrosis of the femoral head [57]. Downregulation of HOTAIR led to decreased methylation of the miR-17-5p promoter and increased expression of miR-17-5p. Subsequently, the expression of SMAD7, a target gene of miR-17-5p, decreased and osteogenic differentiation was ultimately promoted (Fig. 3d). Interestingly, HOTAIR binds to the histone H3K27 methylase PRC2, guiding PRC2 to specific regions of the HOXD locus to negatively regulate gene expression [13]. This mechanism suggests that HOTAIR may regulate the osteogenic differentiation of MSCs through another epigenetic pathway in addition to interacting with miRNAs. Some lncRNAs regulate post-transcriptional processes by binding to miRNAs. During the past few years, miRNAs have been extensively studied, and remarkable progress has been made in determining their role in osteoblast differentiation and bone formation. With their complexity in structure and diversity in function, lncRNAs have been postulated to play a leading role and have a greater influence on the regulation of osteoblast differentiation of MSCs than miRNAs.
Schematic illustration of miRNA-related mechanisms. a MiR-675 downregulates TGF-β1 expression and subsequently suppresses Smad3 phosphorylation. Meanwhile, miR-675 inhibits the recruitment of HDAC(s) to the Runx2-bound DNA sequence and subsequently promotes osteogenic differentiation. b H19 downregulates β-catenin expression and inhibits osteogenic differentiation by acting as a miRNA sponge of miR-141 and miR-22. MiR-675 directly targets H19 and inhibits osteoblast differentiation. c MEG3 overexpression downregulates SLC39A1 expression by directly binding to miR-133a-3p and elevating its expression, resulting in inhibition of osteogenic differentiation. d Downregulation of HOTAIR leads to reduced methylation of the miR-17-5p promoter and increased miR-17-5p expression. Subsequently, the expression of SMAD7, a target gene of miR-17-5p, was decreased and osteogenic differentiation was ultimately promoted
In fact, compared to the diverse functions of lncRNAs that have already been reported, the researches on the mechanisms by which lncRNAs regulate MSC osteogenic differentiation may seem less sufficient. Many more aspects of lncRNAs remain to be explored in the future.
Future Prospects
lncRNAs are emerging as new stars in cellular processes and display great potential in bone tissue engineering. On one hand, they function as biomarkers for the detection of early states of bone disease. On the other hand, they are candidate drug targets for intervention and regeneration in bone disorders. Furthermore, lncRNAs are abundantly expressed in exosomes [88, 89], which are spherical membrane vesicles secreted from multi-vesicular endosomes by a variety of cell types [90]. Exosomes function as transport vesicles for functional lncRNAs, which has offered new insights into the regulatory mechanisms of cell development, the immune system, and tumour metastasis [89, 91]. With large amounts of lncRNAs, exosomes will be applied as a mediator to further investigate the molecular mechanisms of osteogenic differentiation in MSCs. Nevertheless, many important questions remain. Not all lncRNAs downregulated during osteogenic differentiation necessarily function in the sophisticated regulatory network. Therefore, the identification of potentially functional lncRNAs will be a challenging task. In addition, more work still needs to be done to characterise the specific roles of lncRNAs and the concrete mechanisms by which they modulate the osteogenic differentiation of MSCs.
Conclusions
During the past few years, a variety of lncRNAs have been shown to be involved in the osteogenic differentiation of MSCs in a wide repertoire of cellular contexts. In this review, we summarised the lncRNAs that are reportedly involved in the osteogenic differentiation of BMSCs and ADSCs. In addition, we discussed their molecular regulatory mechanisms in chromatin modification, transcription factor binding, and interactions with miRNAs, providing a theoretical and experimental basis for clinical applications in bone tissue engineering. With the development of high throughput and next-generation sequencing technologies, the roles of lncRNAs as regulators of biological processes have attracted increasing attention. However, additional research will be necessary to better illuminate the structures and specific mechanisms by which lncRNAs regulate both normal and pathological conditions. We expect that the use of these and other future techniques will enable researchers to finally uncover the fascinating aspects of lncRNAs and make further progress in conquering human disease.
