Abstract
Exosomes from senescence cells play pivotal roles in endothelium dysfunction. We investigated the exosomal angiogenic cargo of endothelial cells (ECs) in a model of senescence in vitro. After inducing aging by H2O2, the expression of P53, P21, and P16 was investigated by western blotting, while the expression of FMR1, miR-21, and miR-126 were measured by real-time PCR (q-PCR). Oil Red O dye was used to stain cells. Acetylcholinesterase (AChE) assay, transmission electron microscopy (TEM), and western blotting characterized Exosomes. Exosomal miR-21, miR-126, matrix metallopeptidase-9 (MMP-9), and tumor necrosis factor- ɑ (TNF-ɑ) proteins were measured by Q-PCR and western blotting. A wound-healing assay was used to explore the effect of exosomes on ECs migration rate. The results showed that the expression of P53, P21, P16, FMR1, and miR-21 was increased in treated cells as compared with control cells (P < 0.05). In addition, the expression of miR-126 was decreased in treated cells (P < 0.05). The number of Oil Red O-positive-treated cells increased (P < 0.05). The AChE activity of exosomes from treated cells was increased (P < 0.05). In comparison with control cells, an increase in the expression levels of exosomal miR-21 and TNF-ɑ of treated cells coincided with a decrease in the expression levels of miR-126 and MMP-9 levels (P < 0.05). We found that the migration rate of ECs co-cultured with exosomes from treated cells was decreased (P < 0.05). The data indicate ECs under H2O2 condition produce exosomes with distinct cargo that may be useful as a biomarker of age-related vascular disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The issue of aging has received considerable critical attention because aging is associated with age-related morbidities including cardiovascular disease (CVD), chronic kidney disease, osteoarthritis, neurodegeneration, osteoporosis, diabetes, and cancer worldwide [1]. Aging is a multifarious process, characterized by losing physiological function both at the organelle and cellular levels that is also associated with cellular senescence [2]. This process is known as the state of permanent cell cycle arrest in response to different types of cellular stresses [2]. Senescent cells act as bystander cells, which produce many biological soluble factors named the senescence-associated secretory phenotype (SASP), promoting senescent phenotypes [2]. Accordingly, SASP factors like extracellular vesicles (EVs) may affect the biological environment and induce cellular senescence [3]. EVs are a heterogeneous population of bilayer membrane-bound vesicles that contribute to cell-to-cell communication with pivotal roles in several physiological and pathological processes [4]. These vesicles contain many types of biological cargo including RNAs, proteins, and lipids transferring them to target cells [4]. The term exosome referred to 30–150 nm EVs originating from the endosomal compartment, multivesicular bodies (MVBs), located at cytoplasm [4, 5]. Exosomes were shown to be produced by many various cell types, and their associations in intercellular communication in pathological and normal conditions are currently well-known [4, 5]. Exosomes are present in the majority of bio-fluids that can mediate signaling pathways in recipient cells upon interaction with them [4, 5]. miRNAs (miRs) are abundantly transferred by exosomes to recipient cells. miRs, 20–22 nt non-coding RNAs can mediate physiological functions of endothelial cells (ECs) (like angiogenesis and regeneration) and vascular function in response to stress stimuli through targeting mRNAs form of genes [6]. Previous studies showed that exosomes cargo differs under various pathological conditions [5, 7]. Endothelial senescence is associated with a risk factor of age-associated CVD [8]. Exosomes derived from aged ECs may contribute to endothelium dysfunction that ultimately results in age-related vascular disorders [9]. For example, Wong et al. reported that exosomes from senescent ECs cause endothelial barrier dysfunction in young ECs in vitro [10]. Exosomes cargo of senescent cells is different from those released from normal cells [3], accordingly, the function of exosomes released by senescent cells differs from exosomes secreted by young cells [11]. Exosomes from distinct source cells may rearrange the tissue microenvironment and modulate inflammation, thrombosis, and angiogenesis [12]. In addition, exosomes from various cells/tissues are present in serum or other body fluids with different cargo and functions/outcomes; therefore knowledge of the aging process that governs exosomes biology, especially those of ECs, is essential to uncover the exact functions of these vesicles as well as clinical applications involving their biomarker use. The aim of the research was therefore to investigate the effect of hydrogen peroxidase, an aging inducer, on the cargo of exosomes derived from human umbilical vein cells (HUVECs) in vitro.
Materials and Methods
Cell Culture
HUVECs were achieved from the (Pasteur Institute, Iran) and incubated in 10% Fetal Bovine Serum (FBS), complete high-glucose Dulbecco’s Modified Eagle’s Medium (DMEM/LG; Gibco), containing 1% penicillin until confluent. Cells were incubated under 5% CO2 at 37 °C and the medium was changed every two days. For subcultures, cells were trypsinized with trypsin 0.25% EDTA and cells passages 4–6 were used for all experiments.
