Abstract
Stem cells provide an invaluable tool to develop cell replacement therapies for a range of serious disorders caused by cell damage or degeneration. Much research in the field is focused on the identification of signals that either maintain stem cell pluripotency or direct their differentiation. Understanding how stem cells communicate within their microenvironment is essential to achieve their therapeutic potentials. Gap junctional intercellular communication (GJIC) has been described in embryonic stem cells (ES cells) and various somatic stem cells. GJIC has been implicated in regulating different biological events in many stem cells, including cell proliferation, differentiation and apoptosis. This review summarizes the current understanding of gap junctions in both embryonic and somatic stem cells, as well as their potential role in growth control and cellular differentiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past years, stem cell biology has emerged as one of the most active areas in biomedical research. Stem cells possess two remarkable characteristics: they are capable of self-renewal, while retaining the ability to differentiate into mature daughter cells [74]. Two categories of stem cells are discussed here, embryonic stem cells (ES cells) and somatic stem cells. ES cells are derived from the inner cell mass of the blastocyst stage of embryo [30, 69, 96, 111]. ES cells are pluripotent: they can give rise to most, if not all, tissue types of the body [91]. On the other hand, somatic stem cells persist in various niches throughout postnatal life and are critical to maintaining tissue homeostasis by replacing cell losses due to turnover, damage, and degeneration [77]. Generally somatic stem cells are restricted to differentiation into lineage(s) that comprise the tissue of origin. However, in some cases somatic stem cells can differentiate into multiple lineages, showing more developmental potential than previously thought [92, 119]. This multi- or pluri-potent developmental potential of stem cells has spurred great interest in their potential implications in cell replacement therapy.
The microenvironment that stem cells reside in is critical to their maintenance, and communications between neighbouring cells play an important part in determining cell fate. While most studies of the stem cell niche focus on paracrine or juxtacrine cell interactions, intercellular communication through gap junctions represents an understudied area. This review aims to summarize the current understanding of gap junctions in both somatic stem cells and ES cells.
Gap Junctions
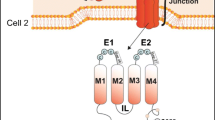
The term ‘gap junction’ comes from histological studies performed in the 1960s, where a distinct ‘gap’ between two plasma membranes was observed by electron microscopy [97]. Gap junctions are hydrophilic channels made up of two hemichannels termed connexons, each of them localized in the membrane of adjacent cells. In turn, each connexon consists of six integral membrane proteins termed connexins [53, 104] Connexons can be assembled from either a single type of connexins (homomeric) or multiple types of connexins (heteromeric). Subsequently, the gap junction can be either homotypic, consisting of two identical homomeric connexons, or heterotypic, consisting of two different heteromeric or homomeric composition (Fig. 1
). For a detailed discussion on connexin trafficking and gap junction assembly, please refer to an excellent review by Martin et al. [70].
Connexin genes belong to a highly conserved multigene family [103, 125]. To date, 21 connexins have been identified in human and 20 in the mouse. Connexins generally consist of four transmembrane regions, two extracellular loops and three intracellular domains [104]. The extracellular and transmembrane domains are conserved among connexin family members, whereas their intracellular sequences are more diverse [35]. The cytoplasmic C-terminal tail contains multiple serine and tyrosine residues which allow phosphorylation of connexins, resulting in regulation of molecular diffusion through gap junctions [57]. The interaction of other cellular proteins with connexins is the subject of an exhaustive review [33]. Connexins can be regulated at the transcriptional, translational and post-translational levels. This in turn can affect biogenesis, assembly, intracellular transport, docking, channel gating, internalization and degradation of gap junctions [56, 57, 79, 84, 103].
Traditionally, connexins are believed to be the only proteins capable of forming gap junctions in vertebrates, while innexins form gap junctions in invertebrates [60]. Recently three innexin orthologs, termed pannexins PANX1, PANX2 and PANX3, have been described in the mouse and human [86, 87]. At least one pannexin, PANX1 is able to form functional gap junctions in mammalian cells, rendering pannexin as the second class of gap junction proteins in vertebrates [43, 55]. However, it remains controversial whether pannexins play an overlapping role with connexins in mediating gap junctional intercellular communication (GJIC).
Gap Junctional Intercellular Communication
Gap junctions are the only intercellular junctions that allow direct transfer of signaling molecules and metabolites to adjacent cells [1, 53]. Atomic force microscopy studies have demonstrated that gap junctions can exist in an ‘opened’ conformation that allows molecular diffusion through their pore, or a ‘closed’ conformation that restricts molecular diffusion [28]. Moreover, gap junctions made up from different connexins can have different pore sizes, giving rise to different permeability for the transfer of molecules [103]. However, it is generally accepted that only molecules less than 1–1.5 kDa with a maximum diameter of ∼1.5 nm can diffuse through gap junctions in the ‘opened’ state [22, 31].
Gap junctional intercellular communication (GJIC) refers to the diffusion of intracellular molecules through gap junctions to the neighboring cell. Molecular movement through the gap junctions is believed to occur by passive diffusion [53]. As illustrated in Fig. 1, numerous cytoplasmic molecules can diffuse through gap junction channels, including small ions (Na+, K+, Ca2+, H+, Cl−), second messengers (cyclic nucleotides, inositol triphosphate), amino acids (glycine, glutamate), metabolites (glucose, glutathione, adenosine, AMP, ADP, ATP), short interfering RNA and peptides involved in cross-presentation of major histocompatibility complex class I molecules [1, 52, 80, 115]. GJIC is involved in various cellular mechanisms, including intercellular buffering of cytoplasmic ions, electrical synchronization, control of cell migration, cell proliferation, cell differentiation, metabolism, apoptosis and carcinogenesis [22, 52, 75, 88, 112, 121].
The functional state of GJIC can be determined experimentally by demonstrating gap junction-mediated transfer of either small biochemical dyes (e.g. Lucifer yellow, 6-carboxyfluorescein) or ions between adjacent cells (microelectrode impalements to detect electrical voltage across adjacent cells). It is important to recognize that GJIC should not be considered a simple all-or-none phenomenon. In some situations, gap junctions can close to a pore size that allows ions to pass through but not relatively larger biochemical dyes [54, 63]. Furthermore, different connexins can form gap junctions with dramatically different permeability and selectivity to specific molecules. For example, gap junctions comprised of Cx43 mediate transfer of glucose metabolites much better than Cx32 channels, while Cx32 channels transfer adenosine with greater efficiency than Cx43 channels [1]. The basis of this selectivity of molecular transfers is thought to be related to the ability of different connexins to assemble channels with different sizes, charges and binding affinities to specific molecules [1]. Gap junctions made of multiple types of connexins (heterotypic or heteromeric) can have different permeability and selectivity to diffused molecules compared to homotypic gap junctions, in a manner that is not yet fully understood.
