Abstract
The aim of the present study was to investigate the effects of recombinant human anti-mullerian hormone (rhAMH) on Stem Cell Factor (SCF) expression in human granulosa cells (GCs). GCs were obtained from infertile patients undergoing IVF-ET cycles and cultured with 20 ng/ml of rhAMH. The levels of SCF mRNA and protein were detected in both matched and experimental group by real-time PCR, immunofluorescence, and ELISA, respectively, on day 4 of culture. We found that human GCs expressed SCF mRNA and protein, and SCF expression in the experimental group was significantly lower than that in the matched group (p < 0.05). We further showed that rhAMH inhibited SCF expression at mRNA and protein levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anti-mullerian hormone (AMH) and Stem Cell Factor (SCF) are important cytokines secreted by granulosa cells (GCs), and they play an important role in the recruitment of the primordial follicle pool and oocyte maturation [1–3]. It is reported that AMH is currently the only cytokine that can inhibit growth of the primordial follicle in vivo. GCs in the ovary express the specific AMH type II receptors (AMHRII). Nilsson et al. [4] showed that AMH can regulate important transcription factors though Smad protein in the signaling pathways in GCs, and then to modulate gene transcription of other cytokines. SCF is an important growth factor for localized signaling within the ovary. In the ovary, SCF generated by GCs can directly stimulate the growth and differentiation of oocytes and theca cells, and trigger steroid hormone production. By using in vitro rat ovary culture, Reynaud et al. [5] found that AMH inhibited the SCF-stimulated growth of oocytes. Based on these findings, we speculated that SCF may be one of the factors acting on oocytes, which is regulated by AMH. However, whether AMH regulates SCF expression remains unknown. In the present study, we treated human GCs with 20 ng/ml rhAMH to examine the effects of AMH on SCF expression and explored the mechanism of follicle collection that is inhibited by AMH though the regulation of SCF secretion.
Materials and Methods
Patients
This study was conducted in 15 patients who underwent COH as part of their IVF treatment. The inclusion criteria were female age under 35 years with infertility due to male or tubal factors. Patients with endometriosis and polycystic ovarian syndrome were excluded. Patients received an intramuscular injection of 1.25 mg of long-acting Diphereline (Ipsen) during the middle of their luteal phase to downregulate the pituitary and an intramuscular injection of 150–450 U of FSH-HP on the 3rd day of their menstrual cycle to superstimulate ovulation. Intramuscular HCG (10,000 IU) was administered when 3 or more follicles were ≥16 mm in diameter, and after 34-36h follicles were aspirated by vaginal oocyte retrieval under the guidance of ultrasound. The study was approved by the local institutional review board of hospital of Ningxia medical university. A written informed consent was obtained from each participating subject.
Isolation of GCs
Granulosa cells surrounding the oocyte were stripped carefully under a microscope, retained in the follicular fluid and centrifuged at 400×g for 5 min at room temperature and the pellets were resuspended in phosphate buffered solution (PBS). Then the precipitate was transferred to 50 % (volume fraction) Percoll gradient (TBD) and centrifuged at 400×g for 20 min. The GCs in the interface layer were digested with hyaluronidase at a 2:1 ratio for 30 min. Following centrifugation, the cells were washed with PBS. Total cell viability was confirmed to be 75 % by Trypan Blue staining.
Primary Granulosa Luteal Cell Culture
Purified cells were seeded in a 24-well culture plate at a density of l × 105/mL. Each well contained 1 ml of M199 medium (GIBCO) supplemented with 10 % fetal bovine serum (FBS) (GIBCO), 2 mM l-Glutamine, 1 mM sodium pyruvate, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were divided into 2 groups and cultured at 37 °C in a humidified atmosphere of 95 % air and 5 % CO2 incubator. In our initial study, we combined human GC with 20 ng/ml rhAMH (R&D American). Cells without rhAMH treatment served as negative control. The cells were stored for total RNA extraction and immunofluorescence, and the cell culture supernatants were stored for ELISA assay.