Treatment Protocol
HUVECs were cultured into proper tissue culture plates with DMEM/LG containing 10% FBS and 1% penicillin–streptomycin. After a 24 h incubation, cells were adopted to treatment protocol and kept over one day. Two groups were considered to do the in vitro analyses as follows: Nor-HUVECs: complete medium; H2O2-HUVECs: medium containing 100 µM H2O2 [13]. For monitoring morphological changes, images were taken from cells using a light microscopy. Exosomes from Nor-HUVECs and from H2O2-HUVECs named Nor-Exo and H2O2-Exo, respectively. Experiments are set in triplicate.
RNA Extraction
Total RNA, including mRNAs and miRNA from either cell lysate or exosomes pellet, was purified using TRIZOL reagent (Sigma) according to a standard protocol. Briefly, 1 mL TRIzol reagent was mixed with cell samples on ice, followed by 5–10 s of the vortex. To each sample, 200 μL chloroform was added, shacked for 30 s and incubated for 5 min at room temperature. Samples were then centrifuged at 12,000×g for 15 min at 4 °C to isolate RNA into the aqueous phase. Then, 500 μL isopropanol was mixed with 350 μL of the aqueous phase and kept 30 min at 4 °C. Samples were centrifuged at 12,000×g for 15 min at 4 °C to yield total RNA. The RNA pellet was washed twice with 750 µl ethanol 70%, followed by centrifugation at 7000×g for 5 min. Finally, RNA samples were resuspended in 30 μL DEPC-treated water and saved to -80 °C for further analysis. RNA quantification was done using a Nanodrop spectrometer (Biotek) using 1 μl aliquot from each sample.
Real-Time PCR (Q-PCR)
To investigate the expression of FMR1 and miRNAs including miR-21 and miR-126, 1 μg of total RNA was reverse-transcribed to cDNA by a cDNA reverse transcription kit (YTZ, Iran) and miRNA First-Strand cDNA Synthesis Kit (Cat No: BN-0011.17, Iran) according to kits recommendation. Q-PCR was performed with MIC Real-Time PCR System (Swiss) using SYBR Green PCR Master Mix (Cat No: YT2551, Iran) for mRNA analysis and SYBR Green High ROX Master mix (Cat No: BN-0011.17, Iran) for miRs. For mRNA, forty cycles were conducted as follows: 95 °C for 5″, 95 °C for 10 s, 60 °C for 30 s, 72 °C for 20 s; the same cycles were considered for miRNAs as, 95 °C for 2 min, 95 °C for 5 s, 60 °C for 40 s. Primer sequences for FMR1, forward: 5′-CTGAACTCAAGGCTTGGCAG-3′, reverse: 5′-TAGCTCCAATCTGTCGCAACT-3′; GAPDH, forward: 5′ CAAGTTCAACGGCACAGTCAAG-3′, reverse: 5′-ATACTCAGCACCAGCATCACC-3′ and miRNA-126: 5′-CAGCGTACCGTGAGTA-3′; miRNA-21: 5′-GGCTTGTCAGACTGATG-3′; Snord-47: 5′-ATCACTGTA AAACCGTT-3′. GAPDH and Snord-47, housekeeping genes, were used as a positive control due to their constitutive expression.
Western Blotting for Senescence Markers
A western blotting investigation was performed to measure the expression of proteins in cells and exosomes as well as to confirm that isolated exosomes contained proteins markers usually associated with exosomes. The protein samples were extracted from cells by RIPA buffer (Sigma) with Protease Inhibitor Cocktail (Sigma). Then, protein samples were denatured in 5 × Laemmli buffer at 95 °C for 10 min and 100 μg of cells protein was separated by 10% SDS-PAGE. Then transferred to PVDF membranes (Millipore), blocked with 5% skim milk (Sigma), membranes washed in TTBS (27 mM KCl, 0.05% Tween-20, 1.37 M NaCl, 25 mM Tris Base). Membranes incubated in primary antibodies P53, P21, and P16 (Santa cruz, Inc) after washing with TTBS. Then, HRP-conjugated secondary antibody was added to membranes and incubated with chemiluminescent substrate and protein bands imaged and analyzed using an Image J software ver.1.4. Relative expression was calculated against β-actin.
Oil Red O Staining
To explore the lipotoxicity level in HUVECs exposed to H2O2, the Oil Red O staining experiment was used to display the increase of lipid drops in senescent cells. Briefly, HUVECs were kept in methanol for 10 min and then fixed by 4% paraformaldehyde solution and kept with 0.1% Oil Red O staining solution (Sigma) for 15 min. After washing with phosphate buffer saline (PBS), images were taken by optical microscopy. The number of Oil Red O-positive cells was calculated in five random area of a field (× 10) [14].
