Abstract
Evidences have been provided that long noncoding RNAs (lncRNAs) act as key molecules in epigenetic regulation and are involved in the development process of cancer in recent studies. HOX transcript antisense RNA (HOTAIR), a long intergenic noncoding RNA (lincRNA), functions as a molecular scaffold to link and target two histone modification complexes PRC2 and LSD1, then reprograms chromatin states by couples histone H3K27 methylation and H3K4 demethylation for epigenetic gene silencing to promote cancer metastasis. HOTAIR, regarded as an oncogene, is pervasively overexpressed in most solid cancers and correlated with tumor invasion, progression, metastasis, and poor prognosis, and HOTAIR has been proven to play a critical role in most biological process of cancer and would be a potential new target in cancer therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long noncoding RNAs (lncRNAs) have been found to be pervasively transcribed in the genome recently [1, 2]. They are transcripts longer than 200 nts and without a functional open reading frame (ORF) in most cases [3]. But they might play widespread roles in gene regulation and other cellular processes, including their involvement in the integrity of epigenetic regulation, gene transcription, and post-transcription regulation. The recent studies also suggest that lncRNAs are involved in the development of human diseases, particularly in cancer [4–8]. Among all kinds lncRNAs, HOX transcript antisense RNA (HOTAIR), a paradigm for long noncoding RNA function in cancer, is transcribed from the HOXC cluster in an antisense manner, functions as a molecular scaffold to link polycomb repressive complex 2 (PRC2) and lysine-specific demethylase 1/REST corepressor 1/RE1-silencing transcription factor (LSD1/CoREST/REST) protein complexes, and regulates gene expression by mediating the modulation of chromatin structures in trans across the 40-kb HOXD locus2 [9–11]. Evidences have been provided that HOTAIR is pervasively overexpressed in many cancers and involved in the process of the tumor invasion, progression, metastasis, and poor prognosis in vitro and in vivo. Here, we summarize the current knowledge about HOTAIR and introduce its biogenesis and regulation mechanism and its functions in different cancers.
Discovery, identification, and characterization of HOTAIR
HOTAIR, which is about 2.2 k nucleotides, spliced, and polyadenylated transcript, was originally discovered by Rinn and colleagues in 2007 and named it HOTAIR for HOX antisense intergenic RNA [12]. It has only one strand transcribed antisense to HOXC genes and lacks protein-coding potential. HOTAIR is transcribed from the HOXC locus on chromosome 12 and represses transcription in trans across 40 kb of the HOXD locus on chromosome 2 by interacting and recruiting the PRC2 and LSD1/CoREST/REST complexes.
HOTAIR has poorly conserved sequences and considerably conserved structures, and exons of HOTAIR show distinct evolutionary features in mammals. HOTAIR evolved faster than nearby HOXC genes, and the HOTAIR exons exist only in mammals. Of the six HOTAIR exons, exon 1, exon 3, exon 4, exon 5, and domain B of exon 6 are better conserved than exon 2 and domain A of exon 6, such as exon 2 is absent in mouse, rat, and kangaroo, and a 239-bp domain is especially conserved in exon 6 in mammals [7, 13]. In human genome, there are five short exons (exon 1 to exon 5) and one long exon (exon 6) which divided into domains A and B of exon 6 [14]. PRC2 binding activity mapped to nucleotides 1 to 300 of HOTAIR, while the LSD1 complex binding activity mapped to nucleotides 1500 to 2146 [10]. Therefore, two fragments in the 5′ end of exon 1 and the 3′ end domain B of exon 6 may have invariable and special sequence and structure binding to PRC2 and LSD1. However, the secondary structure of HOTAIR did not reveal obvious stem loops suggestive of premiRNAs [14].
