Abstract
The objective of the study is to explore change and significance of IL-8, IL-4 and IL-10 in the pathogenesis of terminal Ileitis in SD rat. 60 male SD rats were divided into model group, suture group, and control group equally. The rats subjected to ileum-cecum side-to-side anastomosis in terminal ileum in model group, suture in terminal ileum in suture group, and the control group accepted no special treatment. The terminal ileum tissue which was 1–3 cm from anastomotic stoma was collected at 2 and 8 weeks after surgery in each group. The pathological slice was observed under microscope, and PCR was applied to detect the expression of IL-4, IL-8, and IL-10 at different times. Pathological result showed that neutrophils significantly increased in model group and suture group at 2nd week, showing acute inflammatory reaction; model group showed chronic inflammation at 8th week. The change of IL-8, IL-4, and IL-10 expression level at 2 weeks after surgery: The IL-8 expression level of SD rat terminal ileum tissue in model group was significantly higher than in control and suture groups (P < 0.01), and it was higher in suture group compared to control group (P < 0.01); the expression level of IL-4 in control group was higher than model and suture groups (P < 0.05); there was no statistical significance between model group and suture group (P = 0.363); the expression level of IL-10 in control group was higher than in model and suture groups (P < 0.01), and it was higher in suture group compared to model group (P < 0.01). The change of IL-8, IL-4, IL-10 expression level at 8 weeks after surgery: The expression level of IL-8 significantly decreased in model group, and there was no significantly difference between three groups (P > 0.05); the expression level of IL-4 was higher in model group and suture group compared to 2nd week; there was no significance between three groups (P < 0.05); the expression of IL-10 was higher in model group compared to 2nd week (P < 0.01), it was lower than control group and suture group (P < 0.01); there was no significant difference between suture group and control group (P > 0.05). The chronic terminal ileum model could be successfully established by ileum-cecum side-to-side anastomosis in terminal ileum in SD rats; IL-8 can induce the inflammatory reaction in terminal ileitis and chemokines aggregation and mediate inflammatory reaction by mediating other inflammatory factors; as a proinflammatory cytokine, IL-8 can inhibit IL-10; IL-10 and IL-4 can inhibit the inflammatory reaction of terminal ileum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Terminal ileitis [5, 7, 23, 27] is a disease, which is different from intestinal tuberculosis [15] and Crohn’s disease [1]. The pathogenesis is still unclear, stimulating the abundant lymphoid tissue in terminal ileum to cause immunoreaction and damage the local mucosa to further cause the damage of terminal ileum tissue may be the key pathogenesis [13, 22]. As a proinflammatory factor, IL-8 has chemotactic effect on inflammatory cells such as neutrophil [10], macrophage [12], and monocyte [31] and also has mediation on inflammatory factors such as IL-1, IL-2, IL-6, IL-12, TNF, and interferon [2, 6, 17, 20, 26, 29]; it has significant mediating effect on immune response. There have been few of researches about the mechanism of cytokine action in the morbidity of CTI. We selected IL-8 and IL-10 to explore the mechanism in CTI by the change of expression level in CTI pathogenesis.
Materials and Methods
Establishment of Animal Model
All 60 male SD rats, we used, were all provided by experimental animal department in the University of South China; the average weight was 250–300 g/rat. The establishment of animal model [15]: there were 20 rats in model group, suture group, and control group, respectively. Rats were subjected preoperative fasting for 12 h, then accepted abdominal anesthesia by 0.3–0.4 ml 3 % pentobarbital sodium. The abdominal surgery was done under aseptic conditions, and the laparotomy was performed to expose ileocecal junction to perform ileum-cecum side-to-side anastomosis in terminal ileum. Suture group was treated the same as the model group except performing surgical suture at terminal ileum; the control group accepted no special treatment. Besides, rats in three groups accepted the same diet composition and timing. They subjected fasting for 1 day after surgery and were given one in third amount of daily routine diet. Then their diet increased to three-fourth of routine diet 1 week after surgery. The rats were killed at 2nd or 8th week after surgery.