Finally, recent evidence has also suggested that unpaired connexon hemichannels can also mediate intercellular communication without forming gap junctions [25, 31, 36]. Under resting conditions, connexon hemichannels in the plasma membrane are closed. However, in certain situations, connexon can be stimulated to open and release intracellular signalling molecules. Please refer to Goodenough and Paul for an extensive review on this topic [36].
Gap Junctions in Growth Control
Gap junctions have an important role in cellular growth control. Early studies led to a simple view that up-regulation of GJIC inhibits cellular proliferation, while down-regulation of GJIC stimulates proliferation [68, 114]. The diffusion of putative growth inhibitory factors through the gap junction is thought to cause this effect [68]. Previous studies showed that GJIC become undetectable as the cells enter mitosis [34, 106]. Decreasing GJIC is also observed during normal cell cycle transit and is disrupted during neoplastic growth [101]. In the 1960’s, some pioneering works from Loewenstein and Kanno demonstrated that rat hepatomas do not communicate via gap junctions [66, 67], contrary to normal hepatocytes which possess functional GJIC [90]. This work led to the hypothesis that gap junctions are involved in growth control, where deficiency in GJIC results in the deregulated growth of cancer cells [65]. This hypothesis is supported by the fact that GJIC deficiency is described in many cancer cell types as opposed to their normal counterpart [75, 76, 121]. Other evidences supportive of this hypothesis in cancer cells include the following:
-
I.
In many types of tumour cells, GJIC is not functional as a result of deficient in connexin expression, or inability to assemble functional gap junctions into the membrane [121].
-
II.
Cx32−/− knockout mice have a higher incidence of liver cancer and leukemia, and Cx43+/− knockout mice have a higher susceptibility to lung cancer [3, 38, 39, 110].
-
III.
Tumour-promoting agents (e.g. phorbol 12-myristate 13-acetate) and oncogenes (e.g. Src) can regulate gap junctional communication, further suggesting a role of GJIC in carcinogenesis [33, 130].
-
IV.
A direct relationship between gap junction expression and the malignant phenotype is demonstrated by a number of transfection studies, where transfection of cancer cells with certain connexins is followed by the subsequent restoration of GJIC and growth suppression [121, 130]. In addition, isolated HeLa clones with Cx43 expression and GJIC restoration exhibit marked decrease in cellular growth and tumorigenicity compared to normal HeLa that lack Cx43 and GJIC [49].
Thus, early studies of gap junctions in cancer cells supported a view where down-regulation of GJIC promotes cell proliferation, while up-regulation of GJIC inhibits cell proliferation [65]. This view is almost certainly an oversimplification, since cell proliferation and functional GJIC are correlated in some cell types. Certain cancer cells possess functional GJIC, such as embryonal carcinoma cells and rat bladder carcinoma cells [4, 6, 51]. Moreover, ES cells and early embryonic cells which proliferate rapidly also form functional GJIC. Increasing evidence also hints that tumour suppression by connexin transfection may act via a GJIC-independent pathway [95]. It appears that the relationship between the functional state of GJIC and cell proliferation may be more complicated than previously thought. Nevertheless, the data do indicate that gap junctions serve as a powerful regulator of growth control in a cell type-dependent fashion.
Gap Junctions in Mediating Cellular Differentiation in the Early Embryo
The early mouse embryo provides an excellent model to study the role of gap junctions in mediating cellular differentiation. The pre-implantation embryo expresses multiple connexin transcripts, including Cx30, Cx30.3, Cx31, Cx31.1, Cx36, Cx40, Cx43, Cx45 and Cx57, but not Cx26 and Cx32 [21, 42, 81]. In the human blastocyst, Cx26, Cx31, Cx43 and Cx45 transcripts are detected, however connexin expression patterns can be inconsistent between different embryos [12, 37].
GJIC between blastomeres appears from the eight-cell stage during compaction in the mouse [64], or much later at day 5–6 blastocyst stage in human [20]. At the blastocyst stage of the mouse embryo, GJIC was observed between cells within the inner cell mass [64]. Upon in vitro implantation of mouse blastocysts (equivalent to embryonic day 6.5, E6.5), dye coupling is progressively lost between the inner cell mass and the trophectoderm, but ionic coupling remains between these two populations [63]. By the egg cylinder stage (E7.5), ionic coupling also ceases and GJIC is completely lost between the cells that originate from the inner cell mass (ectoderm, endoderm, mesoderm and extraembryonic endoderm) and those originate from the trophectoderm (extraembryonic ectoderm and ectoplacental cone), thus giving rise to two different ‘communication compartments’ [45, 46]. Such changes in GJIC are consistent with the spatial re-organisation of connexin expression. For example, Cx43 and Cx31 are expressed in both the inner cell mass and trophectoderm in the pre-implantation mouse embryo, but Cx43 expression becomes restricted to derivatives of the inner cell mass while Cx31 expression is confined to derivatives of trophectoderm after implantation (E6.5) [19] Since Cx43 and Cx31 are not able to form functional heterotypic gap junctions [27], their distribution patterns effectively divide the derivatives of the inner cell mass and trophectoderm into two communication compartments. Further subdivision into smaller communication compartments occurs within both the embryo proper and extra-embryonic tissues, with each germ layer comprising a separate communication compartment [45, 46]. Curiously, in some cases, ionic coupling may persist between some germ layers [45, 46]. In summary, the current theory is that during early post-implantation development, GJIC is maintained within cells of the same lineage but lost between tissues whose fate diverges [62, 122]. Thus the formation of gap junction communication compartments is proposed to mediate specific intercellular signalings between cells with similar cell fates.
