Abstract
During the process of development, neural crest cells migrate out from their niche between the newly formed ectoderm and the neural tube. Thereafter, they give rise not only to ectodermal cell types, but also to mesodermal cell types. Cell types with neural crest ancestry consequently comprise a number of specialized varieties, such as ectodermal neurons, melanocytes and Schwann cells, as well as mesodermal osteoblasts, adipocytes and smooth muscle cells. Numerous recent studies suggest that stem cells with a neural crest origin persist into adulthood, especially within the mammalian craniofacial compartment. This review discusses the sources of adult neural crest-derived stem cells (NCSCs) derived from the cranium, as well as their differentiation potential and expression of key stem cell markers. Furthermore, the expression of marker genes associated with embryonic stem cells and the issue of multi- versus pluripotency of adult NCSCs is reviewed. Stringent tests are proposed, which, if performed, are anticipated to clarify the issue of adult NCSC potency. Finally, current pre-clinical and clinical data are discussed in light of the clinical impact of adult NCSCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the last two decades, a number of emerging studies have identified the presence of neural crest-derived stem cells (NCSCs) within different adult craniofacial tissues, such as the skin, periodontal ligament and the lamina propria of the palate, oral cavity and nasal turbinates. Growing evidence currently indicates that these adult stem cell types can differentiate into cells of more than just one germ layer.
Importantly, the persistence of such highly plastic cells into adulthood could provide an explanation for the vast regenerative potential demonstrated by many mammalian craniofacial tissues. Although several reviews are available concerning the neural crest and NCSCs in general (e.g., [1–4]), a current summary of the knowledge concerning craniofacial NCSCs and their relationship to the embryonic cranial neural crest, potential pluripotency, and clinical potential is missing. In this review, the various sources of adult craniofacial NCSCs are therefore presented. Furthermore, adult NCSCs are discussed in light of their ancestry, pattern of expression of key stem cell markers, and possible clinical impact.
The Neural Crest and the Development of Vertebrate Craniofacial Tissues
During the development of craniofacial tissues in vertebrates, cranial neural crest cells give rise to ectodermal cell types (e.g., peripheral sensory and autonomic neurons, glial cells and pigment cells), as well as to mesodermal cell types (e.g., perivascular muscle cells and connective tissues) (see [5] and [6] for reviews). The neural crest was first described with respect to the development of the chick embryo as the ‘Zwischenstrang’ (German, ‘zwischen’: between; ‘Strang’: cord), or the ‘intermediate chord’, because it appeared between the neural chord and the future ectoderm [7] (see also Fig. 1). The emergence of the neural crest is closely aligned with the process of neurulation and the formation of the neural tube. The transient neural crest arises precisely at the fusion line between the invagination of the neural tube and its covering epidermis during vertebrate embryonic development. While the neural tube later gives rise to the brain and the spinal cord, neural crest cells migrate soon after neurulation and engender various populations of cells in the adult body (see Fig. 1). As described above, uncommitted neural crest cells differentiate into cells of both mesodermal and ectodermal types after reaching their target tissues, giving the neural crest its description as a probable fourth germ layer (reviewed in [6, 8, 9]). An overview of the developmental origin of neural crest cells and an illustrative genealogical chart of craniofacial cells and tissues with neural crest ancestry is shown in Fig. 1.
Developmental origin of adult neural crest-derived stem cells and their ancestry. The neural crest arises between the newly formed ectoderm and the neural tube during the development of the vertebrate embryo. While the neural tube gives rise to the central nervous system (brain and spinal cord), embryonic NCSCs migrate away from the neural tube and differentiate into cells of both mesodermal and ectodermal nature. Importantly, new data suggest that embryonic NCSCs not only contribute to terminally differentiated tissues, but also persist as uncommitted adult NCSCs. Adult NCSCs can undergo self-renewal, as well as multi-lineage differentiation
Underlining the developmental importance of neural crest cells, deficiencies in neural crest gene expression result in malformations and defects of the murine and human head. Such genetic deficiencies include Twist -/- (failure of neural tube closure, Saethre-Cotzen Syndrome); Tcof1 -/- (neural crest apoptosis, Treacher Collins-Franceschetti Syndrome); Pax9 -/-, Sox10 -/-, MITF -/-, Slug -/-, EDN3 -/- and EDNRB -/- (cleft secondary palate, absent teeth, oligodontia) [10]; Pax3 -/- (neural tube defects, deficiency of Schwann cells and dorsal root ganglia, Waardenburg Syndrome type 1); and Tgfb2 -/- (cleft palate, defects in neural crest skeletogenesis) (reviewed in [11]). In addition, premature gliogenesis (Hirschsprung’s disease) has recently been attributed to deficiencies in Hedgehog and Notch 1 [12].
Adult Neural Crest-Derived Stem Cells
Despite their capability to give rise to various types of cells during development, cells derived from the embryonic neural crest also persist into adulthood in the human, as described in a number of reports [13–21]. In particular, these reports suggest that NCSCs may exist as a dormant multipotent stem cell population in the adult, as their pluripotent state becomes gradually more restricted after migration. The number of highly multipotent adult NCSCs that persist long-term is largely unknown and may depend on the source of craniofacial tissue from which they are derived (see Fig. 2). However, it is known that such cells have a high capacity for both self-renewal and the generation of multiple kinds of progeny under the appropriate conditions in vitro and in vivo. In light of these characteristics, highly multipotent adult cells derived from the embryonic neural crest represent an extremely important stem cell population [22] with a differentiation potential that is only surpassed by that of pluripotent embryonic stem cells (ESCs). Indeed, these sphere-forming adult NCSCs seem to harbour a fascinatingly broad differentiation potential, especially in regard to the generation of neuronal and glial cells [13, 17, 18, 23–26], α-smooth muscle actin (SMA)-positive mesodermal cells [13, 19, 23, 24, 27], osteogenic cells [21, 26–32], adipocytes [13, 26, 27, 31, 32], chondrocytes [13, 23, 26, 27, 31, 32], melanocytes [23, 31], keratinocyte-like cells [13] and multinucleated MyoD-positive myotubes [17].
Sources of neural crest-derived stem cells within the craniofacial compartment of mammalia. EPI-NCSCs: epidermal neural crest stem cells; SKPs: skin-derived progenitors; COPs: corneal precursors; MCCs: murine corneal cells; OECs: olfactory ensheathing cells; OE-MSCs: olfactory mucosa mesenchymal stem cells; ITSCs: inferior turbinate stem cells; nm-MSCs: nasal mucosa mesenchymal stem cells; pNCSCs: palatal neural crest-derived stem cells; OMLP-PCs: oral mucosa lamina propria progenitor cells; hOMSCs: human oral mucosa stem cells; DPSCs: dental pulp stem cells; SHEDs: stem cells derived from exfoliated human deciduous teeth; PDLSCs: periodontal ligament stem cells; pNSCs: periodontium-derived neural stem cells
With respect to their gene expression profiles, the majority of adult NCSCs express high levels of nestin, an intermediate filament that is essential for the self-renewal of neural stem cells [33]. However, the expression of several additional markers, such as the low-affinity p75 neurotrophin receptor (p75NTR), seems to differ between populations of NCSCs and is dependent, up to a certain extent, on the applied cultivation method (Table 1). Furthermore, when cultivated under serum-free conditions in vitro, adult NCSCs demonstrate a common ability to grow as free-floating neurospheres.