Biogenesis and regulation of HOTAIR
HOTAIR is a long intergenic noncoding RNA (lincRNA), which is one kind of lncRNAs classified by the manner of transcription. At present, little is known about their biological roles and how they carry out those roles of lincRNAs. However, based on a few relatively well-studied examples, several potential mechanisms for lincRNAs have been reported [15]. The functions of lncRNA include cis-tether, trans-regulation, allosteric modification, decoy, etc. [16]. In contrast to the group of cis-regulatory lncRNAs, one example of lncRNA HOTAIR alerts its transcriptional effects across chromosomes in trans [12]. HOTAIR binding sites that were found occur on multiple chromosomes and are enriched in genic regions, notably enhancers and introns, when 832 HOTAIR occupancy sites genome-wide were identified by ChIRP-seq, and these binding sites were enriched in GA-rich homopurine motif [9]. As we know, HOTAIR represses transcription in trans across 40 kb of the HOXD locus and maps to the intergenic region between HOXD3 and HOXD4, in which domains both H3K27me3 and SUZ12 occupancies were lost corresponding to the HOTAIR depletion [10, 11]. HOTAIR ChIRP peaks show focal HOTAIR peaks in association with broad domains PRC2 occupancy and H3K27me3. So in fact, HOTAIR could be regarded as an active recruiter of chromatin-modifying complexes and recruit PRC2 to its targets. PRC2 is specifically required for HOTAIR to promote cellular invasiveness. Enforced expression of HOTAIR in breast cancer cell lines induced localization of H3K27me3 and PRC2 subunits SUZ12 and EZH2 on 854 new genes, and these HOTAIR-PRC2 target genes are coordinately downregulated in aggressive breast tumors [11].
Evidences suggest that HOTAIR epigenetics regulates gene transcription by competing with other molecules, such as BRCA1. HOTAIR binds to and recruits EZH2 occupancy on its target gene loci [11]. And HOTAIR binds to EZH2 in a region that is known to be competitively bound by BRCA1. Overexpression of HOTAIR increases the binding of EZH2 to the target gene HOXA9 promoter in breast cancer cells; however, forced expression of BRCA1 blocks this function by inhibiting the binding of HOTAIR to EZH2 and abolishes HOTAIR-enhanced recruitment of PRC2 to its target genes. The similar results were obtained in mouse embryonic stem (ES) cells, which suggested that lncRNA HOTAIR binding to EZH2 could be involved in the mechanisms of BRCA1 mediating PRC2 targeting in both ES and breast cancer cells [17].
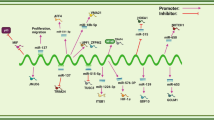
HOTAIR is a modular bifunctional RNA and interacts with PRC2 and LSD1 complexes through its 5′ and 3′ binding domains, respectively, and the domains possess extensive but distinct secondary structures. Only less than 5 % of the two complexes that physically interact with each other indicated that HOTAIR bridges the two complexes. Moreover, a higher-order large complex including HOTAIR, PRC2, and LSD1 was formed since enforced expression of HOTAIR shifts the PRC2 submits in FL-HeLa cells. HOTAIR knockdown decreased SUZ12 and LSD1 occupancy, and correspondingly, H3K27me3 loss and H3K4me2 gain in the region proximal promoters of HOXD genes, and ultimately, H3K27 methylation and H3K4 demethylation influence epigenetic silencing of HOXD genes (Fig. 1) [10]. These epigenetic changes result in a series of upregulation or downregulation genes altering and corresponding changes of cancer cell proliferation, invasion, apoptosis, and migration. These facts have been proven in breast cancer [11], colorectal cancer [18], pancreatic cancer [19], etc. (Table 1).