Despite many attempts to dissect the functional significances of GJIC during pre-implantation embryo development, to date whether GJIC is a functional requirement remains controversial [41]. Inhibition of GJIC by antibodies targeting multiple connexins [5, 58] or antisense RNA [9] prevents compaction and further development in mouse embryos. However, pharmacological inhibition of GJIC using α-glycyrrhetinic acid (α-GA) does not affect development of the blastocyst, suggesting GJIC may be dispensable during pre-implantation development in mouse [42, 118]. More studies are needed to elucidate the role of gap junctions in the early embryo development. In particular, knockout study of multiple connexins will help address this question in the future.
Gap Junctions in Embryonic Stem Cells
ES cells are traditionally derived from the inner cell mass of the blastocyst stage of embryo [30, 69, 96, 111]. Since then, recent reports have demonstrated successful derivation of ES cells from different stages of embryo, including single blastomere from eight–ten cell stage [18], morula and late stage embryo [107, 108]. ES cells are pluripotent and capable of proliferating indefinitely in vitro, thus potentially serve as an unlimited source of healthy tissue for cell replacement therapy [91].
Mouse Embryonic Stem Cells
Similar to cells from the inner cell mass, undifferentiated mouse ES cells (mESC) express transcripts of Cx43 and Cx45 and display functional GJIC [81, 85]. Furthermore, it was recently shown that pannexin-1 and five additional connexin transcripts are present in mESC: Cx26, Cx30.3, Cx31, Cx32 and Cx37. Curiously, only Cx31, Cx43 and Cx45 proteins were detected in mESC [129], suggesting that a regulatory system may exists in mESC to suppress translation of many connexin isoforms.
Several studies have attempted to study the function of gap junctions in mESC by knocking out or knocking down specific connexins. Cell proliferation of Cx43-knock down mESC is reduced significantly, but cell survival remains unchanged upon Cx43 knockdown [112, 129]. Moreover, Cx43 knock-down mESC exhibit down-regulation of several stem cell markers as well as up-regulation of differentiation markers [112]. Interestingly, these mESC are also unable to form embryoid bodies [112]. This suggests that GJIC is essential for the regulation of mESC to maintain undifferentiated as well as initiating differentiation. In addition, gene expression profiling indicates that Cx43-null mESC undergoing neural differentiation exhibit a deficiency in oligodendrocyte differentiation and an increase in astrocyte differentiation [88]. Although more detailed studies are needed to confirm these data, this study is the first to indicate an essential role of gap junction for proper neuroectodermal specification. Together, these results indicate an essential role of gap junction in mESC biology.
In contrast, Cx45-null mESC are morphologically similar to the wildtype and can readily differentiate into cells of the three germ layers following embryoid body formation [26]. One explanation for this lack of phenotype is that Cx43 is still expressed in Cx45-null mESC and may compensate for the loss of Cx45 [26]. Interestingly, when Cx45-null mESC were injected into the blastocyst, they failed to form chimeric mice or post-implantation embryos. Subsequent studies of chimeric blastocysts cultured in vitro showed that Cx45-null mESC were unable to incorporate into the recipient inner cell mass [26]. Cx45+/− heterozygous mutant mESC also show a decreased efficiency in forming chimeric mice [26]. From this study, it is proposed that Cx45 may play a distinct role in the incorporation of ES cells into inner cell mass by mediating intercellular communication.
Human Embryonic Stem Cells
Many transcriptome studies of human embryonic stem cells (hESC) are now available. These studies initially aimed to search for ‘stemness’ genes in hESC, and interestingly their results hinted at a role of gap junctions in maintaining hESC pluripotency. Multiple studies have demonstrated that Cx43 is enriched in undifferentiated hESC when compared to embryoid bodies [10, 61] or a pooled sample of adult human tissues [11, 98]. Moreover, the pluripotent factors Oct4, sox2 and nanog can co-occupy the upstream region of Cx43 to activate its expression, suggesting its role in the maintenance of hESC pluripotency [13]. Other microarray analysis have also shown that Cx43 and Cx45 mRNA are both highly enriched in hESC compared to a range of somatic tissues or spontaneously differentiated hESC [7, 105]. Interestingly, Cx45 is up-regulated in hESC compare to human embryonal carcinoma cells [105]. Furthermore, Enver et al. showed that Cx45 is also up-regulated in SSEA-3 positive undifferentiated hESC compare to its SSEA-3 negative differentiated counterpart [29]. Finally, Assou et al. summarized 38 previous reports of microarray studies in hESC and devised a ‘consensus list’ of undifferentiated hESC markers, including Cx43 and Cx45 [2]. Together, these transcriptome studies hinted an understudied role of connexins in the maintenance of hESC pluripotency.
Our previous study demonstrated that hESC express at least two connexins, Cx43 and Cx45 [128], a result consistent with others [15]. Two phosphorylated forms of Cx43 were observed in undifferentiated hESC, although the role of connexin phosphorylation in regulating GJIC is not clear [128]. Moreover, a recent study demonstrated the mRNA expression of an additional 16 connexin types in hESC, including Cx25, Cx26, Cx30, Cx30.2, Cx30.3, Cx31, Cx31.1, Cx31.9, Cx32, Cx36, Cx37, Cx40, Cx46, Cx47, Cx59 and Cx62 [43]. It appears that hESC express almost all transcripts of human connexin isoforms, except Cx40.1 and Cx50 [44]. Furthermore, GJIC was observed in undifferentiated hESC, as determined by dye coupling [15, 128, 132] and ionic coupling [44]. Our previous studies showed that such GJIC can be inhibited by BMP stimulation, protein kinase C activation and Erk1/2 inhibition [127, 128]. However, exogenous calcium does not modulate GJIC in hESC. In addition, hESC cultured in serum-containing, serum-free or feeder-free conditions possess similar gap junction properties [127]. Together, these studies suggest that functional GJIC is a common characteristic of hESC maintained in different culture conditions [127, 128]. We also showed that hESC do not communicate with mouse embryonic fibroblast feeder cells through gap junctions, a result consistent with studies in mESC [85]. Finally, the functional role of gap junctions in hESC was studied using the gap junction blocker α-GA. We utilized a serum-free culture system for hESC previously established in our laboratory using S1P and PDGF [89]. In hESC cultured in S1P and PDGF, inhibition of GJIC by α-GA resulted in reduced colony growth and increased apoptosis [127]. This result indicates an important role of gap junctions in the maintenance of hESC. However, it must be noted that α-GA had no significant effect on hESC cultured in fetal calf serum, suggesting that under certain conditions the effect of GJIC inhibition may be compensated by other unidentified signals.