Collection and Preservation of Tissue
The terminal ileum tissue (small intestine tissue which was 1–3 cm from anastomotic stoma) was obtained for general observation and sliced for microscope at 2nd or 8th week after surgery [27]; besides, 50 mg tissue was obtained for RNA extraction or immediately transferred in liquid nitrogen and preserved in −80 °C fridge for further use.
Making and Scoring Criteria of Pathological Section
We obtained intestine tissue in the size of 0.3 × 1.5 cm at low temperature to do conventional paraffin embedding to make 5-μm thick section. HE staining was applied to do histologic examination; Theresa evaluation criteria were used to score histological morphology of ileum tissue.
The Expression of IL-8, IL-4, and IL-10 Detected by PCR
Fifty mg tissue was obtained for RNA extraction or immediately transferred in liquid nitrogen and preserved in −80 °C fridge for further use. Real-time PCR: The IL-8, IL-4, and IL-10 gene sequences of SD rat were downloaded from NCBI. They were quantitatively analyzed by RNA extraction, reverse transcription reaction (synthesis of cDNA), and real-time PCR amplification.
Statistical Method
All data were analyzed by SPSS 18.0. Measurement data were shown as mean ± standard deviation (\(\overline{x} \pm {\text{S}}\)); t test was applied to compare two samples. Comparison of multiple samples was performed by One-Way ANOVA. LSD t test was used for pairwise comparison of any two means. Count data were tested by \(x^{ 2}\) test. Correlation analysis was conducted by the linear correlation analysis. Differences in values with P < 0.05 were considered significant.
Results
General Change of SD rats 2 Weeks After Surgery
SD rats showed decreased appetite, poor hair luster, hair loss, obvious weight loss. The weight loss was most obvious 3 days after surgery. Along with the oral intake increase, the hair luster and hair loss obviously improved; Weight of SD rats in model group had no significant difference from the other groups 8 weeks after surgery (P > 0.05). Four rats in model group died at 7–10 days after modeling, and after laparotomy, three rats were found to have intestinal obstruction and one rat to have intestinal perforation, one rat died because of the intestinal obstruction in suture group. No one died in control group, the difference had no statistical significance among three groups (P > 0.05).
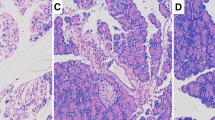
Pathological Result of Terminal Ileum Tissue: Observation Under Microscope
The result of tissue section comparison among three groups was shown in Fig. 1. Suture group and model group both showed acute inflammatory manifestation; the inflammatory scoring was lower in suture group than in model group (P < 0.05). However, only model group showed chronic inflammatory reaction, suture group was almost recovered. The inflammatory score of model group was obviously higher than suture group (P < 0.05).
Expression Level Change of IL-8, IL-4 and IL-10 at Different Time Point After Surgery
Comparison of IL-8, IL-4, and IL-10 expression after surgery was shown in Fig. 2. IL-8 showed high expression level in terminal ileum tissue of model group and suture group in result (P < 0.01). IL-4 and IL-8 showed lower expression level in terminal ileum tissue of model group (P < 0.05). IL-8 and IL-4 showed same expression level in terminal ileum tissue of three groups at 8th week (P < 0.01). However, IL-10 expression was obviously lower in model group than in suture group and control group.
The expression of IL-8 (a), IL-4 (b), and IL-10 (c) of different groups at 2 weeks or 8 weeks. Representative electrophoresis images were displayed underneath the diagraphs. C2 = control group at 2 weeks; C8 = control group at 8 weeks; S2 = suture group at 2 weeks; S8 = suture group at 8 weeks; M2 = model group at 2 weeks, M8 = model group at 8 weeks
Discussion
CTI occurs mostly in adult patients [11] and occasionally seen in children [18]. It has been troubling patients and affecting health of patients for a long time. After a long period of clinical and animal experimental study, dysfunction of ileocecal valve was found to be the initial factor of CTI, which inducing colon-ileum reflux to cause infection of terminal ileum [30].