Exosomes Isolation
Cells were washed twice with PBS and the medium was changed to DMEM depleted FBS for 48 h. Then, the supernatant was harvested for exosome purification and spun first at 3000 g for 15 min to eliminate cell debris, and then filtered by 0.22 Micron syringe filters. Then, 1 volume of exosomes isolation reagent (EXOCIB, Iran) mixed with 5 volumes of the supernatants left on a shaker overnight at 4 °C. To pellet exosomes, samples were centrifuged at 3000×g for 40 min and then the exosomes pellet was resuspended in PBS. The purified exosomes were measured for protein and RNAs contents by a Nanodrop spectrometer as an indicator of the number of exosomes purified. The characterization and size distribution of exosomes was analyzed by western blotting and transmission electron microscope (TEM), the surface protein markers of exosomes were identified by Western blotting.
Transmission Electron Microscopy
Isolated exosomes fractions were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4). Fixed samples containing exosomes were loaded on copper grids (Canada) for 1 min. Samples were also negatively stained with 2% uranyl acetate in deionized water for 5 min and dried out with filter paper. Exosomes were imaged on a TEM (Philips BioTwin, the Netherlands) run at 80 kV [15].
Characterization of Exosomal Proteins and Markers
Marker-based evaluation of isolated exosomes was performed using the western blotting analysis of CD63 and CD81 known exosome markers. In addition, the expression of matrix metallopeptidase-9 (MMP-9) and tumor necrosis factor-ɑ (TNF-ɑ) inside exosomes were evaluated. Briefly, exosomes were lysed in RIPA buffer (Sigma) containing protease inhibitor cocktail (Sigma), and equal total protein (20 µg) was separated by 10%SDS-PAGE as described previously [16]. Primary antibodies, including anti-CD63 (Santa Cruz, Inc, 1:10,000), anti-CD81 (Santa Cruz, Inc, 1:1000), and a secondary antibody (Biolegend, 1:5000) were used to detect exosomes markers and exosomal MM-9 and TNF-ɑ.
Exosomal Acetylcholinesterase Colorimetric Assay
To investigate the number of exosomes, acetylcholinesterase (AChE) assay, a simple colorimetric assay was used [17]. The following method was done according to the manufacturer’s instructions (Cat no. BXC080, Iran). Briefly, AChE reagent 1 and exosomes samples were mixed and added to a 96-well plate in triplicate. After five minutes, AChE reagent 2 was added to the reaction mixture and kept for 20 min at room temperature. The optical density was monitored by a microplate reader (Biotek) at points: 0 s, 30 s, 60 s, and 90 s using a 405 nm wavelength. AChE activity was reported by formula: Activity (U/l) = ΔAbs/min × 65,800.
In Vitro Scratch Assay
The in vitro cell healing assay was done by scratch assay to measure cell mobility. HUVECs were seeded in each well of a 96-well plate with a cell density of (5000). At 80% of confluence, the scratch was done using a white pipette tip. Then cells were washed twice with PBS and treated with exosomes from control and treated cells and were further incubated for 24 h and 48 h. Several images were taken at 0 h, 24 h, and 48 h after the treatment. Finally, the percentage of migration was calculated using the following formula: % healing rate = (surface of scratch at 0 h − the surface of scratch at 24 h or 48 h)/(surface of scratch at 0 h) × 100.
Statistical Analyses
For each experiment, the biological replicates were three. We completed statistical analyses with GraphPad Prism software ver. 8.0.2 (California USA) by One-tailed paired t-test. A difference at P < 0.05 was considered significant.
Results
Oil Red O Staining and Morphological Changes
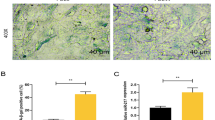
Using Oil Red O staining, we observed many Oil Red O-positive cells incubated with H2O2, indicating accumulation of lipids inside cells and cellular lipotoxicity (Fig. 1A). We found that the number of Oil Red O-positive cells treated with H2O2 was increased compared to control cells (P < 0.05; Fig. 1B). In addition, the HUVECs were observed under the microscope after 24 h. As shown by Fig. 1C, the H2O2-treated cells showed an enlarged nucleus and expanded cytoplasm, indicating cellular senescence [18].
Oil Red O staining confirmed lipotoxicity of cells treated with H2O2 (A). The number of Oil Red O-positive cells was increased in after treatment with H2O2 (B). Morphological change in HUVECs following exposure to H2O2 is shown (C). H2O2-treated HUVECs exhibited the enlarged nuclei and expanded cytoplasm with processes (× 10)
Protein Levels of P53, P21, and P16 were Increased in Treated Cells
To study the impact of H2O2 on the protein levels of P53, P21, and P16 (markers for cellular senescence), we performed Western blotting assay. Results showed that the protein level of P53 (~ 1.47 fold; P < 0.05), P21 (~ 1.49 fold; P < 0.05), and P16 (~ 1.22 fold; P < 0.05) was increased in treated cells as compared to control cells (Fig. 2).