HOTAIR is transcribed from the HOXC cluster in an antisense manner, functions as a molecular scaffold to link PRC2 complex and LSD1 complexes, and regulates gene expression by mediating the modulation of chromatin structures in trans across the 40-kb HOXD locus2. Then, it regulates the metastasis-related genes and promotes cancer metastasis
HOTAIR in human cancer
Breast cancer
HOTAIR expression involvement in human cancer was first found in breast cancer. In 2010, Gupta A et al. [11] found that the expression of HOTAIR in primary breast tumors and metastasis tissue was higher than that in normal breast epithelia, and high HOTAIR level was a significant predictor of metastasis and poor survival. Then, enforced expression of HOTAIR in breast carcinoma cells promoted cell invasion in vivo and lung metastasis in vitro and induced the genome-wide re-targeting of PRC2, altered H3K27 methylation pattern, and therefore altered target gene expression [11]. HOTAIR was highly correlated with EZH2, one of the PRC2 complex subunits, and associated with a worse outcome. HOTAIR overexpression was related to estrogen receptor (ER) and progesterone receptor (PR) positivity [20]. Moreover, HOTAIR is transcriptionally induced by estradiol (E2) in breast cancer and is an estrogen-responsive gene, E2, coordinated by ERs, ER coregulators, chromatin modification marks such as histone H3K4 trimethylation and histone acetylation, and general transcription factors associated with RNAP II transcription, play critical roles in the antisense transcription activation of HOTAIR [21]. Therefore, being crucial for the growth, viability and invasion of breast cancer cell, reducing apoptosis [21], promoting metastasis, and predicting poor survival [11], HOTAIR may be a potential target for novel therapy of breast cancer [21].
Colorectal cancer
HOTAIR is highly overexpressed in patients with stage IV colorectal cancer (CRC) who have liver metastases and a poor prognosis and tightly correlated with the liver metastasis. EZH2 and SUZ12 are overexpressed in CRC and several other cancers [22–25]. And HOTAIR might be correlated to multipotent differentiation of CRC cells through the PRC2 complex interaction with multipotential stem cells. For HOTAIR, downregulated E-cadherin target genes by gene pathway analysis suggest that HOTAIR, cooperated with the PRC2 complex, might maintain mesenchymal and undifferentiated cancer cells [18].
Lung cancer
The changes of host microenvironment play a crucial determinant role in tumor progression. Type I collagen (Col-1), a kind of interstitial extracellular matrix (ECM), aberrantly enriched in the tumor microenvironment in non-small cell lung cancer (NSCLC) and promotes tumor progression. Col-1 concurrently induced the expression of HOTAIR in NSCLC, and Col-1 inhibition by a neutralizing antibody α2β2 integrin diminished the function of induction of HOTAIR. Functional studies implied that the Col-1 transcriptionally activated a reporter gene controlled by the human HOTAIR promoter. Moreover, the expression of HOTAIR and Col-1 was concurrently upregulated in human NSCLC [26] and was associated with advanced stage, lymph node metastasis or lymph-vascular invasion and prognosis, and induced NSCLC cell migration and anchorage-independent cell growth in vitro [27].
Pancreatic cancer
HOTAIR is a negative prognostic factor for pancreatic cancer patients and exhibits pro-oncogenic activity. HOTAIR is more highly expressed in more advanced and invasive pancreatic tumors, and HOTAIR knockdown reduces pancreatic cancer cell invasion, inhibits cell growth, modulates cell cycle progression, and induces apoptosis in vitro [19]. In addition, HOTAIR also plays a distinct pro-oncogenic activity in pancreatic cancer which is associated with increased cell survival and proliferation and repression of interferon-related genes (IL29, IL28A, IL28B, and IFTM1). Although modulated expression of HOTAIR in pancreatic cancer cell and breast/colon cancer cell results in a series of enhanced and suppressed expression of genes, there is minimal overlap between HOTAIR-regulated genes in pancreatic cells and breast cancer cells [11, 18, 19]; this result indicates that modulation of HOTAIR in pancreatic cancer may be different from other cancers, and the potential mechanisms need further studies.
Liver cancer
HOTAIR correlated with hepatocellular carcinoma (HCC) progression and prognosis [28–30]. Knockdown of HOTAIR reduced cell proliferation [29], cell invasion, sensitized TNF-α-induced apoptosis, and the chemotherapeutic sensitivity of cancer cells to cisplatin and doxorubicin in vitro [30], and was also associated with reductions in levels of matrix metalloproteinase-9 (MMP9) and vascular endothelial growth factor (VEGF) [29, 31]. Besides, overexpression of HOTAIR was an independent prognostic factor for predicting HCC recurrence in patients following liver transplantation [30].