Future studies that utilise RNAi techniques to knockdown specific connexins will be helpful in determining the role of specific connexins in regulating cell pluripotency, proliferation and survival in hESC. Research in this area will prove useful in maintaining hESC in an undifferentiated state or enhancing hESC differentiation along specific lineage. In addition, hESC survive poorly as single cell, which is vital to many genetic modification procedures. It is speculated that cell–cell interaction between hESC is important to their survival, thus a better understanding of the role of gap junctions may lead to improved methodology for cloning of hESC.
Gap Junctions in Somatic Stem Cells
In contrast to ES cells, a hallmark feature of somatic stem cells is that they exist in specific niches where they remain in a quiescence state [82]. Somatic stem cells can undergo symmetric division to self-renew, or asymmetric division to generate a differentiated daughter cell [50]. GJIC is often important in regulating the cell fates of somatic stem cells, a phenomenon possibly conserved from lower organism to mammalians. For example, Ovieda et al. recently demonstrated that the inhibition of GJIC in somatic stem cells prevents regeneration of the planarian flatworm, suggesting a conserved role of gap junctions in regulating stem cell fate [83]. Here we will discuss the current understanding of gap junctions in a number of somatic stem cells.
Hematopoietic Stem Cells
Hematopoietic stem cells (HSC) are the most extensively studied somatic stem cell population in both humans and mice [73]. HSC are capable of giving rise to all blood cell types, including red blood cells, B lymphocytes, T lymphocytes, neutrophils, natural killer cells, basophils, eosinophils, monocytes, macrophages and platelets [113]. During adulthood, HSC reside primarily within niches in bone marrow, where cell–cell communication with the surrounding stromal cells is critical for regulating HSC maintenance [48]. In particular, it has been proposed that GJIC may be involved in this process [94, 100]. Recent studies suggested that Cx32 is vital to HSC differentiation. Cx32 expression can be readily detected in Lin−c-kit+ HSC-enriched cells [39]. Interestingly, Cx32 knockout mice exhibit more undifferentiated HSC and fewer progenitor cells, suggesting a role of Cx32 in maturation of HSC to progenitor cells [38, 39]. In addition, Cx43 is also implicated in hematopoiesis. During the quiescent state, undifferentiated HSC (Lin−, Sca1+, C-kit+) do not express Cx43 mRNA [78]. However, Cx43 expression can be massively up-regulated in adult mouse bone marrow upon forced stem cell division [99]. Cx43 deficient mice also demonstrate clear defects in blood cell formation [78]. However, it is not clear whether this effect is mediated by functional gap junctions or by connexon hemichannels. Moreover, it remains unclear whether GJIC among HSC or between HSC and stromal cells is important in defining the stem cell niche. Thus, further research is needed to provide a definitive role of GJIC in HSC.
Mesenchymal Stem Cells
Mesenchymal stem cells (MSC) have been isolated from various tissues, including bone, umbilical cord blood and adipose [8, 123]. They can readily differentiate into adipose tissue, tendon, cartilage and bone [93]. Human MSC express Cx40, Cx43 and Cx45, and can communicate among themselves via gap junctions [59, 116]. However, a rare population of MSC was shown to be deficient in GJIC [59]. The exact identity of this MSC population remains unknown. Moreover, human MSC were demonstrated to form Cx43-mediated GJIC with umbilical vein endothelial cells, where this communication is important for osteogenic differentiation of MSC [120].
Neural Stem Cells
Neural stem cells exist in the developing or adult nervous system and can differentiate into all neural cell types, including neurons, astrocytes and oligodendrocytes [32]. Neural stem cells isolated from the rat developing brain express Cx43 and Cx45 and are capable of forming GJIC, where GJIC is essential for survival and proliferation in these cells [14]. Moreover, Cx43 and Cx32 levels were observed to increase temporarily during differentiation in these cells [131]. Future studies on human neural stem cells will help address whether this role of gap junctions is conserved among species. Similarly in mouse fetal neural progenitors, the closure of gap junctions decreases cell proliferation and diminishes cell survival [17, 24]. On the other hand, overexpression of Cx43 stimulates cell proliferation in these cells [17]. In addition, neural progenitors from other species have been demonstrated to express connexin and communicate via GJIC, possibly suggesting a conserved role of gap junction in neural stem cell biology. The astroglial progenitor cell line TB2, isolated from the fish brain, expresses Cx43 and Cx35 [124]. In the turtle, the spinal cord neural progenitors express Cx43 and are electrically and metabolically coupled via gap junctions [102]. Together, it appears that gap junctions are critical to maintaining neural stem cell homeostasis, both in self-renewal and early stages of differentiation.
Other Somatic Stem Cells
Although GJIC seems to play a role in regulating many somatic stem cell types, gap junctions are absent in some somatic stem cells. This included two somatic stem cells that give rise to the epithelium, keratinocyte stem cells [71] and corneal epithelial stem cells [72], both of which lack connexins and functional GJIC. A number of presumptive somatic stem cells were also demonstrated to lack functional GJIC, including pancreatic ductal epithelial stem cells [109], neural–glial stem cells [23], bovine mammary gland progenitor cells [40], human breast epithelial stem cells [47] and human kidney epithelial stem cells [16]. However, in some cases the identification of the cell populations studied as stem cells is questionable, and further characterization is needed to confirm their potential for self renewal and differentiation. Altogether, it appears that intercellular communication via gap junctions is not a universal feature of all somatic stem cells. Although the function of gap junctions seems to be highly dependent on the cell type, previous reports demonstrated that functional GJIC plays a critical role in regulating the cell fates of many somatic stem cells.