Western blotting data on molecular cellular senescent markers (A). Representative western blotting analysis for expression of senescence markers including P53, P21, and P16 in HUVECs (B). Results showed that expression of P53, P21, and P16 in treated cells was increased. Normalization was performed to β-actin. Data are presented as mean ± SD. T- test was used to compare means. *P < 0.05 versus control cells
Expression of FMR1, miR-21, and miR-126 was Changed in Treated Cells
To assess the effects of H2O2 on the expression of FMR1, miR-21, and miR-126, a q-PCR assay was performed. We found that expression of FMR1 (Fold change ~ 1.33; P < 0.05) and miR-21 (Fold change ~ 1.75; P < 0.01) were increased, while expression of miR-126 (Fold change ~ 0.75; P < 0.05) was downregulated in treated cells (Fig. 3).
Expression of miR-21, miR-126, and FMR1 in HUVECs were measured by real-time PCR. Our result showed an upregulation rate for miR-21 and FMR1 and a downregulation level for miR-126 in treated cells. Data are presented as mean ± SD. T test was used to compare means. *P < 0.05 and **P < 0.01 versus control cells
Exosomes Confirmation
The western blotting analysis confirmed the expression of exosomal common markers; CD63 and CD9 in isolated exosomes (Fig. 4A). Images from TEM showed that isolated exosomes are nano-sized and have a round shape (Fig. 4B).
Western blotting analysis confirmed expression of exosomal marker CD63 and CD9 in exosomes samples (A). Transmission electron microscopy (TEM) confirmed the size and morphology of the exosomes (B). Acetylcholinesterase (AChE) assay for measuring amount of exosomes (C). Data are presented as mean ± SD. T test was used to compare means. *P < 0.05 and **P < 0.01 versus control cells
AChE Activity of Exosomes from Treated Cells Increased
AChE activity is recognized to be associated with exosomes. To examine the possible effect of H2O2 on the exosome secretion rate, we performed AChE activity assay 24 h treatment. As shown by Fig. 4C, AChE activity of exosomes isolated from H2O2-induced cells was significantly increased compared to control cells (200 ± 10.4 vs. 227.3 ± 7.54; P < 0.01).
The Expression Level of miR-21 and miR-126 was Changed in Exosomes Derived from H2O2-HUVECs
Exosomes, a biological carrier particle, play an important in cellular communication by the interchanging of miRNAs and proteins between cells. Whether H2O2would impact the miRs expression in exosomes from HUVECs was assessed by q-PCR. Compared to the control group, we observed that expression of exosomal miR-21 increased (1.17 ± 0.09-fold change, < 0.05) but the expression of exosomal miR-126 was decreased (0.43 ± 0.18-fold change, P < 0.01; Fig. 5A).
Real-time PCR assay for exosomal miR-21 and miR-126 (A). Expression of miR-21 was increased, while expression of miR-126 was decreased in treated cells. Western blotting analysis for exosomal proteins matrix metallopeptidase-9 (MMP-9) and tumor necrosis factor-ɑ (TNF-ɑ) (B). Data are presented as mean ± SD. T test was used to compare means. *P < 0.05 and **P < 0.01 versus control cells
The Protein Level of Exosomal MMP-9 and TNF-ɑ was Changed in Treated Cells
We also monitored the protein level of exosomal MMP-9 and TNF-ɑ in exosome samples. As shown by Fig. 5B, we found that the protein level of MMP-9 was decreased (P < 0.05), and the protein level of TNF-ɑ was increased (P < 0.05) in exosomes of treated cells.
The Wound-Healing Rate of Cells was Decreased Upon Incubation with Exo-H2O2
We used the in vitro scratch assay to investigate whether exosomes from H2O2-HUVECs (H2O2-Exo) had a consequence on the migration ability of ECs, which is a pivotal process in angiogenesis. The results showed that the wound-healing rate of cells treated with H2O2-Exo decreased both at points of 24 h and 48 h compared to Nor-Exo group (P < 0.05; Fig. 6).