Gastric cancer
High expression of HOTAIR has association with advanced stage, lymphatic node metastasis, and poor overall survival [32, 33]. And in high-HOTAIR patients, most of the epithelial markers were downregulated while most of the mesenchymal markers were upregulated. Moreover, knockdown of HOTAIR in gastric cancer cell decreased cell invasion potency, altered the expression of surfacial molecular markers of gastric cancer (GC) cells, and reversed the EMT progression, which might be through regulating snail expression [32]. HOTAIR overexpression GC cells injected into the tail vein of mice formed more liver metastases while downexpression of HOTAIR suppressed peritoneal dissemination [34].
Gastrointestinal stromal tumors
In gastrointestinal stromal tumors (GIST), microarray expression analysis revealed that HOTAIR, HOXC gene, and miR-196a were coordinately upregulated and miR-196a genes were located within the HOXC gene clusters in which HOTAIR was located in an antisense orientation. And overexpression of them was associated with high-risk grade, metastasis, and poor survival among GIST specimens, and overexpression of miR-196a and HOTAIR corporately may contribute to the malignant progression of GISTs by modulating expression of their target genes and could be useful biomarker as well as novel therapeutic and prognostic targets in malignant GISTs. However, inhibition of miR-196a had no effect on HOTAIR expression and knockdown of HOTAIR had no affect on miR-196a expression in GIST-T1 cells suggesting that overexpression of miR-196a or HOTAIR is not a simple downstream effect of their dysregulation [35].
Head and neck carcinoma
The study of the relationship of lncRNAs and head and neck carcinoma was relatively limited. Loss of imprinting at the IGF2 and H19 loci plays a role in the oncogenesis of head and neck carcinoma [36], and metastasis-associated lung adenocarcinoma transcript-1 (MALAT1) is correlated with progression and apoptosis of laryngeal squamous cell carcinoma (LSCC) [37]. HOTAIR is involved in nasopharyngeal carcinoma progression (NPC) [38] and LSCC progression and prognosis [38]. In LSCC, HOTAIR was overexpressed and related with T grade, differentiation, neck nodal metastasis, clinical stage, and prognosis. And HOTAIR promotes invasiveness and resistance to apoptosis in Hep-2 cells in vitro and in vivo, and these effects were related to promotion of PTEN methylation by HOTAIR. As in NPC, HOTAIR overexpression correlated with NPC progression (clinical stage, lymph node classification, metastasis, etc.) and prognosis in the clinic and HOTAIR mediated the migration and invasion of NPC cells in vitro.
Other cancers
A study of six metastasis-related lncRNAs in melanoma indicated that HOTAIR was the most highly expressed in lymph node metastasis tissue and promoted melanoma cell motility and invasion in vitro [39]. In prostate cancer (PCa), HOTAIR is overexpressed and knockdown of HOTAIR decreased PCa cell proliferation, migration, and invasion and induced apoptosis and cell cycle arrest. Further study indicated that HOTAIR was also targeted directly by a tumor suppressor miR-34 and downregulated by genistein, a protein tyrosine kinase inhibitor, which inhibited PCa cell growth [40]. High coexpression of MTDH/AEG1 and HOTAIR in primary sarcoma was correlated with a high probability of metastasis. And low expression of both MTDH/AEG-1 and HOTAIR was correlated with increasing necrosis of sarcoma treated with radiation and chemotherapy, indicating that they may be potential biomarkers for treatment sensitivity [41]. HOTAIR is overexpressed in esophageal squamous cell carcinoma (ESCC) tissue and correlated with clinical stage, TNM classification, histological differentiation, and prognosis. And HOTAIR mediated proliferation, colony formation, and migratory capacity in ESCC cells in vitro [42]. As HOTAIR promotes cancer invasion and metastasis, high expression of HOTAIR in cancer indicates poor prognosis. HOTAIR expression levels were significantly correlated with local recurrence-free survival (LRFS), distant metastasis-free survival (DMFS), disease-free survival (DFS), and overall survival (OS) of NPC [38]. DFS and OS were significantly shorter in the patients with high HOTAIR expression compared to those with low expression in NSCLC [27, 43], liver cancer [29], colorectal cancer [18], cervical cancer [44], and epithelial ovarian cancer [45], and HOTAIR expression was an independent prognostic factor for OS in LSCC [46], GC [32–34], and breast cancer [11, 20] patients. Moreover, high expression level of HOTAIR was an independent prognostic factor for predicting HCC recurrence in LT patients [30].