References
Djebali, S., Davis, C. A., Merkel, A., et al. (2012). Landscape of transcription in human cells. Nature, 489(7414), 101–108.
Esteller, M. (2011). Non-coding RNAs in human disease. Nature Reviews Genetics, 12(12), 861 – 74.
Fu, X. D. (2014). Non-coding RNA: a new frontier in regulatory biology. National Science Review, 1, 190–204.
Sone, M., Hayashi, T., Tarui, H., et al. (2007). The mRNA-like noncoding RNA Gomafu constitutes a novel nuclear domain in a subset of neurons. Journal of Cell Science, 120(Pt 15), 2498 – 506.
Guttman, M., Donaghey, J., Carey, B. W., et al. (2011). LincRNAs act in the circuitry controlling pluripotency and differentiation. Nature, 477(7364), 295–300.
Wapinski, O., & Chang, H. Y. (2011). Long noncoding RNAs and human disease. Trends in Cell Biology, 21(6), 354 – 61.
Guttman, M., & Rinn, J. L. (2012). Modular regulatory principles of large non-coding RNAs. Nature, 482(7385), 339 – 46.
Ma, L., Bajic, V. B., & Zhang, Z. (2013). On the classification of long non-coding RNAs. RNA Biology, 10(6), 925 – 33.
Cabili, M. N., Trapnell, C., Goff, L., et al. (2011). Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes & Development, 25(18), 1915–1927.
Ulitsky, I., & Bartel, D. P. (2013). lincRNAs: genomics, evolution, and mechanisms. Cell, 154(1), 26–46.
Bartolomei, M. S., Zemel, S., & Tilghman, S. M. (1991). Parental imprinting of the mouse H19 gene. Nature, 351(6322), 153–155.
Willingham, A. T., Orth, A. P., Batalov, S., et al. (2005). A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science, 309(5740), 1570–1573.
Rinn, J. L., Kertesz, M., Wang, J. K., et al. (2007). Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell, 129(7), 1311–1323.
Martianov, I., Ramadass, A., Serra Barros, A., Chow, N., & Akoulitchev, A. (2007). Repression of the human dihydrofolate reductase gene by a non-coding interfering transcript. Nature, 445(7128), 666 – 70.
Lee, J. T. (2009). Lessons from X-chromosome inactivation: long ncRNA as guides and tethers to the epigenome. Genes & Development, 23(16), 1831–1842.
Lv, D., Sun, R., Yu, Q., & Zhang, X. (2016). The long non-coding RNA maternally expressed gene 3 activates p53 and is downregulated in esophageal squamous cell cancer. Tumour Biology.
Rumman, M., Dhawan, J., & Kassem, M. (2015). Concise review: quiescence in adult stem cells: biological significance and relevance to tissue regeneration. Stem Cells, 33, 2903–2912.
Bianco, P., Riminucci, M., Gronthos, S., & Robey, P. G. (2001) Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells, 19, pp. 180 – 92.
Pittenger, M. F., Mackay, A. M., Beck, S. C., et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science, 284(5411), 143–147.
da Silva Meirelles, L., Chagastelles, P. C., & Nardi, N. B. (2006). Mesenchymal stem cells reside in virtually all post-natal organs and tissues. Journal of Cell Science, 119, 2204–2213.
Quarto, R., Mastrogiacomo, M., Cancedda, R., et al. (2001). Repair of large bone defects with the use of autologous bone marrow stromal cells. The New England Journal of Medicine, 344(5), 385–386.
Rastegar, F., Rastegar, F., Shenaq, D., et al. (2010). Mesenchymal stem cells: molecular characteristics and clinical applications. World Journal of Stem Cells, 2(4), 67–80.
Gaur, T., Lengner, C. J., Hovhannisyan, H., et al. (2005). Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. The Journal of Biological Chemistry, 280(39), 33132–33140.
Chen, G., Deng, C., & Li, Y. P. (2012). TGF-beta and BMP signaling in osteoblast differentiation and bone formation. International Journal of Biological Sciences, 8(2), 272 – 88.