According to the pathological observation result of postoperative terminal ileum in our study, besides the factor of surgery itself [24], bacterial infection caused by colon-ileum reflux inducing proliferation of inflammatory cells such as neutrophil and monocyte and secretion of inflammatory factors by these inflammatory cells may be the reason of acute inflammation [14, 19]. Along with the proliferation of lymphocyte, immunity of local intestine tissue increased and the inflammation tended to be chronic. Maybe decrease of protective factors and strong immune reaction of local terminal ileum can cause damage of local intestine mucosa tissue in the process of inflammatory reaction [25, 28], which was in accordance with histopathological change we observed in different periods.
Expression level of IL-8 in pathological mucosa of terminal ileum was significantly higher in model group than in control group; furthermore, this was positively correlated with the neutrophil increase in mucosa tissue and general inflammation of local mucosa lesion; this result was in accordance with what Daig et al. [8] found in clinical research and animal experiment. IL-4 mRNA showed highest expression in control group, and it was lower in model group and suture group at 2nd week (P < 0.05). In acute inflammatory stage of intestine tissue, significant decrease of IL-10 expression might be caused by inhibition by a variety of increased inflammatory factors such as IL-8 or TNF [21]. Low expression level of IL-10 and IL-4 which is unable to inhibit proinflammatory cytokines and chemokines further promotes the inflammatory reaction of intestine mucosa [16]; Another possibility is that change of Th1/Th2 ratio caused by proliferation of various inflammatory cells in acute inflammatory stage of SD rat terminal ileum tissue can affect IL-10 secretion by Th2 cells in SD rat terminal ileum tissue [3]. IL-8 expression in SD rat terminal ileum in suture group was higher than in control group 2 week after surgery, and IL-10 expression was lower than in control group. Together with corresponding pathological manifestations, this may be related to surgical injury.
IL-8 expression level in SD rat terminal ileum tissue in model group at 8th week after surgery was significantly decreased compared to 2nd week, there was no significant difference compared with the other two groups. Expression level of IL-10 and IL-4 in SD rat terminal ileum tissue significantly increased at 8th week compared to 2nd week (P < 0.01). This may because decrease of inflammatory factors in local terminal ileum along with the recovery of intestinal anastomosis reduces the inhibition on IL-10 and IL-4 [4], or macrophages and monocytes in local terminal ileum activated by inflammation increase the secretion of IL-10 and IL-4. This is possibly related with the increased immunity of local terminal tissue. There is abundant lymphoid tissue in terminal ileum to inhibit the further development of inflammation, and just because of this, expression levels of anti-inflammatory factors such as IL-10 and IL-4 were inhibited to decrease inflammatory cells and inhibit the expression of inflammatory factors such as IL-8. Danase et al’s research confirmed that IL-8 could induce transmigration of microvascular endothelial cell in intestine to prompt angiogenesis [9]. This is in accordance with that we found capillary proliferation in inflammatory intestine mucosa under microscope, and this is recognized as one of factors which induce inflammatory reaction in terminal ileum tissue.
Our research provides great help to improve the treatment, diagnosis, and prevention of CTI. At present, many cytokine receptors have been successfully cloned including IL-10R. According to the anti-inflammatory effect of IL-10 and IL-4, we are going to intervene IL-10R and IL-4R in experimental animal to explore its applicable value on CTI.
References
Almadi, M. A., Ghosh, S., & Aljebreen, A. M. (2009). Differentiating intestinal tuberculosis from Crohn’s disease: A diagnostic challenge. The American journal of gastroenterology, 104(4), 1003–1012.
Azevedo, V. F., Faria-Neto, J. R., Stinghen, A., Lorencetti, P. G., Miller, W. P., Goncalves, B. P., et al. (2013). IL-8 but not other biomarkers of endothelial damage is associated with disease activity in patients with ankylosing spondylitis without treatment with anti-TNF agents. Rheumatology International, 33(7), 1779–1783.
Bashyam, H. (2007). Th1/Th2 cross-regulation and the discovery of IL-10. The Journal of Experimental Medicine, 204(2), 237.
Borowiec, A. M., Sydora, B. C., Doyle, J., le Guan, L., Churchill, T. A., Madsen, K., et al. (2012). Small bowel fibrosis and systemic inflammatory response after ileocolonic anastomosis in IL-10 null mice. The Journal of Surgical Research, 178(1), 147–154.