Molecular functions
HOTAIR and cancer invasion and metastasis
HOTAIR functions in the recruitment and binding of the PRC2 and LSD1 complex to the HOXD locus on chromosome 2 where genes involved in metastasis are regulated through H3K27 methylation and H3K4 demethylation. So HOTAIR induces tumor metastasis through regulation of the tumor metastasis-related molecules. Hundreds of PRC2 target genes were induced or repressed when HOTAIR was overexpressed in breast cancer cell line MDA-MB-231. HOTAIR-repressed genes such as JAM2, PCDH10, and PCDHB5 were de-repressed upon PRC2 depletion. HOTAIR-induced genes such as ABL2, SNAIL, LAMB3, and LAMC2 were upregulated in aggressive breast tumors that tend to cause metastasis and death but were repressed upon PRC2 depletion [11]. The likely regulation model and target genes exist in colorectal cancers [18]. However, only 9 of the 854 HOTAIR-PRC2 coregulated genes in MDA-MB-231 cells were also regulated by knockdown of HOTAIR in Panc1 cells [19], which suggested that HOTAIR may regulate different target genes in diverse tumors. Expression of HOTAIR correlated with ER and PR status and was transcriptionally induced by estradiol [21]. HOTAIR downregulated E-cadherin target genes in CRC [18] and was associated with matrix metalloproteinase-9 (MMP9) and vascular endothelial growth factor (VEGF) in HCC [29], promoted PTEN methylation in LSCC [46], and coexpressed with MTDH/AEG1 in sarcoma [41], which ultimately led to tumor cell proliferation, invasion, metastasis, and bad clinical outcome.
Epithelial-mesenchymal transition (EMT) is a biologic process in which epithelial cells lose their cell polarity and cell-cell adhesion and gain migratory and invasive properties to become mesenchymal cells. EMT, which is essential for numerous developmental processes, leads to cancer cells with stem cell-like characteristics, increases resistance to apoptosis and chemotherapy, and is involved in tumor invasion and metastasis [31, 47, 48]. As the same function of cancer invasion and metastasis, the relationship between HOTAIR and EMT has been unrevealed in two studies. Most of the epithelial markers were downregulated and most of the mesenchymal markers were upregulated in the high expression of HOTAIR group of GC. And knockdown of HOTAIR led to decreased expression of some mesenchymal markers (such as vimentin and N-cadherin), while the expression of epithelial markers (such as E-cadherin and ZO-1) was increased. Moreover, HOTAIR promoted EMT through regulating snail expression [32]. Another is related to TGFβ1 and maintenance of cancer stem cell (CSC) pathway. Treatment of TGF-β1 in breast and colon cancer cells resulted in increased HOTAIR expression in vivo, while knockdown of HOTAIR prevented E-cadherin reduction and inhibited fibronectin upregulation, which suggested that HOTAIR triggered the EMT program [49]. However, Liu et al. [43] found that EMT markers E-cadherin, N-cadherin, and vimentin had no significant difference between HOTAIR knockdown and control NSCLC cells, while MMP2, MMP9, and HOXA5 had relation to HOTAIR. And they draw a conclusion that HOTAIR may regulate the expression of MMPs and HOXA5 to influence the invasive and metastatic potential of NSCLC cells, but not the expression of EMT-induced markers. Therefore, the relationship and regulating pathways between HOTAIR and EMT need further studies.