Craniofacial Skin
The mammalian craniofacial skin has a very high contribution of neural crest cells during development (reviewed in [2]), as demonstrated by lineage tracing using the neural crest-specific Wnt1-Cre/Rosa26R-lacZ mouse model [34–36]. Fernandes et al. employed this model to demonstrate that many dermal cells were β-gal-positive [37], emphasizing the possibility that neural crest-derived cells can persist as stem cells in adult craniofacial skin. In a ground-breaking study, Toma and colleagues definitively described, for the first time, the presence of neural crest-related stem cells in the dermis of adult mice and the skin of the human scalp [38]. The investigators termed this cellular population skin-derived progenitors (SKPs). Isolated SKPs displayed characteristic stem cell expression of nestin, as well as the capability to undergo multi-lineage differentiation into ectodermal and mesodermal progeny in vitro. The differentiated SKPs also expressed several specific markers for both neural and mesodermal differentiation, e.g., β-III-tubulin and neurofilament (neuronal cell markers); glial fibrillary acidic protein (GFAP), 2’,3’-cyclic nucleotide 3’-phosphodiesterase (CNPase) and A2B5 (glial cell markers); and α-SMA (a smooth muscle-like cell marker). In addition, the production of lipid drops was demonstrated for adipocyte-like cells. Moreover, scalp SKPs were positive for fibronectin, whereas no expression of the mesenchymal stem cell marker vimentin was detected. SKPs also expressed several neural-crest markers, such as Snail, Sox9, Slug and Twist [37].
In vivo, transplanted yellow fluorescent protein (YFP)-expressing SKPs migrated into the sympathetic ganglia, the dorsal root and the spinal nerve. These cells were positive for β-III-tubulin in developing chick embryos, revealing their broad differentiation potential [37]. Furthermore, Joannides et al. highlighted the neurogenic potential of human SKPs [39]. Exposure of SKPs pre-cultivated as spheres to an astrocyte conditioned medium led to a highly efficient neuronal differentiation, whereby the differentiated cells demonstrated the presence of depolarization-evoked calcium transients. In addition to their expression of nestin and the neural crest markers Snail, Slug, Twist and Sox9, scalp-derived SKPs were also shown in 2006 to be positive for octamer-binding transcription factor 4 (Oct4) and Nanog, both transcription factors associated with pluripotency [31].
Sieber-Blum and colleagues discovered a further population of adult cranial NCSCs located in the epidermal niche, particularly within the bulge region of whisker hair follicles [23, 40] (reviewed in [14]). With respect to their endogenous niche and neural crest ancestry, which was clearly demonstrated using the Wnt-1Cre/Rosa26R reporter system, these cells were termed epidermal neural crest stem cells (EPI-NCSCs). EPI-NCSCs were positive for nestin and the neural crest-specific transcription factor Sox10. In addition, gene profiling using the LongSAGE system demonstrated a panel of 19 transcripts that were unique to EPI-NCSCs in a comparison of EPI-NSCs, embryonic neural crest cells and SKPs [41]. A demonstration of the self-renewal of EPI-NCSCs and serial cloning experiments revealed their enormous differentiation potential; the cells successfully generated ectodermal cell types such as neurons, melanocytes and Schwann cells, as well as mesodermal smooth muscle cells and chondrocytes in vitro. Furthermore, transplanted EPI-NCSCs integrated into the lesioned murine spinal cord and differentiated into β-III-tubulin/glutamate decarboxylase 67 (GAD67)-positive GABAergic neurons in vivo. Additionally, a subset of transplanted EPI-NCSCs showed expression of myelin basic protein (MBP), as well as the glial marker RIP. Remarkably, no signs of proliferation or migration were observed after transplantation. Furthermore, the transplanted EPI-NCSCs reversed sensory defects resulting from spinal cord injury to control values in the Semmes-Weinstein touch test (reviewed in [42]). Meanwhile, in contrast to transplanted ESCs, no tumour formation attributable to transplanted EPI-NCSCs was observed in the murine spinal cord [40].
Concerning their expression of pluripotency factors, Sieber-Blum and colleagues demonstrated that murine trunk EPI-NCSCs expressed c-Myc, Klf4 and Sox2 at a similar level. However, the ESC markers Lin28, Oct4 and Nanog were expressed at a significantly lower level in EPI-NCSCs than in ESCs [43]. Of great interest, recent findings from the same group demonstrated the successful isolation of human trunk EPI-NCSCs from a bulge of hair follicles. While keeping their multipotent status, these easily accessible adult human NCSCs could be expanded ex vivo with high purity and underwent efficient differentiation into osteocytes and melanocytes [44]. Importantly, both the easy accessibility of hair follicle EPI-NCSCs and their efficient differentiation makes these cells promising candidates for potential therapeutic uses. Klf4, Nanog and c-Myc were shown to be expressed at similar levels in hair follicle EPI-NSCs and human embryonic stem cell line h9, whereas Oct4, Lin28 and Sox2 displayed pronounced differences in expression between the two cell types.
Palate
The palate is a richly innervated and highly regenerative craniofacial tissue. The development of the palate requires the direct contribution of neural crest cells. It is well recognized that wounds within oral mucosa heal rapidly [45–47]. This capability for rapid regeneration may be explained either by the presence of growth factors, e.g., FGF-2 in the saliva, or by presence of at least one stem cell source within the palatal mucosa. Our group recently identified nestin-positive cells adjacent to Meissner’s corpuscles (touch receptors) and Merkel cell-neurite complexes within the palatal ridges (palatal rugae/rugae palatinae) of adult rats [19]. Due to their niche within the adult palate, these cells were termed palatal neural crest-derived stem cells (pNCSCs).
Cultivated pNCSCs were positive for a set of stem cell markers including nestin, p75, Sox9, Notch1, Slug and Snail. Since 84.8% of the cells also expressed Sox2, a further analysis was conducted of pluripotency-associated gene products (e.g., Klf4, Oct4 and c-Myc). Of particular interest, pNCSCs were not only positive for Sox2, but also for c-Myc, Klf4 and Oct4. Directed neuronal differentiation resulted in a high frequency of cells positive for the neuronal markers β-III-tubulin and the neuron-specific intermediate filament, neurofilament-M (NF-M). In addition, 22.6 ± 10.9% of the cells showed high expression of Map2. The highly efficient neuronal differentiation of pNCSCs in our study provided further evidence for the stemness of this cell population. Finally, in order to identify more closely the potential stem cell pools within the human palate, anterior and posterior samples from the hard palate were investigated, in addition to samples from the papilla incisiva and the distal processus alveolaris maxillae. Reverse transcription polymerase chain reaction (RT-PCR) was employed to demonstrate the highest expression of the human stem cell markers CD133 and nestin within the papilla incisiva and the distal processus alveolaris. These palatal regions also expressed the highest levels of Sox2, Klf4, Oct4 and c-Myc.
Although pNCSCs are a cell population of potential clinical interest, in our hands it was difficult to cultivate human pNCSCs from clinical material without contamination from yeast. About 75% of the cultures contained yeast contaminates, even in the presence of fungicides. Furthermore, cultures without contamination showed a slow doubling time and could not generate secondary neurospheres, which is in sharp contrast to our observations obtained with rat pNCSCs.