Wound-healing assay for HUVECs treated with exosomes derived from control and treated HUVECs at points 24 h and 48 h. Percentage of wound-healing area of cells treated with H2O2-Exo was decreased after 24 h and 48 h. Scratch bands on cell culture plates (A). Analysis of wound-healing area (B). Data are presented as mean ± SD. T test was used to compare means. *P < 0.05 versus control cells
Discussion
In this study, we examined the hypothesis that H2O2 may affect exosome secretion and angiogenic cargo of HUVECs. Here, we focused on exosomes of HUVECs, a model of endothelium cells. Oxidative stress has been known as an important factor in several steps in CVD, Kidney diseases, the central nervous system (CNS) diseases, and cancer [19]. As shown by Fig. 1, we found that the morphology of cells altered under light microscopy, and many Oil Red O-positive cells were present in cell culture plates, representing intercellular lipid accumulation and lipid toxicity [20]. For further inquiry, we did western blotting and found that protein levels of P53, P16, and P21 in H2O2-HUVECs were increased, indicating cellular senescence in cells [21]. P21, the target of P53, has frequently been known essential for inducing cellular senescence, while P16 may contribute to the maintenance of the cellular phenotype [22], a consequence also done by an increase in cellular oxidative stress [23]. Besides the evidence that H2O2 is associated with oxidative stress on cells and different pathways, some findings support the link between cellular senescence and exosomes kinetic. Previous studies showed that exosomes secretion has been increased under oxidative stress like H2O2 treatment [24], which was in agreement with our finding of AChE assay [24, 25]. In parallel, in our previous study, we found that AChE activity of exosomes samples of H2O2-induced ECs significantly increased (results unpublished). Previous studies showed that under stress condition P53 participates in the biogenesis of exosomes. We think that increased levels of AChE activity and P53 level may indicate elevated exosomes biogenesis [26]. Then, we also investigated the key miRNAs involved in the biological process under oxidative stress including miR-21 and miR-126 in ECs. We found an increase in miR-21 and a decrease in miR-126 in cells exposed to H2O2. These results are consistent with previous studies on H2O2 treatment experiment [27] and miRs profiling [28]. Cheng and co-workers, using cardiac myocytes, found that H2O2 treatment caused a significant upregulation of miR-21 and apoptosis, however, a miR-21 inhibitor increased the apoptosis rate of cells [27]. In the same way, Alique et al. reported that expression of miR-126 downregulated in senescent ECs in vitro model [29]. MiR-21 is a multi-functional molecule whose expression is increased in different oxidative stress conditions with a role in several cell responses including inflammation, apoptosis, proliferation, autophagy, immunosuppression, and angiogenesis [30]. Although we did not focus, conversely, previous studies showed that an elevated miR-21 in senescent cells was correlated with progress in cell growth arrest and induction in impaired angiogenesis [31], which may correlate with our findings of reduced angiogenesis. In addition, miR-126 is ECs-specific, we supposed the age-related miR-126 alternation could be associated with ECs senescence. Among the age-related miRs, miR-126 has been considered an aging biomarker with a high level of expression [32]. In ECs, miR-126 supports angiogenesis through inhibiting PIK3R2 and endogenous vascular endothelial growth factor (VEGF) repressors sprout-related, EVH1 domain-containing protein 1 (Spread-1) [33]. Regarding these data, it can therefore be assumed that the expression pattern of this miRs was attributed to ECs dysfunction and senescence. We also observed that the expression of FMR1, a gene implicated in loading/sorting miRs into exosomes, increased upon H2O2 treatment. FMR1, which contains small miR-interacting motifs is up-regulated in oxidative stress and such pathological conditions [34]. Further studies are essential to uncover the relationship between this gene expression level and miRs loading into exosomes under H2O2 treatment. In keeping, we investigated the expression of these miRs in exosomes of HUVECs by q-PCR. We found a similar expression pattern as was observed for cellular one. Lee et al. reported that the expression of some miRs such as mmu-miR-466c-5p and mmu-miR-126-5p were decreased in serum exosomes and lungs and liver tissues of aged mice. In keeping, they showed that expression of mmu-miR-126b-5p and telomerase-related genes in the aged lungs and liver tissues was up-regulated when young exosomes injected intravenously [35]. Consistent with our results, it was demonstrated that exosomes released from senescent cells contain a high level of miR-21 and a low level of miR-126 in [36]. Consequently, these results indicate that cells under oxidative stress distribute abundantly miR-21 copies with a low level of miR-126 copies within exosomes, which may back to the condition of cells that were grown. Whether there was a connection between increased FMR1 and miRs loading rate in our study needs further confirmation. In addition, we found that exosomal MMP-9 was decreased, whereas exosomal TNF-ɑ was increased. This is important in the context of the angiogenic functions of exosomes of senescent ECs. MMP-9 is a key proangiogenesis factor that induces the angiogenesis switch on via degradation of the extracellular matrix [37]. TNF-ɑ is a main inflammatory factor that also facilitates angiogenesis through inducing local production of proangiogenic factors [38]. Angiogenesis, the forming new blood vessels, plays main roles in wound-healing several progresses [39]. Angiogenesis changes are related to age, as the wound-healing procedure is continuously hindered in older persons compared to younger persons [40]. Vascular remodeling and angiogenesis are complex and need a structured balance between different available pro-and anti-angiogenesis factors [41]. Our results suggest that angiogenic cargo of exosomes differ under oxidative stress and this may contribute to impaired angiogenesis [42], which we tested by in vitro wound-healing assay. We co-cultured exosomes with non-treated HUVECs and observed that exosomes from H2O2-treated cells negatively affected the wound-healing rate of HUVECs. Besides this, our findings from q-PCR and western blotting support the idea that exosomes from H2O2-treated cells transfer biological components that inhibited angiogenesis in the recipient cells. Overall, to our knowledge, for the first time, we showed that HUVECs produced more exosomes with distinct angiogenic cargo that may negatively regulate angiogenesis. Furthermore, these exosomes may be useful for further biomarker discoveries in age-related diseases. However, we think these finding are preliminary on HUVECs-derived exosomes and therefore further examinations should focus on exosomal cargo and angiogenesis under cellular senescence.