Interestingly, another deductive pathway between EMT and HOTAIR maybe through miR-10b, which is a microRNA transcribed from the HOXD locus. HOXD10, which is regulated by HOTAIR in breast cancer, is involved in cell migration, extracellular matrix remodeling, and metastasis by controlling a series of genes [50]. And miR-10b binds to the 3′UTR of HOXD10 to inhibit HOXD10 protein translation. Moreover, miR-10b is induced by twist, which is a key transcription factor for epithelial-mesenchymal transition [51]. All the facts indicate that miR-10b may play an important role between HOTAIR and EMT.
HOTAIR and chemoresistance
Chemoresistance is one of most difficult and complicated problems in the area of tumor therapy, and the mechanisms of progression of chemoresistance are still not fully understood. However, the relationship between drug resistance and epigenetic regulations (miRNA and lncRNA are included) has been significantly proven in previous studies [52]. Evidence has been accumulated to indicate that HOTAIR has participated in several chemotherapeutic drugs. High expression level of both MTDH/AEG-1 and HOTAIR is correlated with a low percentage of necrosis in sarcoma samples exposed to chemotherapy and/or radiation. In contrast, low expression of these samples indicated a good response to treatment [41]. Knockdown of HOTAIR increased the chemotherapeutic sensitivity of the cancer cells to cisplatin and doxorubicin in HepG2 cells [30]. A latest study suggested that the expression level of HOTAIR in lung adenocarcinoma tissues and cell line was negatively correlated with the responses of cancer cells to cisplatin-based chemotherapy, while the p21 expression was positively correlated with the chemotherapy responses in terms of apoptosis and the percentage of cells in G0/G1 phase of cell cycle. And the expression of HOTAIR and p21 was inversely correlated with each other, and knockdown of each of them reduced the upregulation of the other. Moreover, the promoter region of p21 enriched with EZH2 and H3K27me3 [53], which involved in the HOTAIR pathways. And p21 could be significantly increased in non-small cell lung cancer cells after suppression of EZH2 [54]. The above evidence indicated that HOTAIR might induce the cisplatin resistance of lung adenocarcinoma cells through the regulation of p21 expression. However, the mechanism of how HOTAIR regulates chemotherapy resistance is far from clear.
Outlook
LncRNAs have been proven to be important regulators of epigenetics in physiological processes and disease. However, we have barely begun to scratch the surface of the lncRNA world; only individual examples have been functionally studied in detail at present and many pivotal questions remain to be addressed. As a paradigm example of guides in trans, HOTAIR regulates histone-modifying enzymes to HOXD loci and epigenetic gene silencing. However, there are still plenty of questions that need to be resolved and much work to be done. Although the sequence of human HOTAIR and the binding domains of PRC2 and LSD1 have been discovered, the spatial structure of HOTAIR, whether the other domains of HOTAIR are useless or non-functional, and whether the two complexes PRC2 and LSD1 bind to HOTAIR directly or indirectly are still unknown. Studies of HOTAIR mostly focus on the PRC2 complex and the following altering of biological molecules and pathways; however, what are the exact functions and regulating biomolecules of LSD1 complex when binding to HOTAIR? In pancreatic cancer, the altering genes of HOTAIR is different from the studies in breast cancer and colorectal cancer, so the differences are due to the different tumor response to the function of HOTAIR or HOTAIR acts on diverse tumors by different pathways or other possible mechanisms are also still unknown. And more interaction or/and regulation biomarkers like miRNA, mRNA, lncRNA, and protein will be found and new mechanisms will be explored.
In conclusion, the present studies have indicated that HOTAIR, defined as an oncogene, functions as a molecular scaffold to link PRC2 and LSD1 complexes and modulation of chromatin structures in trans for epigenetic gene silencing, and is involved in cancer cell proliferation, invasion, apoptosis, progression, metastasis, etc. Further studies are looking forward to explore the precise molecular mechanisms of HOTAIR in cancer and expect HOTAIR to be a biomarker and/or therapeutic targets for tumor diagnosis and treatment.