Oral Mucosa
The presence of nestin-positive cells within the lamina propria of the mammalian oral mucosa was first demonstrated in rats [48]. Although this study revealed that such cells are non-epithelial, as demonstrated by the absence of the epithelial marker cytokeratin, and multipotent, as suggested by the ability of the cells to differentiate into osteoblast-, adipocyte- and astrocyte-like cells, a clear link to the neural crest was not provided. More recently, Davies et al. reported the isolation of a multipotent neural crest-derived progenitor cell population from the human buccal mucosa lamina propria [49]. The expression of Oct4, Nanog, Sox2, Klf4 and hTERT was demonstrated within this tissue by PCR. Isolated cells were cultivated in medium containing 10% fetal calf serum (FCS) and formed colonies, with the cells displaying a fibroblast-like morphology. Further PCR studies revealed the neural crest origin of the cultivated cells by demonstrating that they expressed Snail, Slug, Sox10 and Twist. However, the expression of nestin was only investigated in cells after their differentiation into the neural lineage. Importantly, such human oral mucosa lamina propria progenitor cells (OMLP-PCs) could be differentiated into different lineages, including neurons, astrocytes and Schwann cells, as well as mesenchymal cell types, such as osteoblasts and chondrocytes.
A further recent study identified a stem cell source and the efficient isolation of the cells from the gingival and alveolar lamina propria of human oral mucosa [26]. In this study, the authors isolated human oral mucosa stem cells (hOMSCs) by an explant culture method in the presence of 10% FCS. More than 65% of the cells were positive for pluripotency-related ESC markers, such as SSEA4, Oct4 and Sox2, whereas 40% of the cells expressed Nanog. In addition, similar to cells of ESC lineage, the hOMSCs expressed the surface antigens Tra-2-54 and Tra-49. Surprisingly, alkaline phosphatase expression was two-fold higher in these cells than in human ESCs. Quantitative RT-PCR revealed that Oct4, Sox2 and Nanog were all expressed at a level that was several hundred-fold higher than baseline, but an order of magnitude lower than the expression level in ESCs. The neural crest origin of the hOMSCs was suggested by their expression of p75. These cells could be differentiated into neural, mesodermal and endodermal lineages. In addition, in vivo studies detected chord-like structures within the endogenous niche of the cells (i.e., the lamina propria of the mucosa) that were positive for p75, Oct4 and Sox2 [26].
Periodontium
The periodontal ligament represents a cell renewal system in steady state. In 2004, Seo and co-workers identified a multipotent stem cell population within the human periodontal ligament. In their study, the authors isolated periodontal stem cells using single colony selection and magnetic cell sorting based on cellular expression of the STRO-1 antigen [50]. Although differentiation of the periodontal ligament stem cells (PDLSCs) into cementoblasts, adipocytes and collagen-forming cells was demonstrated, there was no direct link to the neural crest.
Using sphere culture conditions that included FGF-2, epidermal growth factor (EGF) and leukaemia inhibitory factor (LIF) in the culture medium, the group of Miura was able to efficiently expand nestin-positive rat-derived periodontal stem cells. Moreover, PCR analysis revealed the expression of Slug, Twist, Sox9, ABCG2 and SSEA-1 by the cells. Interestingly, in contrast to other NCSC populations, rat-derived periodontal stem cells showed no expression of Oct4 [17]. However, this cell population was able to differentiate into neurofilament-positive neuron-like cells, multinucleated myotube-like structures and GFAP-positive cells with a glial phenotype. Using periodontal biopsies as the source material, which were obtained during routine, minimally-invasive access surgery, our group was able to isolate sphere-forming stem cells from the adult human periodontium. These cells were termed periodontium-derived neural stem cells (pdNSCs) [18]. The pdNSCs expressed high levels of nestin and Sox2 and were negative for the hematopoietic stem cell markers CD133 and CD34. No expression of typical neural crest markers such as p75NTR was detected when the cells were cultured in serum-free neurosphere medium containing EGF and FGF-2. Furthermore, in accordance with the study by Techawattanawisal et al., human pdNSCs cultivated as spheres were negative for Oct4.
Although the expression of neural crest markers was absent in pdNSCs under proliferative conditions, a highly efficient neuronal differentiation of periodontal stem cells was still achieved in our study. This was demonstrated by the up-regulated expression of synaptophysin, MAP2, neurofilament-M, neurofilament-H and neurofilament-L. Moreover, GFP-transfected cells integrated into hippocampal slice cultures, and proper depolarization-induced calcium transients were observed after neuronal differentiation. In addition, human pdNSCs were shown to generate osteopontin-positive, osteoblast-like progeny cells [29].
In contrast to our study, Coura and colleagues reported the expression of neural crest cell markers p75NTR and HNK-1 in adherent cultures of human periodontal stem cells. This study employed neural crest-inductive media containing FCS, chicken embryo extract, transferrin, hydrocortisone, glucagon, insulin, triiodothyronine, EGF and FGF-2 [51]. These culture conditions therefore seem to be more efficient in regard to the maintenance and/or stimulation of the neural crest-typical gene expression profile in human periodontal ligament stem cells. Importantly, the so-cultivated cells were able to differentiate into adipogenic, osteogenic, myofibroblastic and neuronal phenotypes.
A study in 2009 demonstrated than human periodontal stem cells isolated from impacted wisdom teeth of young adults expressed nestin, Slug, p75 and Sox10. The stem cells were successfully differentiated into neuronal, cardiomyogenic, chondrogenic and osteogenic lineages [52]. A subset of the wisdom tooth-derived periodontal stem cells expressed the ESC markers Oct4, Sox2, Nanog and Klf4. In a recent study, Kawanabe et al. showed that human PDLSCs also expressed SSEA-1, SSEA-3, SSEA-4, TRA-1-60 and TRA-1-81 [53].
Dental Pulp
In addition to the periodontal ligament, progenitor cells exhibiting features of NCSCs have been identified in the dental pulp (dental pulp stem cells/DPSCs) and exfoliated human deciduous teeth (stem cells from human exfoliated deciduous teeth/SHEDs) [54–56] (reviewed in [57]). Although cells from dental pulp and the dental follicle are often defined as ectomesenchymal, it is noteworthy that implantation and tissue recombination studies have demonstrated that dental tissues, including cementum, are tooth-related and neural crest-derived [58]. This result is demonstrated by a study using genetic lineage tracing in Wnt1-Cre/Rosa26R mice, which revealed that the dental pulp originates from the neural crest [59]. Cultivated DPSCs were shown to form nestin-positive spheres under appropriate culture conditions [60]. In an animal model, DPSCs survived, integrated and differentiated into functional neurons after injection into avian embryos [61]. Using magnetic cell sorting, Waddington and colleagues isolated DPSCs based on their expression of p75 [32]. However, both p75+ and p75− populations differentiated into osteoblasts, adipocytes and chondrocytes. Recent studies also demonstrated that subsets of DPSCs isolated from human deciduous and natal teeth expressed the markers Oct4, Sox2, Nanog and Rex1 [62, 63]. Although the plasticity of DPSCs is promising, the practical usefulness of human dental pulp cells is limited by the need to exfoliate third molars, if still obtainable, or to access the dental pulp of permanent teeth.
Olfactory Mucosa
A well-studied adult stem cell type within the nasal cavity is the so-called olfactory ensheathing cell (OEC) [64–66]. OECs are glial cells that ensheath the olfactory nerve [67]. Genetic lineage tracing with Wnt1-Cre/Rosa26R-YFP mice showed that OECs directly originate from mouse neural crest cells. Neural crest-derived OECs can be found within both the embryonic [68] and adult olfactory mucosa [24]. OECs express nestin [69], GFAP, p75, S100, laminin and N-CAM [70]. They also possess a sphere-forming ability [71].
However, to our knowledge, there are no reports on the expression of Oct4 or other pluripotency markers by OECs.