Summary
As the reader can appreciate, the role of gap junctions in stem cells requires further research and remains an important research question. Emerging evidence suggests that gap junctions seem to play an important role in regulating the cell fate of ES cells, neural stem cells, mesenchymal stem cells and possibly hematopoietic stem cells. However, the precise role of gap junctions appears to be highly dependent on the type of stem cells. This is not surprising, given the biological differences between somatic stem cells and ES cells. To date, several key questions remain to be addressed: Is GJIC a defining feature of the stem cell niche that maintains homeostasis of somatic stem cells? During commitment and differentiation, do stem cells form ‘communication compartments’ where GJIC is restricted to only cells with similar cell fate? If so, what is the role of the specific connexin isoforms? Does the formation of communication compartment enhance cellular commitment and differentiation? Which intracellular molecules that diffuse through gap junction are responsible for such effects? One interesting candidate is microRNA that diffuses through gap junctions [117], given the increasing appreciation of the important role of microRNA in directing stem cell fate [132]. Indeed, recently it was demonstrated in hESC that siRNA are able to move through gap junctions to affect gene expression of the neighbouring cells [126]. The identification of molecules that mediate intercellular communication through gap junctions will be an important step towards the elucidation of regulatory mechanisms of stem cell survival, differentiation and proliferation. Studies on gap junctions in stem cells can potentially lead to the development of novel methods in expanding stem cells in vitro, directing their differentiation into functional mature cells or cancer treatment that target particular cancer stem cells.
Abbreviations
- α-GA:
-
α-glycyrrhetinic acid
- BMP:
-
Bone morphogenetic protein
- ES cells:
-
Embryonic stem cells
- HSC:
-
Hematopoietic stem cells
- MSC:
-
Mesenchymal stem cells
- GJIC:
-
Gap junctional intercellular communication
- hESC:
-
Human embryonic stem cells
- mESC:
-
Mouse embryonic stem cells
- PDGF:
-
Platelet-derived growth factor
- S1P:
-
Sphingosine-1-phosphate
References
Alexander, D. B., & Goldberg, G. S. (2003). Transfer of biologically important molecules between cells through gap junction channels. Current Medicinal Chemistry, 10(19), 2045–2058.
Assou, S., Lecarrour, T., et al. (2007). A meta-analysis of human embryonic stem cells transcriptome integrated into a web-based expression atlas. Stem Cells, 25, 961–973.
Avanzo, J. L., Mesnil, M., et al. (2004). Increased susceptibility to urethane-induced lung tumors in mice with decreased expression of connexin43. Carcinogenesis, 25(10), 1973–1982.
Bani-Yaghoub, M., Bechberger, J. F., et al. (1997). Reduction of connexin43 expression and dye-coupling during neuronal differentiation of human NTera2/clone D1 cells. Journal of Neuroscience Research, 49(1), 19–31.
Becker, D. L., Evans, W. H., et al. (1995). Functional analysis of amino acid sequences in connexin43 involved in intercellular communication through gap junctions. Journal of Cell Science, 108(Pt 4), 1455–1467.
Belliveau, D. J., Bechberger, J. F., et al. (1997). Differential expression of gap junctions in neurons and astrocytes derived from P19 embryonal carcinoma cells. Developmental Genetics, 21(3), 187–200.
Beqqali, A., Kloots, J., et al. (2006). Genome-wide transcriptional profiling of human embryonic stem cells differentiating to cardiomyocytes. Stem Cells, 24(8), 1956–1967.
Bernacki, S. H., Wall, M. E., et al. (2008). Isolation of human mesenchymal stem cells from bone and adipose tissue. Methods in Cell Biology, 86, 257–278.
Bevilacqua, A., Loch-Caruso, R., et al. (1989). Abnormal development and dye coupling produced by antisense RNA to gap junction protein in mouse preimplantation embryos. Proceedings of National Academy of Sciences of United States of America, 86(14), 5444–5448.
Bhattacharya, B., Cai, J., et al. (2005). Comparison of the gene expression profile of undifferentiated human embryonic stem cell lines and differentiating embryoid bodies. BMC Developmental Biology, 5, 22.
Bhattacharya, B., Miura, T., et al. (2004). Gene expression in human embryonic stem cell lines: unique molecular signature. Blood, 103(8), 2956–2964.
Bloor, D. J., Wilson, Y., et al. (2004). Expression of connexins in human preimplantation embryos in vitro. Reproductive Biology and Endocrinology, 2, 25.
Boyer, L. A., Lee, T. I., et al. (2005). Core transcriptional regulatory circuitry in human embryonic stem cells. Cell, 122(6), 947–956.
Cai, J., Cheng, A., et al. (2004). Membrane properties of rat embryonic multipotent neural stem cells. Journal of Neurochemistry, 88(1), 212–226.
Carpenter, M. K., Rosler, E. S., et al. (2004). Properties of four human embryonic stem cell lines maintained in a feeder-free culture system. Developmental Dynamics, 229(2), 243–258.
Chang, C. C., Trosko, J. E., et al. (1987). Contact insensitivity of a subpopulation of normal human fetal kidney epithelial cells and of human carcinoma cell lines. Cancer Research, 47(6), 1634–1645.
Cheng, A., Tang, H., et al. (2004). Gap junctional communication is required to maintain mouse cortical neural progenitor cells in a proliferative state. Developments in Biologicals, 272(1), 203–216.
Chung, Y., Klimanskaya, I., et al. (2008). Human embryonic stem cell lines generated without embryo destruction. Cell Stem Cell, 2(2), 113–117.
Dahl, E., Winterhager, E., et al. (1996). Expression of the gap junction proteins connexin31 and connexin43 correlates with communication compartments in extraembryonic tissues and in the gastrulating mouse embryo, respectively. Journal of Cell Science, 109(Pt 1), 191–197.
Dale, B., Gualtieri, R., et al. (1991). Intercellular communication in the early human embryo. Molecular Reproduction and Development, 29(1), 22–28.
Davies, T. C., Barr, K. J., et al. (1996). Multiple members of the connexin gene family participate in preimplantation development of the mouse. Developmental Genetics, 18(3), 234–243.
De Maio, A., Vega, V., et al. (2002). Gap junctions, homeostasis, and injury. Journal of Cellular Physiology, 191(3), 269–282.
Dowling-Warriner, C. V., & Trosko, J. E. (2000). Induction of gap junctional intercellular communication, connexin43 expression, and subsequent differentiation in human fetal neuronal cells by stimulation of the cyclic AMP pathway. Neuroscience, 95(3), 859–868.
Duval, N., Gomes, D., et al. (2002). Cell coupling and Cx43 expression in embryonic mouse neural progenitor cells. Journal of Cell Science, 115(Pt 16), 3241–3251.
Ebihara, L. (2003). New roles for connexons. News in Physiological Sciences, 18, 100–103.
Egashira, K., Nishii, K., et al. (2004). Conduction abnormality in gap junction protein connexin45-deficient embryonic stem cell-derived cardiac myocytes. The Anatomical Record. Part A, Discoveries in Molecular, Cellular, and Evolutionary Biology, 280(2), 973–979.