References
Ponting CP et al. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629–41.
Jeggari A et al. miRcode: a map of putative microRNA target sites in the long non-coding transcriptome. Bioinformatics. 2012;28(15):2062–3.
Jalali S et al. Systematic transcriptome wide analysis of lncRNA-miRNA interactions. PLoS One. 2013;8(2):e53823.
Lee JT. Epigenetic regulation by long noncoding RNAs. Science. 2012;338(6113):1435–9.
Maruyama R, Suzuki H. Long noncoding RNA involvement in cancer. BMB Rep. 2012;45(11):604–11.
Kung JT et al. Long noncoding RNAs: past, present, and future. Genetics. 2013;193(3):651–69.
Mercer TR, Mattick JS. Structure and function of long noncoding RNAs in epigenetic regulation. Nat Struct Mol Biol. 2013;20(3):300–7.
Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9(6):703–19.
Chu C et al. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol Cell. 2011;44(4):667–78.
Tsai MC et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329(5992):689–93.
Gupta RA et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–6.
Rinn JL et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129(7):1311–23.
Schorderet P, Duboule D. Structural and functional differences in the long non-coding RNA HOTAIR in mouse and human. PLoS Genet. 2011;7(5):e1002071.
He S et al. The sequence, structure and evolutionary features of HOTAIR in mammals. BMC Evol Biol. 2011;11:102.
Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154(1):26–46.
Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482(7385):339–46.
Wang L et al. BRCA1 is a negative modulator of the PRC2 complex. EMBO J. 2013;32(11):1584–97.
Kogo R et al. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71(20):6320–6.
Kim K et al. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32(13):1616–25.
Chisholm KM et al. Detection of long non-coding RNA in archival tissue: correlation with polycomb protein expression in primary and metastatic breast carcinoma. PLoS One. 2012;7(10):e47998.
Bhan A et al. Antisense transcript long noncoding RNA (lncRNA) HOTAIR is transcriptionally induced by estradiol. J Mol Biol. 2013;425(19):3707–22.
Kuzmichev A et al. Composition and histone substrates of polycomb repressive group complexes change during cellular differentiation. Proc Natl Acad Sci U S A. 2005;102(6):1859–64.
Sparmann A, van Lohuizen M. Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer. 2006;6(11):846–56.
Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat Res. 2008;647(1–2):21–9.
Kirmizis A et al. Silencing of human polycomb target genes is associated with methylation of histone H3 Lys 27. Genes Dev. 2004;18(13):1592–605.
Zhuang Y et al. Induction of long intergenic non-coding RNA HOTAIR in lung cancer cells by type I collagen. J Hematol Oncol. 2013;6:35.
Nakagawa T et al. Large noncoding RNA HOTAIR enhances aggressive biological behavior and is associated with short disease-free survival in human non-small cell lung cancer. Biochem Biophys Res Commun. 2013;436(2):319–24.
Ishibashi M et al. Clinical significance of the expression of long non-coding RNA HOTAIR in primary hepatocellular carcinoma. Oncol Rep. 2013;29(3):946–50.
Geng YJ et al. Large intervening non-coding RNA HOTAIR is associated with hepatocellular carcinoma progression. J Int Med Res. 2011;39(6):2119–28.
Yang Z et al. Overexpression of long non-coding RNA HOTAIR predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Ann Surg Oncol. 2011;18(5):1243–50.
De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13(2):97–110.
Xu ZY et al. Knockdown of long non-coding RNA HOTAIR suppresses tumor invasion and reverses epithelial-mesenchymal transition in gastric cancer. Int J Biol Sci. 2013;9(6):587–97.
Hajjari M et al. Up-regulation of HOTAIR long non-coding RNA in human gastric adenocarcinoma tissues. Med Oncol. 2013;30(3):670.