Conclusions
Our finding demonstrated that cellular senescence induction coincided with an increase in exosomes secretion and sorting of particular exosomal cargo including miRs and proteins. These exosomes negatively regulated the wound-healing rate of HUVECs, indicating constant cellular senescence under oxidative stress. This research not only extends our knowledge of exosomes kinetics under H2O2 treatment but also supports an idea for biomarker application of these exosomal cargos in age-related diseases. However, more research on this topic needs to be undertaken before the association between cellular senescence and exosomal cargo is more clearly understood.
Data Availability
The data are available upon a request to corresponding author.
References
Suzman, R., Beard, J. R., Boerma, T., & Chatterji, S. (2015). Health in an ageing world—What do we know? The Lancet, 385(9967), 484–486.
Di Micco, R., Krizhanovsky, V., Baker, D., & di Fagagna, F. A. (2021). Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nature Reviews Molecular Cell Biology, 22(2), 75–95.
Urbanelli, L., Buratta, S., Sagini, K., Tancini, B., & Emiliani, C. (2016). Extracellular vesicles as new players in cellular senescence. International Journal of Molecular Sciences, 17(9), 1408.
Zhang, Y., Liu, Y., Liu, H., & Tang, W. H. (2019). Exosomes: Biogenesis, biologic function and clinical potential. Cell & Bioscience, 9(1), 1–18.
Rezaie, J., Aslan, C., Ahmadi, M., Zolbanin, N. M., Kashanchi, F., & Jafari, R. (2021). The versatile role of exosomes in human retroviral infections: From immunopathogenesis to clinical application. Cell & Bioscience, 11(1), 1–15.
Caporali, A., & Emanueli, C. (2011). MicroRNA regulation in angiogenesis. Vascular pharmacology, 55(4), 79–86.
Jafari, R., Rahbarghazi, R., Ahmadi, M., Hassanpour, M., & Rezaie, J. (2020). Hypoxic exosomes orchestrate tumorigenesis: Molecular mechanisms and therapeutic implications. Journal of Translational Medicine, 18(1), 1–14.
Voghel, G., Thorin-Trescases, N., Farhat, N., Nguyen, A., Villeneuve, L., Mamarbachi, A. M., Fortier, A., Perrault, L. P., Carrier, M., & Thorin, E. (2007). Cellular senescence in endothelial cells from atherosclerotic patients is accelerated by oxidative stress associated with cardiovascular risk factors. Mechanisms of Ageing and Development, 128(11–12), 662–671.
Hromada, C., Mühleder, S., Grillari, J., Redl, H., & Holnthoner, W. (2017). Endothelial extracellular vesicles-promises and challenges. Frontiers in Physiology, 8, 275–275. https://doi.org/10.3389/fphys.2017.00275
Wong, P.-F., Kind-Leng Tong, J. J., Khor, E.-S., Lai, S.-L., & Mustafa, M. R. (2019). Senescent HUVECs-secreted exosomes trigger endothelial barrier dysfunction in young endothelial cells. EXCLI Journal, 18, 764.
Lunyak, V. V., Amaro-Ortiz, A., & Gaur, M. (2017). Mesenchymal stem cells secretory responses: Senescence messaging secretome and immunomodulation perspective. Frontiers in Genetics, 8, 220.
Xu, D., & Tahara, H. (2013). The role of exosomes and microRNAs in senescence and aging. Advanced Drug Delivery Reviews, 65(3), 368–375.
Chowdhary, S. (2019). The effects of oxidative stress on inducing senescence in human fibroblasts. Journal of the South Carolina Academy of Science, 16(2), 2.
Rezaie, J., Nejati, V., Khaksar, M., Oryan, A., Aghamohamadzadeh, N., Shariatzadeh, M. A., Rahbarghazi, R., & Mehranjani, M. S. (2018). Diabetic sera disrupted the normal exosome signaling pathway in human mesenchymal stem cells in vitro. Cell and Tissue Research, 374(3), 555–565.