Calabrese, C., Gionchetti, P., Rizzello, F., Liguori, G., Gabusi, V., Tambasco, R., et al. (2008). Short-term treatment with infliximab in chronic refractory pouchitis and ileitis. Alimentary Pharmacology & Therapeutics, 27(9), 759–764.
Candel-Marti, M. E., Flichy-Fernandez, A. J., Alegre-Domingo, T., Ata-Ali, J., & Penarrocha-Diago, M. A. (2011). Interleukins IL-6, IL-8, IL-10, IL-12 and periimplant disease. An update. Medicina oral patologia oral y cirugia bucal, 16(4), e518–e521.
Coon, S., & Sundaram, U. (2000). Mechanism of glucocorticoid-mediated reversal of inhibition of Cl(-)/HCO(-)(3) exchange during chronic ileitis. American Journal of Physiology, 278(4), G570–G577.
Daig, R., Rogler, G., Aschenbrenner, E., Vogl, D., Falk, W., Gross, V., et al. (2000). Human intestinal epithelial cells secrete interleukin-1 receptor antagonist and interleukin-8 but not interleukin-1 or interleukin-6. Gut, 46(3), 350–358.
Danese, S., Sans, M., de la Motte, C., Graziani, C., West, G., Phillips, M. H., et al. (2006). Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology, 130(7), 2060–2073.
de Oliveira, S., Reyes-Aldasoro, C. C., Candel, S., Renshaw, S. A., Mulero, V., & Calado, A. (2013). Cxcl8 (IL-8) mediates neutrophil recruitment and behavior in the zebrafish inflammatory response. J Immunol, 190(8), 4349–4359.
Desmond, A. N., & Shanahan, F. (2012). Managing chronic disease in Ireland: Hospital admission rates and clinical outcomes in a large ulcerative colitis population. Irish Journal of Medical Science, 181(1), 65–71.
Dzierzanowska-Fangrat, K., Michalkiewicz, J., Cielecka-Kuszyk, J., Nowak, M., Celinska-Cedro, D., Rozynek, E., et al. (2008). Enhanced gastric IL-18 mRNA expression in Helicobacter pylori-infected children is associated with macrophage infiltration, IL-8, and IL-1 beta mRNA expression. European Journal of Gastroenterology and Hepatology, 20(4), 314–319.
Fadeev, M., & Smirnov, V. P. (1999). Mucosal morphology in the latent form of terminal ileitis in children based on biopsy data. Arkhiv Patologii, 61(4), 41–43.
Fernandes, E. R., Pagliari, C., Tuon, F. F., De Andrade, H. F., Jr, Averbach, M., & Averbach, M. I. (2008). Chronic colitis associated with HIV infection can be related to intraepithelial infiltration of the colon by CD8+T lymphocytes. International Journal of STD and AIDS, 19(8), 524–528.
Fernandez Bueno, F., Antequera Perez, A., De La Torre Gonzalez, J., Rivera Diaz, A., & Pereira Perez, F. (2009). [Intestinal tuberculosis]. Cirugia espanola, 86(3), 186–188.
Fukatsu, K., Kudsk, K. A., Zarzaur, B. L., Wu, Y., Hanna, M. K., & DeWitt, R. C. (2001). TPN decreases IL-4 and IL-10 mRNA expression in lipopolysaccharide stimulated intestinal lamina propria cells but glutamine supplementation preserves the expression. Shock, 15(4), 318–322.
Katsumata, Y., Harigai, M., Kawaguchi, Y., Fukasawa, C., Soejima, M., Takagi, K., et al. (2007). Diagnostic reliability of cerebral spinal fluid tests for acute confusional state (delirium) in patients with systemic lupus erythematosus: Interleukin 6 (IL-6), IL-8, interferon-alpha, IgG index, and Q-albumin. The Journal of rheumatology, 34(10), 2010–2017.
Kennedy, R., Potter, D. D., Moir, C., Zarroug, A. E., Faubion, W., & Tung, J. (2012). Pediatric chronic ulcerative colitis: Does infliximab increase post-ileal pouch anal anastomosis complications? Journal of Pediatric Surgery, 47(1), 199–203.