SCF RNA Extraction and Real-Time PCR
The total RNA extraction procedure was performed according to the manufacturer’s instructions (Qiagen). We used ReverTra Ace reverse transcriptase to copy RNA into cDNA and stored the RNA samples at −80 °C. Real-time PCR were performed with the ABI Prism 7500 Fast Detection System (ABI). The forward and reverse primers were as follows: SCF total (134 bp), 5′-GTCGATGACCTTGTGGAGTG-3′ and 5′-TTGAAGGCATCAATGGATCT-3′. Action (91 bp), 5′-CGGGACCTGACTGA CTACCTC-3′and 5′-TAATGTCACGCACGATTTCCC-3′. PCR products were examined by agarose (1.5 %) gel electrophoresis. β-actin served as internal control quantified, and used for normalization. The relative quantification of the genes of interest was analyzed with the comparative threshold cycles (CT). The values were used to plot the expression 2−∆∆CT.
Immunofluorescence
The cellular distribution of SCF in GCs was determined by indirect immunofluorescence. Samples were blocked in 3–4 % paraformaldehyde in PBS for 15 min at room temperature, followed by washing with PBS and incubating the samples for 10 min with PBS containing 0.25 % triton X-100. Then the cells were incubated with 1 % BSA in PBST for 30 min at room temperature. Cells were then incubated with SCF antibody (1:1,500 dilution; Abcam) in 1 % BSA in PBST in a humidified chamber overnight at 4 °C, followed by washing with PBS and subsequently incubated with goat anti-rabbit IgG-TRITC (1:5,000 dilution; Santa Cruz) for 1 h at temperature in dark. Finally, cells were incubated with 0.1–1 μg/ml DAPI for 1 min. Images were captured with a NIKON microscope (NIKON TE2000). The flow cytometry analysis (multicolor flow cytometry) was used to measure and analyze SCF fluorescence intensity (BD Tech).
ELISA
The cell culture supernatants were collected by centrifugation and stored at −80 °C. The procedure was performed with an ultrasensitive Human SCF Quantikine ELISA Kit (R&D) following the manufacturer’s instructions. OD values were measured at 450 nm using a spectrophotometer (Bio-rad M680). The concentrations of SCF in the samples were determined by comparing the OD values of the samples to the standard curve.
Statistical Analysis
Experiments were performed for a minimum of three replicates. The data were processed for statistical analysis by spss16.0 and are presented as the mean ± SE of the mean. Statistical comparisons between each group were calculated by independent-sample t test and p < 0.05 were considered statistically significant.
Results
Expression of SCF mRNA
A quantitative SYBR Green real-time PCR method was used for detection of SCF mRNA expression in human GCs, and to examine the effect of rhAMH on SCF mRNA expression. We found that there was a dramatic difference in relative levels of SCF mRNA between control group (without rhAMH) and experimental group (GCs treated with 20 ng/ml rhAMH) (*p < 0.05) (Fig. 1).
Identification of SCF in Human GCs
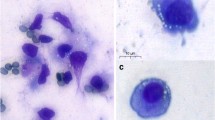
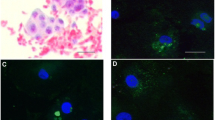
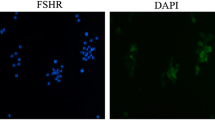
As shown in Fig. 2a–f, SCF staining was localized to cytoplasm of GC cells, and the fluorescence intensity in human GCs treated with 20 ng/ml rhAMH was weaker than that of control cells. It was illustrated in Fig. 3 that SCF protein level was lower in the experimental group than that in the control group (*p < 0.05).