Although OECs are quite plastic and represent a promising NCSC population, the stem cell source is very limited and age-related. OECs are located in humans in the lamina propria of the olfactory epithelium. The total area of this region is only about 3% of the total surface area of the nasal cavity, and as a further complication, OECs are wrapped around bundles of axons [72]. Moreover, the human adult olfactory epithelium is gradually replaced by the respiratory epithelium, leading to a decreased source of material during normal aging [73]. Additionally, the random biopsy of the olfactory region does not assure a pure source of the olfactory epithelium [74, 75]. Another source limitation is governed by the fact that the removal of large tissue biopsies from the human olfactory mucosa may result in the loss of the sense of smell.
A further population of adult stem cells derived from the olfactory epithelium was recently described by Murrel and colleagues [27]. These olfactory mucosa mesenchymal stem cells (OE-MSCs, see Fig. 3 for anatomical localization) were able to form spheres and expressed high levels of nestin, CD54 and CD90, while showing no expression of the hematopoietic markers CD133 and CD45 and the IL-6 receptor CD126. Nestin was expressed in OE-MSCs at a level more than seven-fold higher than that in bone marrow MSCs. On the other hand, Sox9 expression was lower in OE-MSCs compared with bone marrow MSCs. The authors successfully differentiated OE-MSCs in both osteogenic and adipogenic directions, although no chondrogenic differentiation was observed.
Neural crest-derived stem cells within the olfactory mucosa. At least two distinct stem cell populations can be detected within the olfactory mucosa: glia-related olfactory ensheathing cells (OECs) adjacent to nerve fibres, and olfactory epithelium mesenchymal stem cells (OE-MSCs), which harbour similarity to bone marrow mesenchymal stem cells (MSCs). OE: olfactory epithelium; BM: basal membrane; LP: lamina propria
Respiratory Mucosa
Apart from the extensively studied regenerative potential of the olfactory epithelium, regeneration of complex tissues can also be achieved by cell populations residing within the human respiratory mucosa. For instance, Mansour et al. demonstrated that the transplantation of grafts containing human inferior turbinates led to an efficient closure of small- and medium-sized nasal septal perforations [76]. Thus, our group hypothesized that at least one NCSC population is present in the respiratory mucosa of the adult human inferior turbinate. Based on this hypothesis, a novel NCSC type was identified within the respiratory epithelium of the adult human inferior turbinate. These cells were termed inferior turbinate stem cells (ITSCs) [21]. Within their niche, ITSCs were localized within the lamina close to the nerve fibres (see Fig. 4 for anatomical localization).
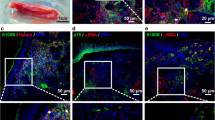
The respiratory mucosa is an easily accessible and stem cell-rich anatomical region. a Similar to the olfactory mucosa, different stem cell populations are present within the respiratory mucosa. Inferior turbinate stem cells (ITSCs) are localized close to nerve bundles within the lamina propria. A further adult stem cell population within the respiratory mucosa is the so-called nasal mucosa mesenchymal stem cell (nm-MSC), which shows MSC-like characteristics. RE: respiratory epithelium; B: basal membrane; LP: lamina propria. b Sections from human inferior turbinates were fixed and stained with antibodies against nestin and p75, and counterstained for DNA with SYTOX green. Confocal microscopy analysis revealed that nestin- and p75-postive ITSCs are localized adjacent to neurofilament-positive nerve fibres
Whereas the isolation of OECs from the superior and middle turbinate cannot be assured in elderly patients, detection of p75-positive ITSCs derived from the respiratory mucosa is not affected by aging. For example, the successful isolation and expansion of these easily accessible NCSCs was demonstrated in our study in an age-independent manner from individuals aged 4 to 76 years. In vitro, ITSCs revealed the capacity to form spheres and expressed high levels of nestin, Slug and Sox10. Interestingly, prolonged cultivation was associated with the down-regulation of p75. However, none of the other markers investigated (nestin, Sox10, Klf4 and c-Myc) were down-regulated with increasing time in culture. Furthermore, cultivated ITSCs were robustly positive for Oct4 and Sox2 at both the transcript and the protein level. Nevertheless, these pluripotency-associated markers, together with Lin28 and Nanog, were expressed at a dramatically lower level compared with the expression level in the human embryonic stem cell line HUES6 and the teratoma-derived cell line NTERA-2. Furthermore, although ITSCs were positive for CD117 [30], flow-cytometric analyses displayed a dramatically low expression levels of CD54 (unpublished data).
In regard to their differentiation capability in vitro, ITSCs were able to give rise to β-III-positive neuronal cells and α-SMA-expressing mesodermal cells in a spontaneous differentiation assay [21]. Moreover, ITSCs successfully differentiated into osteogenic cell types, as demonstrated in our work by both alkaline phosphatase activity and signs of mineralization (shown by alizarin red S and von Kossa staining) [21, 30]. Indicating their potential for in vivo transplantation, ITSCs survived and showed neural crest-typical dorsolateral chain migration after transplantation into avian embryos [21]. Furthermore, our group developed a novel three-dimensional (3D) and animal serum-free cultivation method for ITSCs, which is promising for potential clinical applications of these cells [30]. In particular, human blood plasma was used as a personalizable supplement for the cultivation medium, which led to the formation of a 3D fibrin matrix. This work provided evidence that ITSCs can be efficiently grown in a 3D blood plasma matrix, resulting in increased proliferation, while retaining their ploidy, expression profile of stem cell markers, and potential to differentiate into neural and osteogenic lineages.
In another study, Jakob and co-workers isolated MSC-like stem cells from the adult inferior turbinate. The cells were then cultivated as adherent cultures in presence of 10% FCS without growth factor supplementation [28]. This cell population, termed nasal mucosa MSCs (nm-MSCs), showed fibroblast-like morphology and was negative for p75 (CD271). A minority of the cells were positive for CD117 and STRO-1, whereas CD90 was expressed at a high level. Moreover, in contrast to our work with ITSCs, the authors showed high expression of CD54 in nm-MSCs. Despite their successful differentiation into osteogenic, adipogenic and chondrogenic lineages, neither the capacity of sphere formation, nor the expression of nestin or other NCSC or pluripotency markers by nm-MSCs, was investigated. Therefore, it remains unclear if nm-MSCs are related to the neural crest.
Eye
Using fate mapping, Gage and colleagues demonstrated that numerous cell types are of neural crest ancestry within the anterior part of the vertebrate eye [77].
Moreover, Yoshida et al. reported the successful isolation of NCSCs residing in the murine cornea. When expanded in vitro, such cells derived from the cornea of adult mice expressed nestin, Musashi-1, Notch1 and the neural crest markers Sox9, Twist, Slug and Snail. Terming these cells crest-derived corneal precursors (COPs), the authors further reported that they were positive for Sca1 and the hematopoietic stem cell marker CD34, whereas no expression of CD117 was detected. Furthermore, underlining their multi-lineage differentiation potential, COPs were capable of differentiating into neuroectodermal β-III-tubulin-positive neuron-like cells, as well as mesodermal α-SMA-positive cells [13]. However, only a few β-III-tubulin-expressing COPs were observed in a neuronal differentiation assay, and COPs of a less differentiated nature were also positive for neurofilament, a marker of mature neurons. More recently, neural crest-like cells were isolated from the corneal limbus of juvenile mice using an explant approach [78]. In addition to their ability to undergo osteogenic and neurogenic differentiation, murine corneal cells (MCCs) also expressed nestin, Sox9, Snail, Slug, Twist, Musashi-1 and vimentin.