Jabbari, N., Nawaz, M., & Rezaie, J. (2019). Ionizing radiation increases the activity of exosomal secretory pathway in MCF-7 human breast cancer cells: A possible way to communicate resistance against radiotherapy. International Journal of Molecular Sciences, 20(15), 3649.
Risha, Y., Minic, Z., Ghobadloo, S. M., & Berezovski, M. V. (2020). The proteomic analysis of breast cell line exosomes reveals disease patterns and potential biomarkers. Scientific Reports, 10(1), 13572. https://doi.org/10.1038/s41598-020-70393-4
Feghhi, M., Rezaie, J., Akbari, A., Jabbari, N., Jafari, H., Seidi, F., & Szafert, S. (2021). Effect of multi-functional polyhydroxylated polyhedral oligomeric silsesquioxane (POSS) nanoparticles on the angiogenesis and exosome biogenesis in human umbilical vein endothelial cells (HUVECs). Materials & Design, 197, 109227.
Kiyoshima, T., Enoki, N., Kobayashi, I., Sakai, T., Nagata, K., Wada, H., Fujiwara, H., Ookuma, Y., & Sakai, H. (2012). Oxidative stress caused by a low concentration of hydrogen peroxide induces senescence-like changes in mouse gingival fibroblasts. International Journal of Molecular Medicine, 30(5), 1007–1012.
Liguori, I., Russo, G., Curcio, F., Bulli, G., Aran, L., Della-Morte, D., Gargiulo, G., Testa, G., Cacciatore, F., Bonaduce, D., & Abete, P. (2018). Oxidative stress, aging, and diseases. Clinical Interventions in Aging, 13, 757.
Yao, H. R., Liu, J., Plumeri, D., Cao, Y. B., He, T., Lin, L., Li, Y., Jiang, Y. Y., Li, J., & Shang, J. (2011). Lipotoxicity in HepG2 cells triggered by free fatty acids. American Journal of Translational Research, 3(3), 284.
Lujambio, A. (2016). To clear, or not to clear (senescent cells)? That is the question. BioEssays, 38, S56–S64.
Althubiti, M., Lezina, L., Carrera, S., Jukes-Jones, R., Giblett, S. M., Antonov, A., Barlev, N., Saldanha, G. S., Pritchard, C. A., Cain, K., & Macip, S. (2014). Characterization of novel markers of senescence and their prognostic potential in cancer. Cell Death & Disease, 5(11), e1528–e1528.
Macip, S., Igarashi, M., Berggren, P., Yu, J., Lee, S. W., & Aaronson, S. A. (2003). Influence of induced reactive oxygen species in p53-mediated cell fate decisions. Molecular and Cellular Biology, 23(23), 8576–8585.
Chiaradia, E., Tancini, B., Emiliani, C., Delo, F., Pellegrino, R. M., Tognoloni, A., Urbanelli, L., & Buratta, S. (2021). Extracellular vesicles under oxidative stress conditions: Biological properties and physiological roles. Cells, 10(7), 1763.
Soraya, H., Sani, N. A., Jabbari, N., & Rezaie, J. (2021). Metformin increases exosome biogenesis and secretion in U87 MG human glioblastoma cells: A possible mechanism of therapeutic resistance. Archives of Medical Research, 52(2), 151–162.
Lespagnol, A., Duflaut, D., Beekman, C., Blanc, L., Fiucci, G., Marine, J. C., Vidal, M., Amson, R., & Telerman, A. (2008). Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death and Differentiation, 15(11), 1723.
Cheng, Y., Liu, X., Zhang, S., Lin, Y., Yang, J., & Zhang, C. (2009). MicroRNA-21 protects against the H2O2-induced injury on cardiac myocytes via its target gene PDCD4. Journal of Molecular and Cellular Cardiology, 47(1), 5–14.
Talepoor, A. G., Kalani, M., Dahaghani, A. S., & Doroudchi, M. (2017). Hydrogen peroxide and lipopolysaccharide differentially affect the expression of microRNAs 10a, 33a, 21, 221 in endothelial cells before and after coculture with monocytes. International Journal of Toxicology, 36(2), 133–141.
Alique, M., Bodega, G., Giannarelli, C., Carracedo, J., & Ramírez, R. (2019). MicroRNA-126 regulates Hypoxia-Inducible Factor-1α which inhibited migration, proliferation, and angiogenesis in replicative endothelial senescence. Scientific Reports, 9(1), 1–19.
Zhou, J., Wang, K. C., Wu, W., Subramaniam, S., Shyy, J. Y. J., Chiu, J. J., Li, J. Y. S., & Chien, S. (2011). MicroRNA-21 targets peroxisome proliferators-activated receptor-α in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proceedings of the National Academy of Sciences, 108(25), 10355–10360.