Deng, Z. L., Sharff, K. A., Tang, N., et al. (2008). Regulation of osteogenic differentiation during skeletal development. Frontiers in Bioscience, 13, 2001–2021.
Javed, A., Bae, J. S., Afzal, F., et al. (2008). Structural coupling of Smad and Runx2 for execution of the BMP2 osteogenic signal. The Journal of Biological Chemistry, 283(13), 8412–8422.
Hong, J. H., & Yaffe, M. B. (2006). TAZ: a beta-catenin-like molecule that regulates mesenchymal stem cell differentiation. Cell Cycle, 5(2), 176–179.
Chen, Q., Shou, P., Zheng, C., et al. (2016). Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death and Differentiation, 23(7), 1128–1139.
Li, H., Li, T., Fan, J., et al. (2015). miR-216a rescues dexamethasone suppression of osteogenesis, promotes osteoblast differentiation and enhances bone formation, by regulating c-Cbl-mediated PI3K/AKT pathway. Cell Death and Differentiation, 22(12), 1935–1945.
Zhang, J. F., Fu, W. M., He, M. L., et al. (2011). MiRNA-20a promotes osteogenic differentiation of human mesenchymal stem cells by co-regulating BMP signaling. RNA Biology, 8(5), 829 – 38.
Huang, S., Wang, S., Bian, C., et al. (2012). Upregulation of miR-22 promotes osteogenic differentiation and inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells by repressing HDAC6 protein expression. Stem Cells and Development, 21(13), 2531–2540.
Wang, J., Guan, X., Guo, F., et al. (2013). miR-30e reciprocally regulates the differentiation of adipocytes and osteoblasts by directly targeting low-density lipoprotein receptor-related protein 6. Cell Death & Disease, 4, e845.
Li, Z., Hassan, M. Q., Volinia, S., et al. (2008). A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proceedings of the National Academy of Sciences of the United States of America, 105(37), 13906–13911.
Zhang, W., Dong, R., Diao, S., Du, J., Fan, Z., & Wang, F. (2017). Differential long noncoding RNA/mRNA expression profiling and functional network analysis during osteogenic differentiation of human bone marrow mesenchymal stem cells. Stem Cell Research & Therapy, 8, 30.
Tye, C. E., Gordon, J. A., Martin-Buley, L. A., Stein, J. L., Lian, J. B., & Stein, G. S. (2015). Could lncRNAs be the missing links in control of mesenchymal stem cell differentiation? Journal of Cellular Physiology, 230(3), 526 – 34.
Wang, L., Wang, Y., Li, Z., & Yu, B. (2015). Differential expression of long noncoding ribonucleic acids during osteogenic differentiation of human bone marrow mesenchymal stem cells. International Orthopaedics, 39(5), 1013–1019.
Zuo, C., Wang, Z., Lu, H., Dai, Z., Liu, X., & Cui, L. (2013). Expression profiling of lncRNAs in C3H10T1/2 mesenchymal stem cells undergoing early osteoblast differentiation. Molecular Medicine Reports, 8(2), 463–467.
Song, W. Q., Gu, W. Q., Qian, Y. B., Ma, X., Mao, Y. J., & Liu, W. J. (2015). Identification of long non-coding RNA involved in osteogenic differentiation from mesenchymal stem cells using RNA-Seq data. Genetics and Molecular Research, 14(4), 18268–18279.
Cui, Y., Lu, S., Tan, H., Li, J., Zhu, M., & Xu, Y. (2016). Silencing of long non-coding RNA NONHSAT009968 ameliorates the staphylococcal protein a-inhibited osteogenic differentiation in human bone mesenchymal stem cells. Cellular Physiology and Biochemistry, 39(4), 1347–1359.
Cai, X., & Cullen, B. R. (2007). The imprinted H19 noncoding RNA is a primary microRNA precursor. RNA, 13(3), 313–316.
Huang, Y., Zheng, Y., Jia, L., & Li, W. (2015). Long noncoding RNA H19 promotes osteoblast differentiation via TGF-beta1/Smad3/HDAC signaling pathway by deriving miR-675. Stem Cells, 33(12), 3481–3492.