As reported in a recent study, cells positive for Oct4, Sox2 and Nanog were isolated from different regions of a specialized conjunctive epithelium that covered the surface of the anterior part of the eye. Importantly, even a heterogeneous expression of Oct4, Nanog and Sox2 supported the suggestion of a stem cell population residing in this epithelium [79], although no nestin expression was detected. However, a more detailed analysis of pluripotency-associated markers at the protein level will be necessary to ascertain the potential stemness of these cells because the investigators focused solely on marker expression at the RNA level [79].
In another study, Zhou et al. demonstrated the persistence of an additional population of cells expressing Oct4 within the mammalian eye, particularly in the limbal epithelial basal cell layer [80]. Very recently, isolation of sphere-forming stem cells from the human corneal limbus and the central epithelium was shown by Chang et al. However, the sphere-forming potential of the cells declined with the advancing age of the donor [81].
De-Differentiated Schwann Cells as a Potential Neural Crest-Related Stem Cell Population
Schwann cells are directly related to the neural crest, share several markers with post-migratory NCSCs, and are routinely separated or enriched based on the expression of common receptors, such as p75 [82–86]. Several studies propose that Schwann cell progenitors and Schwann cells themselves possess a highly unstable phenotype [87, 88]. In particular, the differentiated phenotype of Schwann cells can be reversed or de-differentiated, suggesting a potential stemness of these cells. In light of these findings, it is intriguing to note that myelinating Schwann cells were responsible for a marked induction of pigmentation occurring after the lesion of the adult sciatic nerve [89, 90]. Furthermore, Schwann cells and post-migratory NCSCs share their expression of several markers, such as Notch1 and Notch2 [91], Sox10 [92, 93], p75 [82–86, 94] and neuregulin [95], at least during certain developmental stages. Moreover, as a consequence of nerve injury, several reports provide evidence for the re-induction of proliferation and de-differentiation of mature myelinating Schwann cells [87, 96]. Finally, isolated Schwann cells can trans-differentiate into α-SMA-expressing myofibroblasts, as convincingly demonstrated by Dupin and co-workers [88]. Trans-differentiation occurred through the generation of a pluripotent progeny, indicating that the neural crest phenotypes are highly plastic and unstable.
Recently, our group showed successful cellular reprogramming of adult trunk Schwann cells into a multipotent neural crest-like phenotype [97]. In particular, an up-regulated expression of p75, c-Myc, Sox2, Klf4, Oct4, Sox9 and Slug was demonstrated in the Schwann cells after their cultivation as neurospheres, whereas nestin expression had already been observed before the isolation. Importantly, as demonstrated by the generation of ectodermal and mesodermal progeny, adult trunk Schwann cells reprogrammed by culture also revealed the ability to differentiate into multiple lineages in vitro. It is notable that such cellular reprogramming also seems to occur in vivo after injury, as demonstrated in a Wnt1-Cre/lox-EGFP mouse model [98]. Neural crest-derived Schwann cells residing at the nerve roots de-differentiated into proliferating P0−/p75+ immature Schwann cells, followed by their migration into the lesion site. Although these two studies [97, 98] dealt with trunk Schwann cells, a similar reprogramming mechanism is very likely for cranial Schwann cells.
Are Adult NCSCs Pluripotent?
In their pioneering studies in 2006 and 2007, Takahashi and colleagues demonstrated the successful reprogramming of adult cells, e.g., skin fibroblasts, into an ESC-like, pluripotent state using forced expression of Oct4, Sox2, Klf4 and c-Myc [99, 100]. Numerous additional studies have provided evidence for the expression of these pluripotency-related markers in adult NCSCs of cranial origin, as discussed above. Moreover, ESCs and NCSCs share their broad differentiation potential, and it is thus feasible that adult NCSCs exist in an almost pluripotent state. However, several important issues must be addressed to unequivocally demonstrate the pluripotency of NCSCs. First, although NCSCs express several markers of ESCs, such as Oct4, Lin28, Sox2, Nanog, SSEA-1, SSEA-3, SSEA-4, TRA-1-60 and TRA-1-81, only a few studies have compared the expression level of these markers to that in ESCs. Indeed, studies performing this comparison reported significantly lower expression levels of ES markers in NCSCs compared with ESCs [21, 43, 44]. Second, the demonstration of marker expression is only one of several evidences for a potentially pluripotent state of cells. Attempts to demonstrate pluripotency should also focus on, for example, the methylation status of pluripotency-associated genes. Moreover, NCSCs do not form teratomas as a consequence of transplantation into immunodeficient mice [21, 26, 43], which casts doubt on their potentially pluripotent status. Whereas some of these studies did not detect any tumour formation at all, Marynka-Kalmani et al. demonstrated only the formation of bi-lineage tumours by NCSCs [26].
A further unavoidable hallmark of pluripotent cells is their ability to give rise not only to cells of all three germ layers, but also to beget germ cells themselves. To our knowledge, there are currently no reports providing evidence for differentiation into germ cells by adult NCSCs. Moreover, difficulties concerning the proof of pluripotency affect mainly human cells. In particular, the gold standard for proof of pluripotency includes the ability of the cells to form tetraploid embryo chimaeras, as recently discussed by Smith [101]. Ethical concerns provide an obvious hindrance to carrying out the tetraploid embryo complementation test in humans, and therefore, such an assay can only be performed using materials that are derived from an animal source. Thus, the final proof of the potential pluripotent state of NCSCs will be chimaera formation and the birth of fertile animals that are generated using animal NCSCs in a tetraploid embryo complementation assay.
Taken together, we propose that the developmental potential of NCSCs should be classified as “extended multipotency”, at least until further studies verify or falsify the presumption of their pluripotency.
Clinical Potential of Adult NCSCs
Due to their extraordinary plasticity and their on-going presence in the adult organism, craniofacial NCSCs may represent an ideal source of cells for regenerative medicine. Indeed, recent pre-clinical and clinical data with NCSCs raise hope for the treatment of several complex clinical syndromes.
In a pre-clinical study, isolated and cultivated EPI-NCSCs were shown to encourage the recovery of function after experimental spinal cord injury, as demonstrated by the significant improvement of sensory perception in animals that received EPI-NCSC grafts [40, 102] [103]. Central to their regenerative potential and in contrast to transplanted but undifferentiated ESCs, transplanted EPI-NCSCs demonstrated no signs of tumour formation.
OECs are another promising cranial stem cell population that have been used for the treatment of spinal cord lesions. In a study by Li and colleagues, transplanted olfactory bulb OECs mediated the repair of corticospinal lesions in a rat model [104].
Recently, human OECs have been applied in clinical studies for the treatment of spinal cord injury. In 2008, a report demonstrated that the transplantation of OECs resulted in a slight improvement of light touch and pin prick sensitivity in one of three treated patients [105]. Three further studies suggested that OEC transplantation may lead to the improvement of additional sensory and motor scores. However, no controls were applied in these studies, making added clinical investigation mandatory to address the question of the clinical effectiveness of OECs for the treatment of spinal cord injury (reviewed in [106]).
Human OECs have been successfully employed in an experimental rat model of Parkinson’s disease [107]. In this study, the authors showed significantly reduced behavioural asymmetry after the application of human OECs compared with the control group. More recently, Nivet et al. reported that isolated and cultivated human OE-MSCs also improved memory dysfunction and restored neuroplasticity in mice with chemically-induced hippocampal lesions [108]. In this study, human OE-MSCs differentiated into neurons, restored synaptic transmission, and contributed to the restoration of the hippocampal neuronal network.
In addition to these promising reports concerning regeneration after neurological defects, several pre-clinical reports have described the contribution of adult NCSCs to the regeneration of mesodermal tissues. For instance, it has been reported that SKPs participated in bone repair in a tibial bone fracture model [109]. Furthermore, a clinical study showed that transplanted grafts of DPSCs within a collagen matrix induced the repair of large mandible bone defects resulting from the extraction of third molars [110].