Dellago, H., Preschitz‐Kammerhofer, B., Terlecki‐Zaniewicz, L., Schreiner, C., Fortschegger, K., Chang, M. W. F., Hackl, M., Monteforte, R., Kuhnel, H., Schosserer, M., Gruber, F., Tschachler, E., Scheideler, M., Grillari-Voglauer, R., Grillari, J., & Wieser, M. (2013). High levels of oncomi R-21 contribute to the senescence-induced growth arrest in normal human cells and its knock-down increases the replicative lifespan. Aging Cell, 12(3), 446–458.
Freedman, J. E., Gerstein, M., Mick, E., Rozowsky, J., Levy, D., Kitchen, R., Das, S., Shah, V., Danielson, K., Beaulieu, L., Navarro, F. C. P. Wang, Y., Galeev, T. R., Holman, A., Kwong, R. Y., Murthy, V., Tanriverdi, S. E., Koupenova, M., Mikhalev, E., & Tanriverdi, K. (2016). Diverse human extracellular RNAs are widely detected in human plasma. Nature Communications, 7(1), 1–14.
Fish, J. E., Santoro, M. M., Morton, S. U., Yu, S., Yeh, R. F., Wythe, J. D., Lvey, K. N., Bruneau, B. J., Stainier, D. Y. R., & Srivastava, D. (2008). miR-126 regulates angiogenic signaling and vascular integrity. Developmental Cell, 15(2), 272–284.
Wozniak, A. L., Adams, A., King, K. E., Dunn, W., Christenson, L. K., Hung, W.-T., & Weinman, S. A. (2020). The RNA binding protein FMR1 controls selective exosomal miRNA cargo loading during inflammation. Journal of Cell Biology, 219(10), e201912074.
Lee, B.-R., Kim, J.-H., Choi, E.-S., Cho, J.-H., & Kim, E. (2018). Effect of young exosomes injected in aged mice. International Journal of Nanomedicine, 13, 5335.
Rippe, C., Blimline, M., Magerko, K. A., Lawson, B. R., LaRocca, T. J., Donato, A. J., & Seals, D. R. (2012). MicroRNA changes in human arterial endothelial cells with senescence: Relation to apoptosis, eNOS and inflammation. Experimental Gerontology, 47(1), 45–51. https://doi.org/10.1016/j.exger.2011.10.004
Yabluchanskiy, A., Ma, Y., Iyer, R. P., Hall, M. E., & Lindsey, M. L. (2013). Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology, 28(6), 391–403.
Wang, Y., Xu, J., Zhang, X., Wang, C., Huang, Y., Dai, K., & Zhang, X. (2017). TNF-α-induced LRG1 promotes angiogenesis and mesenchymal stem cell migration in the subchondral bone during osteoarthritis. Cell Death & Disease, 8(3), e2715–e2715.
Chen, C. Y., Rao, S. S., Ren, L., Hu, X. K., Tan, Y. J., Hu, Y., Luo, J., Liu, Y.-W., Yin, H., Huang, J., Cao, J., Wang, Z.-X., Liu, Z.-Z., Liu, H.-M., Tang, S.-Y., Xu, R., & Xie, H. (2018). Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics, 8(6), 1607.
Shakeri, H., Gevaert, A. B., Schrijvers, D. M., De Meyer, G. R., De Keulenaer, G. W., Guns, P. J. D., Lemmens., K., & Segers, V. F. (2018). Neuregulin-1 attenuates stress-induced vascular senescence. Cardiovascular Research, 114(7), 1041–1051.
Tonini, T., Rossi, F., & Claudio, P. P. (2003). Molecular basis of angiogenesis and cancer. Oncogene, 22(42), 6549–6556.
Sun, L., Zhu, W., Zhao, P., Zhang, J., Lu, Y., Zhu, Y., Zhao, W., Liu, Y., Chen, Q., & Zhang, F. (2020). Down-regulated exosomal MicroRNA-221–3p derived from senescent mesenchymal stem cells impairs heart repair. Frontiers in Cell and Developmental Biology, 8, 263.
Acknowledgements
None applicable.
Funding
This study was MSc research project that completed in Urmia University.
Author information
Authors and Affiliations
Contributions
Conceptualization and Methodology, VN and JR; Data collection and performance: SAS. Software and Analysis: JR. Writing—Review and Editing: VN and JR.
Corresponding author
Ethics declarations
Competing Interests
The authors declared that there is no conflict of interest to declare. The authors indicate they do have not a financial relationship with the organization that sponsored the research.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Handling Editor: Dipak K Dube.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shaban, S.A., Rezaie, J. & Nejati, V. Exosomes Derived from Senescent Endothelial Cells Contain Distinct Pro-angiogenic miRNAs and Proteins. Cardiovasc Toxicol 22, 592–601 (2022). https://doi.org/10.1007/s12012-022-09740-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-022-09740-y