Elfgang, C., Eckert, R., et al. (1995). Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. Journal of Cell Biology, 129(3), 805–817.
Engel, A., & Muller, D. J. (2000). Observing single biomolecules at work with the atomic force microscope. Nature Structural Biology, 7(9), 715–718.
Enver, T., Soneji, S., et al. (2005). Cellular differentiation hierarchies in normal and culture-adapted human embryonic stem cells. Human Molecular Genetics, 14(21), 3129–3140.
Evans, M. J., & Kaufman, M. H. (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature, 292(5819), 154–156.
Evans, W. H., De Vuyst, E., et al. (2006). The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochemical Journal, 397(1), 1–14.
Gage, F. H. (2000). Mammalian neural stem cells. Science, 287(5457), 1433–1438.
Giepmans, B. N. (2004). Gap junctions and connexin-interacting proteins. Cardiovascular Research, 62(2), 233–245.
Goodall, H., & Maro, B. (1986). Major loss of junctional coupling during mitosis in early mouse embryos. Journal of Cell Biology, 102(2), 568–575.
Goodenough, D. A., Goliger, J. A., et al. (1996). Connexins, connexons, and intercellular communication. Annual Reviews of Biochemical, 65, 475–502.
Goodenough, D. A., & Paul, D. L. (2003). Beyond the gap: functions of unpaired connexon channels. Nature Reviews. Molecular Cell Biology, 4(4), 285–294.
Hardy, K., Warner, A., et al. (1996). Expression of intercellular junctions during preimplantation development of the human embryo. Molecular Human Reproduction, 2(8), 621–632.
Hirabayashi, Y., Yoon, B. I., et al. (2007a). Membrane channel connexin 32 maintains Lin(-)/c-kit(+) hematopoietic progenitor cell compartment: analysis of the cell cycle. Journal of Membrane Biology, 217(1–3), 105–113.
Hirabayashi, Y., Yoon, B. I., et al. (2007b). Protective role of connexin 32 in steady-state hematopoiesis, regeneration state, and leukemogenesis. Experimental Biology and Medicine (Maywood), 232(5), 700–712.
Holland, M. S., Tai, M. H., et al. (2003). Isolation and differentiation of bovine mammary gland progenitor cell populations. American Journal of Veterinary Research, 64(4), 396–403.
Houghton, F. D. (2005). Role of gap junctions during early embryo development. Reproduction, 129(2), 129–135.
Houghton, F. D., Barr, K. J., et al. (2002). Functional significance of gap junctional coupling in preimplantation development. Biology of Reproduction, 66(5), 1403–1412.
Huang, Y. J., Maruyama, Y., et al. (2007). The role of pannexin 1 hemichannels in ATP release and cell–cell communication in mouse taste buds. Proceedings of National Academy of Sciences of the United State of America, 104(15), 6436–6441.
Huettner, J. E., Lu, A., et al. (2006). Gap junctions and connexon hemichannels in human embryonic stem cells. Stem Cells, 24(7), 1654–1667.
Kalimi, G. H., & Lo, C. W. (1988). Communication compartments in the gastrulating mouse embryo. Journal of Cell Biology, 107(1), 241–255.
Kalimi, G. H., & Lo, C. W. (1989). Gap junctional communication in the extraembryonic tissues of the gastrulating mouse embryo. Journal of Cell Biology, 109(6 Pt 1), 3015–3026.
Kao, C. Y., Nomata, K., et al. (1995). Two types of normal human breast epithelial cells derived from reduction mammoplasty: phenotypic characterization and response to SV40 transfection. Carcinogenesis, 16(3), 531–538.
Kiel, M. J., He, S., et al. (2007). Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature, 449(7159), 238–242.
King, T. J., Fukushima, L. H., et al. (2000). Correlation between growth control, neoplastic potential and endogenous connexin43 expression in HeLa cell lines: implications for tumor progression. Carcinogenesis, 21(2), 311–315.
Knoblich, J. A. (2008). Mechanisms of asymmetric stem cell division. Cell, 132(4), 583–597.
Krutovskikh, V. A., Yamasaki, H., et al. (1998). Inhibition of intrinsic gap-junction intercellular communication and enhancement of tumorigenicity of the rat bladder carcinoma cell line BC31 by a dominant-negative connexin 43 mutant. Molecular Carcinogenesis, 23(4), 254–261.
Krysko, D. V., Leybaert, L., et al. (2005). Gap junctions and the propagation of cell survival and cell death signals. Apoptosis, 10(3), 459–469.
Kumar, N. M., & Gilula, N. B. (1996). The gap junction communication channel. Cell, 84(3), 381–388.
Kwak, B. R., & Jongsma, H. J. (1996). Regulation of cardiac gap junction channel permeability and conductance by several phosphorylating conditions. Molecular and Cellular Biochemistry, 157(1–2), 93–99.
Lai, C. P., Bechberger, J. F., et al. (2007). Tumor-suppressive effects of pannexin 1 in C6 glioma cells. Cancer Research, 67(4), 1545–1554.
Laird, D. W. (2005). Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochimica et Biophysica Acta, 1711(2), 172–182.
Lampe, P. D., & Lau, A. F. (2004). The effects of connexin phosphorylation on gap junctional communication. International Journal of Biochemistry & Cell Biology, 36(7), 1171–1186.
Lee, S., Gilula, N. B., et al. (1987). Gap junctional communication and compaction during preimplantation stages of mouse development. Cell, 51(5), 851–860.
Lin, T. M., Chang, H. W., et al. (2007). Isolation and identification of mesenchymal stem cells from human lipoma tissue. Biochemical and Biophysical Research Communications, 361(4), 883–889.
Litvin, O., Tiunova, A., et al. (2006). What is hidden in the pannexin treasure trove: the sneak peek and the guesswork. Journal of Cellular and Molecular Medicine, 10(3), 613–634.
Liu, Y., Shin, S., et al. (2006). Genome wide profiling of human embryonic stem cells (hESCs), their derivatives and embryonal carcinoma cells to develop base profiles of U.S. Federal government approved hESC lines. BMC Developmental Biology, 6, 20.
Lo, C. W. (1996). The role of gap junction membrane channels in development. Journal of Bioenergetics and Biomembranes, 28(4), 379–385.