In conclusion, adult craniofacial NCSCs represent an easily accessible, ethically unambiguous, and highly plastic source of cells with high clinical potential. Adult NCSCs show great promise concerning the treatment of several diseases requiring the regeneration of multiple cell types, and may be of vital importance for the future of regenerative medicine.
References
Crane, J. F., & Trainor, P. A. (2006). Neural crest stem and progenitor cells. Annual Review of Cell and Developmental Biology, 22, 267–286.
Teng, L., & Labosky, P. A. (2006). Neural crest stem cells. Advances in Experimental Medicine and Biology, 589, 206–212.
Dupin, E., Calloni, G., Real, C., Goncalves-Trentin, A., & Le Douarin, N. M. (2007). Neural crest progenitors and stem cells. Comptes Rendus Biologies, 330, 521–529.
Shakhova, O., & Sommer, L. (2010). Neural crest-derived stem cells. In F. H. Gage & F. M. Watt (Eds.), StemBook. Cambridge: Harvard Stem Cell Institute.
Santagati, F., & Rijli, F. M. (2003). Cranial neural crest and the building of the vertebrate head. Nature Reviews Neuroscience, 4, 806–818.
Noden, D. M., & Trainor, P. A. (2005). Relations and interactions between cranial mesoderm and neural crest populations. Journal of Anatomy, 207, 575–601.
His, W. (1868). Untersuchungen über die erste Anlage des Wirbeltierleibes. Die erste Entwicklung des Hühnchens im Ei. Leipzig: Vogel.
Vickaryous, M. K., & Hall, B. K. (2006). Human cell type diversity, evolution, development, and classification with special reference to cells derived from the neural crest. Biological Reviews of the Cambridge Philosophical Society, 81, 425–455.
Slack, J. M. (2008). Origin of stem cells in organogenesis. Science, 322, 1498–1501.
Tachibana, M., Kobayashi, Y., & Matsushima, Y. (2003). Mouse models for four types of Waardenburg syndrome. Pigment Cell Research, 16, 448–454.
Wilkie, A. O., & Morriss-Kay, G. M. (2001). Genetics of craniofacial development and malformation. Nature Reviews Genetics, 2, 458–468.
Ngan, E. S., Garcia-Barcelo, M. M., Yip, B. H., et al. (2011). Hedgehog/Notch-induced premature gliogenesis represents a new disease mechanism for Hirschsprung disease in mice and humans. The Journal of Clinical Investigation, 121, 3467–3478.
Yoshida, S., Shimmura, S., Nagoshi, N., et al. (2006). Isolation of multipotent neural crest-derived stem cells from the adult mouse cornea. Stem Cells, 24, 2714–2722.
Sieber-Blum, M., & Grim, M. (2004). The adult hair follicle: Cradle for pluripotent neural crest stem cells. Birth Defects Research. Part C, Embryo Today, 72, 162–172.
Sasaki, R., Aoki, S., Yamato, M., et al. (2008). Neurosphere generation from dental pulp of adult rat incisor. European Journal of Neuroscience, 27, 538–548.
Nagoshi, N., Shibata, S., Kubota, Y., et al. (2008). Ontogeny and multipotency of neural crest-derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell, 2, 392–403.
Techawattanawisal, W., Nakahama, K., Komaki, M., Abe, M., Takagi, Y., & Morita, I. (2007). Isolation of multipotent stem cells from adult rat periodontal ligament by neurosphere-forming culture system. Biochemical and Biophysical Research Communications, 357, 917–923.
Widera, D., Grimm, W. D., Moebius, J. M., et al. (2007). Highly efficient neural differentiation of human somatic stem cells, isolated by minimally invasive periodontal surgery. Stem Cells and Development, 16, 447–460.
Widera, D., Zander, C., Heidbreder, M., et al. (2009). Adult palatum as a novel source of neural crest-related stem cells. Stem Cells, 27, 1899–1910.
Nagase, T., Matsumoto, D., Nagase, M., et al. (2007). Neurospheres from human adipose tissue transplanted into cultured mouse embryos can contribute to craniofacial morphogenesis: A preliminary report. The Journal of Craniofacial Surgery, 18, 49–53. discussion 60–1.
Hauser, S., Widera, D., Qunneis, F., et al. (2011). Isolation of novel multipotent neural crest-derived stem cells from adult human inferior turbinate. Stem Cells and Developement. doi:10.1089/scd.2011.0419.
Pierret, C., Spears, K., Maruniak, J. A., & Kirk, M. D. (2006). Neural crest as the source of adult stem cells. Stem Cells and Development, 15, 286–291.
Sieber-Blum, M., Grim, M., Hu, Y. F., & Szeder, V. (2004). Pluripotent neural crest stem cells in the adult hair follicle. Developmental Dynamics, 231, 258–269.
Murrell, W., Feron, F., Wetzig, A., et al. (2005). Multipotent stem cells from adult olfactory mucosa. Developmental Dynamics, 233, 496–515.
Hunt, D. P., Morris, P. N., Sterling, J., et al. (2008). A highly enriched niche of precursor cells with neuronal and glial potential within the hair follicle dermal papilla of adult skin. Stem Cells, 26, 163–172.
Marynka-Kalmani, K., Treves, S., Yafee, M., et al. (2010). The lamina propria of adult human oral mucosa harbors a novel stem cell population. Stem Cells, 28, 984–995.
Delorme, B., Nivet, E., Gaillard, J., et al. (2010). The human nose harbors a niche of olfactory ectomesenchymal stem cells displaying neurogenic and osteogenic properties. Stem Cells and Development, 19, 853–866.
Jakob, M., Hemeda, H., Janeschik, S., et al. (2010). Human nasal mucosa contains tissue-resident immunologically responsive mesenchymal stromal cells. Stem Cells and Development, 19, 635–644.
Arnold, W. H., Becher, S., Dannan, A., et al. (2010). Morphological characterization of periodontium-derived human stem cells. Annals of Anatomy.
Greiner, J., Hauser, S., Widera, D., et al. (2011). Efficient animal-serum free 3D cultivation method for adult human neural crest-derived stem cell therapeutics. European Cells and Materials, in press.
Yu, H., Fang, D., Kumar, S. M., et al. (2006). Isolation of a novel population of multipotent adult stem cells from human hair follicles. American Journal of Pathology, 168, 1879–1888.
Waddington, R. J., Youde, S. J., Lee, C. P., & Sloan, A. J. (2009). Isolation of distinct progenitor stem cell populations from dental pulp. Cells, Tissues, Organs, 189, 268–274.
Park, D., Xiang, A. P., Mao, F. F., et al. (2010). Nestin is required for the proper self-renewal of neural stem cells. Stem Cells, 28, 2162–2171.
Soriano, P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genetics, 21, 70–71.
Lee, H. Y., Kleber, M., Hari, L., et al. (2004). Instructive role of Wnt/beta-catenin in sensory fate specification in neural crest stem cells. Science, 303, 1020–1023.
Kleber, M., Lee, H. Y., Wurdak, H., et al. (2005). Neural crest stem cell maintenance by combinatorial Wnt and BMP signaling. The Journal of Cell Biology, 169, 309–320.
Fernandes, K. J., McKenzie, I. A., Mill, P., et al. (2004). A dermal niche for multipotent adult skin-derived precursor cells. Nature Cell Biology, 6, 1082–1093.