Liang, W. C., Fu, W. M., Wang, Y. B., et al. (2016). H19 activates Wnt signaling and promotes osteoblast differentiation by functioning as a competing endogenous RNA. Scientific Reports, 6, 20121.
Ohnishi, Y., Tanaka, T., Yamada, R., et al. (2000). Identification of 187 single nucleotide polymorphisms (SNPs) among 41 candidate genes for ischemic heart disease in the Japanese population. Human Genetics 106(3), 288 – 92.
Vausort, M., Wagner, D. R., & Devaux, Y. (2014). Long noncoding RNAs in patients with acute myocardial infarction. Circulation Research, 115(7), 668 – 77.
Yan, B., Yao, J., Liu, J. Y., et al. (2015). lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circulation Research, 116(7), 1143–1156.
Barry, G., Briggs, J. A., Vanichkina, D. P., et al. (2014). The long non-coding RNA Gomafu is acutely regulated in response to neuronal activation and involved in schizophrenia-associated alternative splicing. Molecular Psychiatry, 19(4), 486 – 94.
Jin, C., Zheng, Y., Huang, Y., Liu, Y., Jia, L., Zhou, Y., et al. (2017). Long non-coding RNA MIAT knockdown promotes osteogenic differentiation of human adipose-derived stem cells. Cell Biology International, 41(1), 33–41.
Shen, Y., Dong, L. F., Zhou, R. M., et al. (2016). Role of long non-coding RNA MIAT in proliferation, apoptosis and migration of lens epithelial cells: a clinical and in vitro study. Journal of Cellular and Molecular Medicine, 20(3), 537 – 48.
Kretz, M., Webster, D. E., Flockhart, R. J., et al. (2012). Suppression of progenitor differentiation requires the long noncoding RNA ANCR. Genes & Development, 26(4), 338 – 43.
Zhu, L., & Xu, P. C. (2013). Downregulated LncRNA-ANCR promotes osteoblast differentiation by targeting EZH2 and regulating Runx2 expression. Biochemical and Biophysical Research Communications, 432(4), 612–617.
Jia, Q., Jiang, W., & Ni, L. (2015). Down-regulated non-coding RNA (lncRNA-ANCR) promotes osteogenic differentiation of periodontal ligament stem cells. Archives of Oral Biology, 60(2), 234 – 41.
Jia, Q., Chen, X., Jiang, W., Wang, W., Guo, B., & Ni, L. (2016). The Regulatory Effects of Long Noncoding RNA-ANCR on Dental Tissue-Derived Stem Cells. Stem Cells International, 2016, 3146805.
Chisholm, K. M., Wan, Y., Li, R., Montgomery, K. D., Chang, H. Y., & West, R. B. (2012). Detection of long non-coding RNA in archival tissue: correlation with polycomb protein expression in primary and metastatic breast carcinoma. PLoS One, 7(10), e47998.
Endo, H., Shiroki, T., Nakagawa, T., et al. (2013). Enhanced expression of long non-coding RNA HOTAIR is associated with the development of gastric cancer. PLoS One, 8(10), e77070.
Yang, Z., Zhou, L., Wu, L. M., et al. (2011). Overexpression of long non-coding RNA HOTAIR predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Annals of Surgical Oncology, 18(5), 1243–1250.
Xing, D., Liang, J. Q., Li, Y., et al. (2014). Identification of long noncoding RNA associated with osteoarthritis in humans. Orthopaedic Surgery, 6(4), 288 – 93.
Wei, B., Wei, W., Zhao, B., Guo, X., & Liu, S. (2017). Long non-coding RNA HOTAIR inhibits miR-17-5p to regulate osteogenic differentiation and proliferation in non-traumatic osteonecrosis of femoral head. PLoS One, 12(2), e0169097.
Anwar, S. L., Krech, T., Hasemeier, B., et al. (2012). Loss of imprinting and allelic switching at the DLK1-MEG3 locus in human hepatocellular carcinoma. PloS One, 7, e49462.