Lo, C. W., & Gilula, N. B. (1979a). Gap junctional communication in the post-implantation mouse embryo. Cell, 18(2), 411–422.
Lo, C. W., & Gilula, N. B. (1979b). Gap junctional communication in the preimplantation mouse embryo. Cell, 18(2), 399–409.
Loewenstein, W. R. (1979). Junctional intercellular communication and the control of growth. Biochimica et Biophysica Acta, 560(1), 1–65.
Loewenstein, W. R., & Kanno, Y. (1966). Intercellular communication and the control of tissue growth: lack of communication between cancer cells. Nature, 209(5029), 1248–1249.
Loewenstein, W. R., & Kanno, Y. (1967). Intercellular communication and tissue growth. I. Cancerous growth. Journal of Cell Biology, 33(2), 225–234.
Loewenstein, W. R., & Rose, B. (1992). The cell–cell channel in the control of growth. Seminars in Cell & Biology, 3(1), 59–79.
Martin, G. R. (1981). Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proceedings of National Academy of Sciences of the United State of America, 78(12), 7634–7638.
Martin, P. E., & Evans, W. H. (2004). Incorporation of connexins into plasma membranes and gap junctions. Cardiovascular Research, 62(2), 378–387.
Matic, M., Evans, W. H., et al. (2002). Epidermal stem cells do not communicate through gap junctions. Journal of Investigative Dermatology, 118(1), 110–116.
Matic, M., Petrov, I. N., et al. (1997). Stem cells of the corneal epithelium lack connexins and metabolite transfer capacity. Differentiation, 61(4), 251–260.
McCulloch, E. A., & Till, J. E. (2005). Perspectives on the properties of stem cells. Natural Medicines, 11(10), 1026–1028.
Melton, D. a., & Cowan, C. (2004). ‘Stemness’: Definitions, criteria and standards. In R. Lanza, J. Gearhart, B. Hogan, D. Melton, R. Pedersen, J. Thomson, & M. West (Eds.), Handbook of stem cells, Vol. 1. Amsterdam: Elsevier.
Mesnil, M., Crespin, S., et al. (2005). Defective gap junctional intercellular communication in the carcinogenic process. Biochimica et Biophysica Acta, 1719(1–2), 125–145.
Mesnil, M., & Yamasaki, H. (2000). Bystander effect in herpes simplex virus-thymidine kinase/ganciclovir cancer gene therapy: role of gap-junctional intercellular communication. Cancer Research, 60(15), 3989–3999.
Mimeault, M., & Batra, S. K. (2006). Concise review: recent advances on the significance of stem cells in tissue regeneration and cancer therapies. Stem Cells, 24(11), 2319–2345.
Montecino-Rodriguez, E., Leathers, H., et al. (2000). Expression of connexin 43 (Cx43) is critical for normal hematopoiesis. Blood, 96(3), 917–924.
Moreno, A. P. (2005). Connexin phosphorylation as a regulatory event linked to channel gating. Biochimica et Biophysica Acta, 1711(2), 164–171.
Neijssen, J., Herberts, C., et al. (2005). Cross-presentation by intercellular peptide transfer through gap junctions. Nature, 434(7029), 83–88.
Nishi, M., Kumar, N. M., et al. (1991). Developmental regulation of gap junction gene expression during mouse embryonic development. Developments in Biologicals, 146(1), 117–130.
Orford, K. W., & Scadden, D. T. (2008). Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nature Reviews. Genetics, 9(2), 115–128.
Oviedo, N. J., & Levin, M. (2007). smedinx-11 is a planarian stem cell gap junction gene required for regeneration and homeostasis. Development, 134(17), 3121–3131.
Oyamada, M., Oyamada, Y., et al. (2005). Regulation of connexin expression. Biochimica et Biophysica Acta, 1719(1–2), 6–23.
Oyamada, Y., Komatsu, K., et al. (1996). Differential regulation of gap junction protein (connexin) genes during cardiomyocytic differentiation of mouse embryonic stem cells in vitro. Experimental Cell Research, 229(2), 318–326.
Panchin, Y., Kelmanson, I., et al. (2000). A ubiquitous family of putative gap junction molecules. Current Biology, 10(13), R473–R474.
Panchin, Y. V. (2005). Evolution of gap junction proteins—the pannexin alternative. Journal of Experimental Biology, 208(Pt 8), 1415–1419.
Parekkadan, B., Berdichevsky, Y., et al. (2008). Cell–cell interaction modulates neuroectodermal specification of embryonic stem cells. Neuroscience Letters, 438(2), 190–195.
Pebay, A., Wong, R. C., et al. (2005). Essential roles of sphingosine-1-phosphate and platelet-derived growth factor in the maintenance of human embryonic stem cells. Stem Cells, 23(10), 1541–1548.
Penn, R. D. (1966). Ionic communication between liver cells. Journal of Cell Biology, 29(1), 171–174.
Pera, M. F., Reubinoff, B., et al. (2000). Human embryonic stem cells. Journal of Cell Science, 113(Pt 1), 5–10.
Phinney, D. G., & Prockop, D. J. (2007). Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells, 25(11), 2896–2902.
Pittenger, M. F., Mackay, A. M., et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science, 284(5411), 143–147.
Ploemacher, R. E., Mayen, A. E., et al. (2000). Hematopoiesis: gap junction intercellular communication is likely to be involved in regulation of stroma-dependent proliferation of hemopoietic stem cells. Hematology, 5(2), 133–147.
Qin, H., Shao, Q., et al. (2002). Retroviral delivery of connexin genes to human breast tumor cells inhibits in vivo tumor growth by a mechanism that is independent of significant gap junctional intercellular communication. Journal of Biological Chemistry, 277(32), 29132–29138.
Reubinoff, B. E., Pera, M. F., et al. (2000). Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nature Biotechnology, 18(4), 399–404.
Revel, J. P., & Karnovsky, M. J. (1967). Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. Journal of Cell Biology, 33(3), C7–C12.
Richards, M., Tan, S. P., et al. (2004). The transcriptome profile of human embryonic stem cells as defined by SAGE. Stem Cells, 22(1), 51–64.
Rosendaal, M., Green, C. R., et al. (1994). Up-regulation of the connexin43+ gap junction network in haemopoietic tissue before the growth of stem cells. Journal of Cell Science, 107(Pt 1), 29–37.