Toma, J. G., Akhavan, M., Fernandes, K. J., et al. (2001). Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nature Cell Biology, 3, 778–784.
Joannides, A., Gaughwin, P., Schwiening, C., et al. (2004). Efficient generation of neural precursors from adult human skin: Astrocytes promote neurogenesis from skin-derived stem cells. Lancet, 364, 172–178.
Sieber-Blum, M., Schnell, L., Grim, M., Hu, Y. F., Schneider, R., & Schwab, M. E. (2006). Characterization of epidermal neural crest stem cell (EPI-NCSC) grafts in the lesioned spinal cord. Molecular and Cellular Neuroscience, 32, 67–81.
Hu, Y. F., Zhang, Z. J., & Sieber-Blum, M. (2006). An epidermal neural crest stem cell (EPI-NCSC) molecular signature. Stem Cells, 24, 2692–2702.
Sieber-Blum, M. Epidermal neural crest stem cells and their use in mouse models of spinal cord injury. Brain Research Bulletin, 83, 189–93.
Sieber-Blum, M., & Hu, Y. (2008). Epidermal neural crest stem cells (EPI-NCSC) and pluripotency. Stem Cell Reviews, 4, 256–260.
Clewes, O., Narytnyk, A., Gillinder, K. R., Loughney, A. D., Murdoch, A. P., Sieber-Blum, M. (2011). Human Epidermal Neural Crest Stem Cells (hEPI-NCSC)-characterization and directed differentiation into osteocytes and melanocytes. Stem Cell Reviews.
Kahnberg, K. E., & Thilander, H. (1982). Healing of experimental excisional wounds in the rat palate. (I) histological study of the interphase in wound healing after sharp dissection. International Journal of Oral Surgery, 11, 44–51.
Kahnberg, K. E., & Thilander, H. (1984). Healing of experimental excisional wounds in the rat palate. II. Histological study of electrosurgical wounds. Swedish Dental Journal, 8, 49–56.
Kahnberg, K. E., & Thilander, H. (1987). Healing of experimental excisional wounds in the rat palate. III. Effects of radiation on wound healing. Swedish Dental Journal, 11, 61–70.
Dong, R., Liu, X., Fan, M., Yang, L., Peng, L., & Zhang, L. (2010). Isolation and differentiation of nestin positive cells from rat oral mucosal lamina propria. Differentiation, 79, 9–14.
Davies, L. C., Locke, M., Webb, R. D., et al. (2010). A multipotent neural crest-derived progenitor cell population is resident within the oral mucosa lamina propria. Stem Cells and Development, 19, 819–830.
Seo, B. M., Miura, M., Gronthos, S., et al. (2004). Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet, 364, 149–155.
Coura, G. S., Garcez, R. C., de Aguiar, C. B., Alvarez-Silva, M., Magini, R. S., & Trentin, A. G. (2008). Human periodontal ligament: A niche of neural crest stem cells. Journal of Periodontal Research, 43, 531–536.
Huang, C. Y., Pelaez, D., Dominguez-Bendala, J., Garcia-Godoy, F., & Cheung, H. S. (2009). Plasticity of stem cells derived from adult periodontal ligament. Regenerative Medicine, 4, 809–821.
Kawanabe, N., Murata, S., Murakami, K., et al. (2010). Isolation of multipotent stem cells in human periodontal ligament using stage-specific embryonic antigen-4. Differentiation, 79, 74–83.
Gronthos, S., Mankani, M., Brahim, J., Robey, P. G., & Shi, S. (2000). Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America, 97, 13625–13630.
Shi, S., Robey, P. G., & Gronthos, S. (2001). Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone, 29, 532–539.
Miura, M., Gronthos, S., Zhao, M., et al. (2003). SHED: Stem cells from human exfoliated deciduous teeth. Proceedings of the National Academy of Sciences of the United States of America, 100, 5807–5812.
Sloan, A. J., & Waddington, R. J. (2009). Dental pulp stem cells: What, where, how? International Journal of Paediatric Dentistry, 19, 61–70.
Lumsden, A. (1988). Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development, 103, 155–170.
Chai, Y., Jiang, X., Ito, Y., et al. (2000). Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development, 127, 1671–1679.
Stevens, A., Zuliani, T., Olejnik, C., et al. (2008). Human dental pulp stem cells differentiate into neural crest-derived melanocytes and have label-retaining and sphere-forming abilities. Stem Cells and Development, 17, 1175–1184.
Arthur, A., Rychkov, G., Shi, S., Koblar, S. A., & Gronthos, S. (2008). Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells, 26, 1787–1795.
Govindasamy, V., Abdullah, A. N., Ronald, V. S., et al. (2010). Inherent differential propensity of dental pulp stem cells derived from human deciduous and permanent teeth. Journal of Endodontics, 36, 1504–1515.
Yalvac, M. E., Ramazanoglu, M., Rizvanov, A. A., et al. (2010). Isolation and characterization of stem cells derived from human third molar tooth germs of young adults: Implications in neo-vascularization, osteo-, adipo- and neurogenesis. The Pharmacogenomics Journal, 10, 105–113.
Franssen, E. H., de Bree, F. M., & Verhaagen, J. (2007). Olfactory ensheathing glia: Their contribution to primary olfactory nervous system regeneration and their regenerative potential following transplantation into the injured spinal cord. Brain Research Reviews, 56, 236–258.
Kocsis, J. D., Lankford, K. L., Sasaki, M., & Radtke, C. (2009). Unique in vivo properties of olfactory ensheathing cells that may contribute to neural repair and protection following spinal cord injury. Neuroscience Letters, 456, 137–142.
Lindsay, S. L., Riddell, J. S., Barnett, S. C. Olfactory mucosa for transplant-mediated repair: A complex tissue for a complex injury? Glia, 58, 125–34.
Barraud, P., Seferiadis, A. A., Tyson, L. D., et al. Neural crest origin of olfactory ensheathing glia. Proceedings of the National Academy of Sciences of the United States of America, 107, 21040–5.
Tome, M., Lindsay, S. L., Riddell, J. S., & Barnett, S. C. (2009). Identification of nonepithelial multipotent cells in the embryonic olfactory mucosa. Stem Cells, 27, 2196–2208.
Savchenko, E. A., Andreeva, N. A., Dmitrieva, T. B., Viktorov, I. V., & Chekhonin, V. P. (2005). Culturing of specialized glial cells (olfactory ensheathing cells) of human olfactory epithelium. Bulletin of Experimental Biology and Medicine, 139, 510–513.
Pellitteri, R., Spatuzza, M., Stanzani, S., & Zaccheo, D. (2010). Biomarkers expression in rat olfactory ensheathing cells. Frontiers in Bioscience (Scholar Edition), 2, 289–298.
Viktorov, I. V., Savchenko, E. A., & Chekhonin, V. P. (2007). Spontaneous neural differentiation of stem cells in culture of human olfactory epithelium. Bulletin of Experimental Biology and Medicine, 144, 596–601.
Sorokin, S. (1988). The respiratory system. In L. Weiss (Ed.), A textbook of histology (pp. 751–814). Baltimore: Urban & Schwarzenberg.
Nakashima, T., Kimmelman, C. P., & Snow, J. B., Jr. (1984). Structure of human fetal and adult olfactory neuroepithelium. Archives of Otolaryngology, 110, 641–646.
Paik, S. I., Lehman, M. N., Seiden, A. M., Duncan, H. J., & Smith, D. V. (1992). Human olfactory biopsy. The influence of age and receptor distribution. Archives of Otolaryngology – Head & Neck Surgery, 118, 731–738.