Zhou, Y., Zhang, X., & Klibanski, A. (2012). MEG3 noncoding RNA: a tumor suppressor. Journal of Molecular Endocrinology, 48(3), R45-53.
Zhuang, W., Ge, X., Yang, S., et al. (2015). Upregulation of lncRNA MEG3 promotes osteogenic differentiation of mesenchymal stem cells from multiple myeloma patients by targeting BMP4 transcription. Stem Cells, 33(6), 1985–1997.
Li, Z., Jin, C., Chen, S., et al. (2017). Long non-coding RNA MEG3 inhibits adipogenesis and promotes osteogenesis of human adipose-derived mesenchymal stem cells via miR-140-5p. Molecular and Cellular Biochemistry.
Wang, Q., Li, Y., Zhang, Y., et al. (2017). LncRNA MEG3 inhibited osteogenic differentiation of bone marrow mesenchymal stem cells from postmenopausal osteoporosis by targeting miR-133a-3p. Biomed Pharmacother, 89, 1178–1186.
Zhao, Y., Feng, G., Wang, Y., Yue, Y., & Zhao, W. (2014). Regulation of apoptosis by long non-coding RNA HIF1A-AS1 in VSMCs: implications for TAA pathogenesis. International Journal of Clinical and Experimental Pathology, 7(11), 7643–7652.
Wang, S., Zhang, X., Yuan, Y., et al. (2015). BRG1 expression is increased in thoracic aortic aneurysms and regulates proliferation and apoptosis of vascular smooth muscle cells through the long non-coding RNA HIF1A-AS1 in vitro. European Journal of Cardio-Thoracic Surgery, 47(3), 439–46.
Xu, Y., Wang, S., Tang, C., & Chen, W. (2015). Upregulation of long non-coding RNA HIF 1alpha-anti-sense 1 induced by transforming growth factor-beta-mediated targeting of sirtuin 1 promotes osteoblastic differentiation of human bone marrow stromal cells. Molecular Medicine Reports, 12(5), 7233–7238.
Wang, Y., Dang, Y., Liu, J., & Ouyang, X. (2016). The function of homeobox genes and lncRNAs in cancer. Oncology Letters, 12(3), 1635–1641.
Zhu, X. X., Yan, Y. W., Chen, D., et al. (2016). Long non-coding RNA HoxA-AS3 interacts with EZH2 to regulate lineage commitment of mesenchymal stem cells. Oncotarget, 7(39), 63561–63570.
Augoff, K., McCue, B., Plow, E. F., & Sossey-Alaoui, K. (2012). miR-31 and its host gene lncRNA LOC554202 are regulated by promoter hypermethylation in triple-negative breast cancer. Molecular Cancer, 11, 5.
Yang, H., Liu, P., Zhang, J., et al. (2016). Long noncoding RNA MIR31HG exhibits oncogenic property in pancreatic ductal adenocarcinoma and is negatively regulated by miR-193b. Oncogene, 35(28), 3647–3657.
Jin, C., Jia, L., Huang, Y. et al. (2016). Inhibition of lncRNA MIR31HG promotes osteogenic differentiation of human adipose-derived stem cells. Stem Cells.
Sheik Mohamed, J., Gaughwin, P. M., Lim, B., Robson, P., & Lipovich, L. (2010). Conserved long noncoding RNAs transcriptionally regulated by Oct4 and Nanog modulate pluripotency in mouse embryonic stem cells. RNA, 16(2), 324 – 37.
Li, H., Li, H., Zhang, Z., Chen, Z., & Zhang, D. (2015). Osteogenic growth peptide promotes osteogenic differentiation of mesenchymal stem cells mediated by LncRNA AK141205-induced upregulation of CXCL13. Biochemical and Biophysical Research Communications, 466(1), 82 – 8.
Cao, B., Liu, N., & Wang, W. (2016). High glucose prevents osteogenic differentiation of mesenchymal stem cells via lncRNA AK028326/CXCL13 pathway. Biomedicine and Pharmacotherapy, 84, 544–551.