Flow cytometry analysis of SCF protein expression in human GCs using TRITC fluorescence-labeled antibody Immunofluorescence System. a–f Immunofluorescence analysis on GCs with anti-SCF (red) antibodies identified the effect of rhAMH in control (a–c) and experimental group (d–f), respectively. g Quantification analysis of SCF expression in TRITC positive cells treated with 0 ng/ml (control) rhAMH and 20 ng/ml rhAMH (*p < 0.05). Data were the average of three independent experiments (Color figure online)
Discussion
AMH, also known as Müllerian-inhibiting substance (MIS), is a member of the transforming growth factor-ß (TGF-ß) superfamily [6]. Studies have shown that the primordial follicle bank of female mice was exhausted at an early stage when AMH was absent, revealing that AMH was bound up with the ovarian reserve [7]. AMH was mainly produced by parental and antral follicles of the GCs, and the function of GCs and follicles might be regulated by AMH through autocrine and paracrine mechanisms. AMH was detected in the serum from the early puberty stages to complete sexual maturity. The concentration of serum AMH could reflect the size of the primordial follicle pool and be a marker of ovarian reserve function [8]. We found that AMH, which is the only cytokine that can inhibit the growth of primordial follicle and is secreted by GCs, exerted its biological effects through autocrine and paracrine signals. AMH bound to its specific receptor (AMHRII) in GCs and was transduced into the nucleus by Smad3, then AMH inhibited the CYP19 mRNA expression in the nucleus, and decreased the activity of cytochrome P450 to downregulate the secretion of estrogen. AMH also inhibited the collection of primordial follicles [9]. Moreover, as a member of the TGF-β family, AMH, can not only modulate important transcription factors found in intracellular signaling pathways through the Smad protein, but also inhibit the expression of most follicle growth stimulating factors, and eventually suppress the collection of primordial follicles. The latter might be the principal mechanism underlying inhibition of follicle collection by AMH [4]. AMH-regulated intracellular signaling pathway for oocyte growth has not been reported. Thus, the identification of a pathway linking AMH and the oocyte growth could ultimately contribute to the preservation and promotion of human fertility.
SCF, which belongs to the colony stimulating factor (CSF) family, is an important cytokine in the local ovary that promotes oocyte growth. SCF is secreted by GCs and binds to Kit on the oocyte membrane, which accelerates the collection of primordial follicles by PI3-AKT [2]. Whereas, few reports detail the expression and regulation of SCF in GCs. Eric Nilsson found that AMH might inhibit SCF-promoted oocyte growth [4]. AMH and SCF play opposite roles in the human local ovary tissue, and a relationship between the two factors has not been reported.
By observing that the expression of SCF mRNA in human luteinized GCs was effected by 20 ng/ml rhAMH, We hypothesized that AMH inhibit primordial follicle collection by down–regulating the expression of SCF. We found that the GCs growth rate was very slow and adherent cells appeared within 24 h of culture. Cell division peaked within 2–5 days of culture, and the cells began to degenerate after 7 days of culture [10]. Therefore, we chose to detect SCF mRNA and protein in GCs on day 4 of culture in the presence of 20 ng/ml rhAMH, and found that AMH can inhibit the expression of SCF mRNA and protein.
We speculated that AMH might reduce the transcription of SCF by inhibiting cAMP. It was found that AMH could inhibit the secretion of estrogen in GCs, which was promoted by FSH when AMH and FSH were used to co-intervene the culture of human GCs in vitro [10]. FSH promoted the transcription of estrogen synthase though the cAMP pathway after it bound to membrane receptors on GCs. However AMH inhibited phosphorylation of CREB in the cAMP pathway through autocrine and paracrine mechanisms, and reduced the transcription of estrogen synthase [11]. In addition, it has been reported that cAMP pathway could enhance the transcription of SCF [12]. Silva et al. [13] reported that there was a binding site for the cAMP response element CRE in the SCF promoter. Based upon these findings, we hypothesized that AMH inhibited the phosphorylation of CREB in the cAMP pathway, thus prohibited CREB entry into nucleus, binding to the SCF promoter, and decreased SCF transcription, thereby weakened the ovular growth-promoted action of SCF and ultimately inhibited the collection of follicles.