Rosendaal, M., Mayen, A., et al. (1997). Does transmembrane communication through gap junctions enable stem cells to overcome stromal inhibition? Leukemia, 11(8), 1281–1289.
Ruch, R. J., & Trosko, J. E. (2001). Gap-junction communication in chemical carcinogenesis. Drug Metabolism Reviews, 33(1), 117–124.
Russo, R. E., Reali, C., et al. (2008). Connexin 43 delimits functional domains of neurogenic precursors in the spinal cord. Journal of Neuroscience, 28(13), 3298–3309.
Saez, J. C., Berthoud, V. M., et al. (2003). Plasma membrane channels formed by connexins: Their regulation and functions. Physiological Reviews, 83(4), 1359–1400.
Sosinsky, G. E., & Nicholson, B. J. (2005). Structural organization of gap junction channels. Biochimica et Biophysica Acta, 1711(2), 99–125.
Sperger, J. M., Chen, X., et al. (2003). Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proceedings of National Academy of Sciences of the United State of America, 100(23), 13350–13355.
Stein, L. S., Boonstra, J., et al. (1992). Reduced cell–cell communication between mitotic and nonmitotic coupled cells. Experimental Cell Research, 198(1), 1–7.
Stojkovic, M., Lako, M., et al. (2004). Derivation of human embryonic stem cells from day-8 blastocysts recovered after three-step in vitro culture. Stem Cells, 22(5), 790–797.
Strelchenko, N., Verlinsky, O., et al. (2004). Morula-derived human embryonic stem cells. Reproductive Biomedicine Online, 9(6), 623–629.
Tai, M. H., Olson, L. K., et al. (2003). Characterization of gap junctional intercellular communication in immortalized human pancreatic ductal epithelial cells with stem cell characteristics. Pancreas, 26(1), e18–e26.
Temme, A., Buchmann, A., et al. (1997). High incidence of spontaneous and chemically induced liver tumors in mice deficient for connexin32. Current Biology, 7(9), 713–716.
Thomson, J. A., Itskovitz-Eldor, J., et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science, 282(5391), 1145–1147.
Todorova, M. G., Soria, B., et al. (2008). Gap junctional intercellular communication is required to maintain embryonic stem cells in a non-differentiated and proliferative state. Journal of Cellular Physiology, 214(2), 354–362.
Traver, D. a. & Akashi, K. (2004). Common myeloid progenitors. In Handbook of Stem Cells (M. a. T. 2005), Elsevier 1.
Trosko, J. E. (2003). Human stem cells as targets for the aging and diseases of aging processes. Medical Hypotheses, 60(3), 439–447.
Valiunas, V., Bukauskas, F. F., et al. (1997). Conductances and selective permeability of connexin43 gap junction channels examined in neonatal rat heart cells. Circulation Research, 80(5), 708–719.
Valiunas, V., Doronin, S., et al. (2004). Human mesenchymal stem cells make cardiac connexins and form functional gap junctions. Journal of Physiology, 555(Pt 3), 617–626.
Valiunas, V., Polosina, Y. Y., et al. (2005). Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. Journal of Physiology, 568(Pt 2), 459–468.
Vance, M. M., & Wiley, L. M. (1999). Gap junction intercellular communication mediates the competitive cell proliferation disadvantage of irradiated mouse preimplantation embryos in aggregation chimeras. Radiation Research, 152(5), 544–551.
Verfaillie, C. M., Pera, M. F., et al. (2002). Stem cells: hype and reality. Hematology (American Society of Hematology. Education Program), 369–391.
Villars, F., Guillotin, B., et al. (2002). Effect of HUVEC on human osteoprogenitor cell differentiation needs heterotypic gap junction communication. American Journal of Physiology. Cell Physiology, 282(4), C775–C785.
Vine, A. L., & Bertram, J. S. (2002). Cancer chemoprevention by connexins. Cancer Metastasis Reviews, 21(3–4), 199–216.
Wei, C. J., Xu, X., et al. (2004). Connexins and cell signaling in development and disease. Annual Review of Cell and Developmental Biology, 20, 811–838.
Weiss, M. L., & Troyer, D. L. (2006). Stem cells in the umbilical cord. Stem Cell Reviews, 2(2), 155–162.
Wen, C. M., Cheng, Y. H., et al. (2008). Isolation and characterization of a neural progenitor cell line from tilapia brain. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology, 149(2), 167–180.
Willecke, K., Eiberger, J., et al. (2002). Structural and functional diversity of connexin genes in the mouse and human genome. Biological Chemistry, 383(5), 725–737.
Wolvetang, E. J., Pera, M. F., et al. (2007). Gap junction mediated transport of shRNA between human embryonic stem cells. Biochemical and Biophysical Research Communications, 363(3), 610–615.
Wong, R. C., Dottori, M., et al. (2006). Gap junctions modulate apoptosis and colony growth of human embryonic stem cells maintained in a serum-free system. Biochemical and Biophysical Research Communications, 344(1), 181–188.
Wong, R. C., Pebay, A., et al. (2004). Presence of functional gap junctions in human embryonic stem cells. Stem Cells, 22(6), 883–889.
Worsdorfer, P., Maxeiner, S., et al. (2008). Connexin expression and functional analysis of gap junctional communication in mouse embryonic stem cells. Stem Cells, 26(2), 431–439.
Yamasaki, H., Krutovskikh, V., et al. (1999). Role of connexin (gap junction) genes in cell growth control and carcinogenesis. Comptes Rendus de l’AcadeÂmie des Sciences III, 322(2–3), 151–159.
Yang, S. R., Cho, S. D., et al. (2005). Role of gap junctional intercellular communication (GJIC) through p38 and ERK1/2 pathway in the differentiation of rat neuronal stem cells. Journal of Veterinary Medical Science, 67(3), 291–294.
Zhang, B., Pan, X., et al. (2006). MicroRNA: a new player in stem cells. Journal of Cellular Physiology, 209(2), 266–269.
Acknowledgements
This work was supported by the California Institute of Regenerative Medicine, the University of Melbourne and the National Health and Medical Research Council of Australia (NHMRC 454723).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wong, R.C.B., Pera, M.F. & Pébay, A. Role of Gap Junctions in Embryonic and Somatic Stem Cells. Stem Cell Rev 4, 283–292 (2008). https://doi.org/10.1007/s12015-008-9038-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-008-9038-9