Pasquinelli, A. E. (2012). MicroRNAs and their targets: recognition, regu- lation and an emerging reciprocal relationship. Nature Reviews Genetics, 13, 271–282.
Quinn, J. J., & Chang, H. Y. (2016). Unique features of long non-coding RNA biogenesis and function. Nature Reviews Genetics, 17, 47–62.
Egger, G., et al. (2004). Epigenetics in human disease and prospects for epigenetic therapy. Nature, 429(6990), 457 –63.
Helin, K., & Dhanak, D. (2013). Chromatin proteins and modifications as drug targets. Nature, 502(7472), 480–488.
Hemming, S., Cakouros, D., senmann, S., et al. (2014). EZH2 and KDM6A act as an epigenetic switch to regulate mesenchymal stem cell lineage specification. Stem Cells, 32(3), 802–15.
Guil, S., Soler, M., Portela, A., et al. (2012). Intronic RNAs mediate EZH2 regulation of epigenetic targets. Nature Structural and Molecular Biology, 19(7), 664–70.
Ghosal, S., Das, S., & Chakrabarti, J. (2013). Long noncoding RNAs: new players in the molecular mechanism for maintenance and differentiation of pluripotent stem cells. Stem Cells and Development, 22(16), 2240–2253.
Khalil, A. M., Guttman, M., Huarte, M., et al. (2009). Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proceedings of the National Academy of Sciences of the United States of America, 106(28), 11667–11672.
Miyoshi, N., Wagatsuma, H., Wakana, S., et al. (2000). Identification of an imprinted gene, Meg3/Gtl2 and its human homologue MEG3, first mapped on mouse distal chromosome 12 and human chromosome 14q. Genes to Cells, 5(3), 211–20.
Tay, Y., Rinn, J., & Pandolfi, P. P. (2014). The multilayered complexity of ceRNA crosstalk and competition. Nature, 505(7483), 344–52.
Xing, C. Y., Hu, X. Q., Xie, F. Y., et al. (2015). Long non-coding RNA HOTAIR modulates c-KIT expression through sponging miR-193a in acute myeloid leukemia. FEBS Letters, 589(15), 1981–1987.
Cai, H., Xue, Y., Wang, P., et al. (2015). The long noncoding RNA TUG1 regulates blood-tumor barrier permeability by targeting miR-144. Oncotarget, 6(23), 19759–19779.
Keniry, A., et al. (2012). The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nature Cell Biology, 14(7), 659–65.
Wu, Y., et al. (2014). Long noncoding RNA HOTAIR involvement in cancer. Tumour Biology, 35(10), 9531–9538.
Kogure, T., Yan, I. K., Lin, W. L., & Patel, T. (2013). Extracellular vesicle-mediated transfer of a novel long noncoding RNA TUC339: a mechanism of intercellular signaling in human hepatocellular cancer. Genes Cancer, 4(7–8), 261–72.
Conigliaro, A., Costa, V., Lo Dico, A., et al. (2015). CD90 + liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Molecular Cancer, 14, 155.
Thery, C., Amigorena, S., Raposo, G., & Clayton, A. (2006). Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Current Protocols in Cell Biology, 3, Unit 3 22.
Hewson, C., & Morris, K. V. (2016). Form and Function of Exosome-Associated Long Non-coding RNAs in Cancer. Current Topics in Microbiology and Immunology, 394, 41–56.
Acknowledgements
This study was financially supported by grants from the National Natural Science Foundation of China (Nos. 81670957, 81772876, and 81700938), a grant from the Peking University (PKU) School and Hospital of Stomatology (No. PKUSS20140104), and seed grants from the PKU School of Stomatology for PostDoc (No. YS0203) and the Beijing Natural Science Foundation (No. 7172239).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Yang, Q., Jia, L., Li, X. et al. Long Noncoding RNAs: New Players in the Osteogenic Differentiation of Bone Marrow- and Adipose-Derived Mesenchymal Stem Cells. Stem Cell Rev and Rep 14, 297–308 (2018). https://doi.org/10.1007/s12015-018-9801-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-018-9801-5