GCs is the main element of somatic cell in follicles and can maintain a favorable microenvironment for mature oocytes [14]. Our findings on interaction among the cytokines secreted by GCs will help to clarify the mechanism of follicle growth and oocyte maturity.
References
Durlinger, A. L., Gruijters, M. J., Kramer, P., Karels, B., Ingraham, H. A., Nachtigal, M. W., et al. (2002). Themmen. Anti-mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology, 143, 1076–1084.
McLaughlin, E. A., & McIver, S. C. (2009). Awakening the oocyte: Controlling primordial follicle development. Reproduction, 137, 1–11.
Hu, R., Zhang, X. M., & Wu, X. (2009). Anti-mullerian hormone (AMH) predict ovarian reserve and reactivity studies. Reproduction and Contraception, 29, 515–519.
Nilsson, E., Rogers, N., & Skinner, M. K. (2007). Actions of anti-mullerian hormone on the ovarian transcriptome to inhibit primordial to primary follicle transition. Reproduction, 134, 209–221.
Reynaud, K., Cortvrindt, R., Smitz, J., Bernex, F., Panthier, J. J., & Driancourt, M. A. (2001). Alterations in ovarian function of mice with reduced amounts of KIT receptor. Reproduction, 121, 229–237.
Pepinsky, R. B., Sinclair, L. K., Chow, E. P., Mattaliano, R. J., Manganaro, T. F., Donahoe, P. K., et al. (1988). Proteolytic processing of mullerian inhibiting substance produces a transforming growth factor-beta-like fragment. Journal of Biolological Chemistry, 263, 18961–18964.
Visser, J. A., & Themman, A. P. (2005). Anti-mullerian hormone and folliculogenesis. Molecular Cellular Endocrinology, 234, 81–86.
Lambalk, C. B., Van Disseldorp, J., De Koning, C. H., & Broekmans, F. J. (2009). Testing ovarian reserve to predict age at menopause. Maturitas, 63, 280–291.
Marca, A. La., Orvieto, R., Giulini, S., Jasonni, V. M., Volpe, A., & De Leo, V. (2004). Mullerian-inhibiting substance in women with polycystic ovary syndrome: relationship with hormonal and metabolic characteristics. Fertility and Sterility, 82, 970–972.
Hu, R., & Zhang, X. M. (2009). Anti-mullerian hormone effects secretory function of human luteinized granulosa in vitro. Ningxia Medical Journal, 31, 868–870.
Kanakkaparambil, R., Singh, R., Li, D., Webb, R., & Sinclair, K. D. (2009). B-vitamin and homocysteine status determines ovarian response to gonadotropin treatment in sheep. Biology of Reproduction, 80, 743–752.
Chang, L. C., Guo, C. L., Lin, Y. S., Fu, H., Wang, C. S., & Jauh, G. Y. (2009). Pollen-specific SKP1-like proteins are components of functional scf complexes and essential for lily pollen tube elongation. Plant Cell Physiology, 50, 1558–1572.
Da Silva, C. A., Kassel, O., Lebouquin, R., Lacroix, E. J., & Frossard, N. (2004). Paradoxical early glucocorticoid induction of stem cell factor (SCF) expression in inflammatory conditions. British Journal of Pharmacology, 141, 75–84.
Gong, Y., Li, S. W., & Fan, W. (2011). Insulin affects on cultured of mice ovarian granulosa cells Foxo1mRNA expression. Practical Obstetrics and Gynecology, 27, 368–370.
Acknowledgments
This work was supported by Grants from National Natural Science Foundation of China (No. 81260109); Natural Science Foundation of Ningxia Province, China (No. NZ10115); and State Key Foundation of Science and Technology of Ningxia, China (No. 2010297).
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, R., Lou, Y., Wang, FM. et al. Effects of Recombinant Human AMH on SCF Expression in Human Granulosa Cells. Cell Biochem Biophys 67, 1481–1485 (2013). https://doi.org/10.1007/s12013-013-9649-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-013-9649-x