Feron, F., Perry, C., McGrath, J. J., & Mackay-Sim, A. (1998). New techniques for biopsy and culture of human olfactory epithelial neurons. Archives of Otolaryngology – Head & Neck Surgery, 124, 861–866.
Mansour, H. A. (2011). Repair of nasal septal perforation using inferior turbinate graft. Journal of Laryngology & Otology, 1–5.
Gage, P. J., Rhoades, W., Prucka, S. K., & Hjalt, T. (2005). Fate maps of neural crest and mesoderm in the mammalian eye. Investigative Ophthalmology & Visual Science, 46, 4200–4208.
Brandl, C., Florian, C., Driemel, O., Weber, B. H., & Morsczeck, C. (2009). Identification of neural crest-derived stem cell-like cells from the corneal limbus of juvenile mice. Experimental Eye Research, 89, 209–217.
Harun, M. H., Sepian, S. N., Chua, K. H., et al. (2011). Human forniceal region is the stem cell-rich zone of the conjunctival epithelium. Human Cell.
Zhou, S. Y., Zhang, C., Baradaran, E., & Chuck, R. S. (2010). Human corneal basal epithelial cells express an embryonic stem cell marker OCT4. Current Eye Research, 35, 978–985.
Chang, C. Y., McGhee, J. J., Green, C. R., Sherwin, T. (2011). Comparison of stem cell properties in cell populations isolated from human central and limbal corneal epithelium. Cornea.
Vroemen, M., & Weidner, N. (2003). Purification of Schwann cells by selection of p75 low affinity nerve growth factor receptor expressing cells from adult peripheral nerve. Journal of Neuroscience Methods, 124, 135–143.
Morrison, S. J., White, P. M., Zock, C., & Anderson, D. J. (1999). Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell, 96, 737–749.
Li, H. Y., Say, E. H., & Zhou, X. F. (2007). Isolation and characterization of neural crest progenitors from adult dorsal root ganglia. Stem Cells, 25, 2053–2065.
Jiang, X., Gwye, Y., McKeown, S. J., Bronner-Fraser, M., Lutzko, C., & Lawlor, E. R. (2009). Isolation and characterization of neural crest stem cells derived from in vitro-differentiated human embryonic stem cells. Stem Cells and Development, 18, 1059–1070.
Stemple, D. L., & Anderson, D. J. (1992). Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell, 71, 973–985.
Dupin, E., Real, C., Glavieux-Pardanaud, C., Vaigot, P., & Le Douarin, N. M. (2003). Reversal of developmental restrictions in neural crest lineages: Transition from Schwann cells to glial-melanocytic precursors in vitro. Proceedings of the National Academy of Sciences of the United States of America, 100, 5229–5233.
Real, C., Glavieux-Pardanaud, C., Vaigot, P., Le-Douarin, N., & Dupin, E. (2005). The instability of the neural crest phenotypes: Schwann cells can differentiate into myofibroblasts. International Journal of Developmental Biology, 49, 151–159.
Adameyko, I., Lallemend, F., Aquino, J. B., et al. (2009). Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell, 139, 366–379.
Rizvi, T. A., Huang, Y., Sidani, A., et al. (2002). A novel cytokine pathway suppresses glial cell melanogenesis after injury to adult nerve. Journal of Neuroscience, 22, 9831–9840.
Woodhoo, A., Alonso, M. B., Droggiti, A., et al. (2009). Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nature Neuroscience, 12, 839–847.
Nonaka, D., Chiriboga, L., & Rubin, B. P. (2008). Sox10: A pan-schwannian and melanocytic marker. The American Journal of Surgical Pathology, 32, 1291–1298.
Britsch, S., Goerich, D. E., Riethmacher, D., et al. (2001). The transcription factor Sox10 is a key regulator of peripheral glial development. Genes & Development, 15, 66–78.
Yamamoto, N., Akamatsu, H., Hasegawa, S., et al. (2007). Isolation of multipotent stem cells from mouse adipose tissue. Journal of Dermatological Science, 48, 43–52.
Garratt, A. N., Britsch, S., & Birchmeier, C. (2000). Neuregulin, a factor with many functions in the life of a Schwann cell. Bioessays, 22, 987–996.
Stewart, H. J., Morgan, L., Jessen, K. R., & Mirsky, R. (1993). Changes in DNA synthesis rate in the Schwann cell lineage in vivo are correlated with the precursor–Schwann cell transition and myelination. European Journal of Neuroscience, 5, 1136–1144.
Widera, D., Heimann, P., Zander, C., et al. (2011). Schwann cells can be reprogrammed to multipotency by culture. Stem Cells and Development.
Nagoshi, N., Shibata, S., Hamanoue, M., et al. (2011). Schwann cell plasticity after spinal cord injury shown by neural crest lineage tracing. Glia, 59, 771–784.
Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126, 663–676.
Takahashi, K., Tanabe, K., Ohnuki, M., et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131, 861–872.
Smith, K. P., Luong, M. X., & Stein, G. S. (2009). Pluripotency: Toward a gold standard for human ES and iPS cells. Journal of Cellular Physiology, 220, 21–29.
Sieber-Blum, M. (2010). Epidermal neural crest stem cells and their use in mouse models of spinal cord injury. Brain Research Bulletin, 83, 189–193.
Hu, Y. F., Gourab, K., Wells, C., Clewes, O., Schmit, B. D., & Sieber-Blum, M. (2010). Epidermal neural crest stem cell (EPI-NCSC)–mediated recovery of sensory function in a mouse model of spinal cord injury. Stem Cell Reviews, 6, 186–198.
Li, Y., Field, P. M., & Raisman, G. (1997). Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science, 277, 2000–2002.
Mackay-Sim, A., Feron, F., Cochrane, J., et al. (2008). Autologous olfactory ensheathing cell transplantation in human paraplegia: A 3-year clinical trial. Brain, 131, 2376–2386.
King-Robson, J. (2010). Encouraging regeneration in the central nervous system: Is there a role for olfactory ensheathing cells? Neuroscience Research, 69, 263–275.
Murrell, W., Wetzig, A., Donnellan, M., et al. (2008). Olfactory mucosa is a potential source for autologous stem cell therapy for Parkinson’s disease. Stem Cells, 26, 2183–2192.
Nivet, E., Vignes, M., Girard, S. D., et al. (2011). Engraftment of human nasal olfactory stem cells restores neuroplasticity in mice with hippocampal lesions. The Journal of Clinical Investigation, 121, 2808–2820.
Lavoie, J. F., Biernaskie, J. A., Chen, Y., et al. (2009). Skin-derived precursors differentiate into skeletogenic cell types and contribute to bone repair. Stem Cells and Development, 18, 893–906.
d'Aquino, R., De Rosa, A., Lanza, V., et al. (2009). Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. European Cells & Materials, 18, 75–83.
Acknowledgments
Work described herein that was performed in our laboratory was supported by a University of Bielefeld FiF (Förderung Innovativer Forschung) grant to DW, grants of the German Research Council (DFG) to CK, and a grant of the German Ministry of Research and Education (BMBF) to BK (grant number 01GN1006A). We thank Johannes Greiner, Stefan Hauser and Jana Mallah for their critical reading of the manuscript, and Janine Müller for the immucytochemical staining of human inferior turbinate tissue.
Conflict of interest disclosure
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaltschmidt, B., Kaltschmidt, C. & Widera, D. Adult Craniofacial Stem Cells: Sources and Relation to the Neural Crest. Stem Cell Rev and Rep 8, 658–671 (2012). https://doi.org/10.1007/s12015-011-9340-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-011-9340-9