Levison, S. E., McLaughlin, J. T., Zeef, L. A., Fisher, P., Grencis, R. K., & Pennock, J. L. (2010). Colonic transcriptional profiling in resistance and susceptibility to trichuriasis: Phenotyping a chronic colitis and lessons for iatrogenic helminthosis. Inflammatory Bowel Diseases, 16(12), 2065–2079.
Martinez, F. O., Sironi, M., Vecchi, A., Colotta, F., Mantovani, A., & Locati, M. (2004). IL-8 induces a specific transcriptional profile in human neutrophils: Synergism with LPS for IL-1 production. European Journal of Immunology, 34(8), 2286–2292.
Martinez-Prado, E. (2010). Camejo Bermudez MI: Expression of IL-6, IL-8, TNF-alpha, IL-10, HSP-60, anti-HSP-60 antibodies, and anti-sperm antibodies, in semen of men with leukocytes and/or bacteria. American Journal of Reproductive Immunology, 63(3), 233–243.
McNamee, E. N., Masterson, J. C., Jedlicka, P., Collins, C. B., Williams, I. R., & Rivera-Nieves, J. (2013). Ectopic lymphoid tissue alters the chemokine gradient, increases lymphocyte retention and exacerbates murine ileitis. Gut, 62(1), 53–62.
McNamee, E. N., Wermers, J. D., Masterson, J. C., Collins, C. B., Lebsack, M. D., Fillon, S., et al. (2010). Novel model of TH2-polarized chronic ileitis: The SAMP1 mouse. Inflammatory Bowel Diseases, 16(5), 743–752.
Mortensen, N. (2000). Fulminant acute and diffuse chronic ulcerative colitis–the argument for surgery. Acta gastro-enterologica Belgica, 63(3), 289.
Ostanin, D. V., Bao, J., Koboziev, I., Gray, L., Robinson-Jackson, S. A., Kosloski-Davidson, M., et al. (2009). T cell transfer model of chronic colitis: Concepts, considerations, and tricks of the trade. American Journal of Physiology, 296(2), G135–G146.
Penson, R. T., Kronish, K., Duan, Z., Feller, A. J., Stark, P., Cook, S. E., et al. (2000). Cytokines IL-1beta, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNFalpha in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. International journal of gynecological cancer, 10(1), 33–41.
Rivera-Nieves, J., Burcin, T. L., Olson, T. S., Morris, M. A., McDuffie, M., Cominelli, F., et al. (2006). Critical role of endothelial P-selectin glycoprotein ligand 1 in chronic murine ileitis. The Journal of Experimental Medicine, 203(4), 907–917.
Rubin, D. C., Shaker, A., & Levin, M. S. (2012). Chronic intestinal inflammation: Inflammatory bowel disease and colitis-associated colon cancer. Frontiers in immunology, 3, 107.
Samaras, V., Piperi, C., Levidou, G., Zisakis, A., Kavantzas, N., Themistocleous, M. S., et al. (2009). Analysis of interleukin (IL)-8 expression in human astrocytomas: Associations with IL-6, cyclooxygenase-2, vascular endothelial growth factor, and microvessel morphometry. Human Immunology, 70(6), 391–397.
Sunami, Y., Sato, E., Ichikawa, K., Yasuda, H., & Komatsu, N. (2011). Hemorrhagic colitis caused by dasatinib following cytomegalovirus enterocolitis in a patient with chronic myelogenous leukemia in the second chronic phase. [Rinsho ketsueki] The Japanese journal of clinical hematology, 52(5), 282–286.
Zhu, K., Shen, Q., Ulrich, M., & Zheng, M. (2000). Human monocyte-derived dendritic cells expressing both chemotactic cytokines IL-8, MCP-1, RANTES and their receptors, and their selective migration to these chemokines. Chinese Medical Journal, 113(12), 1124–1128.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hong-yu Zhou and Jun Yan have contributed equally.
Rights and permissions
About this article
Cite this article
Zhou, Hy., Yan, J., Fang, L. et al. Change and Significance of IL-8, IL-4, and IL-10 in the Pathogenesis of Terminal Ileitis in SD Rat. Cell Biochem Biophys 69, 327–331 (2014). https://doi.org/10.1007/s12013-013-9802-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-013-9802-6