Endo H et al. Enhanced expression of long non-coding RNA HOTAIR is associated with the development of gastric cancer. PLoS One. 2013;8(10):e77070.
Niinuma T et al. Upregulation of miR-196a and HOTAIR drive malignant character in gastrointestinal stromal tumors. Cancer Res. 2012;72(5):1126–36.
El-Naggar AK et al. Frequent loss of imprinting at the IGF2 and H19 genes in head and neck squamous carcinoma. Oncogene. 1999;18(50):7063–9.
Feng J et al. Expression of long non-coding ribonucleic acid metastasis-associated lung adenocarcinoma transcript-1 is correlated with progress and apoptosis of laryngeal squamous cell carcinoma. Head Neck Oncol. 2012;4(2):46.
Nie Y et al. Long non-coding RNA HOTAIR is an independent prognostic marker for nasopharyngeal carcinoma progression and survival. Cancer Sci. 2013;104(4):458–64.
Tang L et al. Long noncoding RNA HOTAIR is associated with motility, invasion, and metastatic potential of metastatic melanoma. Biomed Res Int. 2013;2013:251098.
Chiyomaru T et al. Genistein inhibits prostate cancer cell growth by targeting miR-34a and oncogenic HOTAIR. PLoS One. 2013;8(8):e70372.
Milhem MM et al. Correlation of MTDH/AEG-1 and HOTAIR expression with metastasis and response to treatment in sarcoma patients. J Cancer Sci Ther. 2011;S5:4.
Lv XB et al. Long noncoding RNA HOTAIR is a prognostic marker for esophageal squamous cell carcinoma progression and survival. PLoS One. 2013;8(5):e63516.
Liu XH et al. The long non-coding RNA HOTAIR indicates a poor prognosis and promotes metastasis in non-small cell lung cancer. BMC Cancer. 2013;13(1):464.
Huang L, et al. Overexpression of long noncoding RNA HOTAIR predicts a poor prognosis in patients with cervical cancer. Arch Gynecol Obstet. 2014.
Qiu JJ et al. Overexpression of long non-coding RNA HOTAIR predicts poor patient prognosis and promotes tumor metastasis in epithelial ovarian cancer. Gynecol Oncol. 2014;134(1):121–8.
Li D et al. Long intergenic noncoding RNA HOTAIR is overexpressed and regulates PTEN methylation in laryngeal squamous cell carcinoma. Am J Pathol. 2013;182(1):64–70.
Franco-Chuaire ML et al. Epithelial-mesenchymal transition (EMT): principles and clinical impact in cancer therapy. Invest Clin. 2013;54(2):186–205.
Gao D et al. Microenvironmental regulation of epithelial-mesenchymal transitions in cancer. Cancer Res. 2012;72(19):4883–9.
Alves CP, et al. The lincRNA HOTAIR is required for epithelial-to-mesenchymal transition and stemness maintenance of cancer cells lines. Stem Cells. 2013.
Ma L et al. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449(7163):682–8.
Wan Y, Chang HY. HOTAIR: flight of noncoding RNAs in cancer metastasis. Cell Cycle. 2010;9(17):3391–2.
Haenisch S, Cascorbi I. miRNAs as mediators of drug resistance. Epigenomics. 2012;4(4):369–81.
Chen J et al. Enhancer of zeste homolog 2 is overexpressed and contributes to epigenetic inactivation of p21 and phosphatase and tensin homolog in B-cell acute lymphoblastic leukemia. Exp Biol Med (Maywood). 2012;237(9):1110–6.
Cao W et al. EZH2 promotes malignant behaviors via cell cycle dysregulation and its mRNA level associates with prognosis of patient with non-small cell lung cancer. PLoS One. 2012;7(12):e52984.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yansheng Wu and Li Zhang contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Wu, Y., Zhang, L., Wang, Y. et al. Long noncoding RNA HOTAIR involvement in cancer. Tumor Biol. 35, 9531–9538 (2014). https://doi.org/10.1007/s13277-014-2523-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2523-7