Abstract
Human mesenchymal stem cells were exposed to diabetic sera for 7 days. Cell viability and apoptosis rate were detected by MTT and flow cytometry assays. The expression of key genes such as CD63, Alix, Rab27a, Rab27b, and Rab8b was monitored by real-time PCR. We also measured acetylcholinesterase activity and size and zeta potential of exosomes in the supernatant form diabetic cells and control. The cellular distribution of CD63 was shown by immunofluorescence imaging and western blotting. Any changes in the ultrastructure of cells were visualized by electron microscopy. Data showed a slight decrease in survival rate and an increased apoptosis in diabetic cells as compared to control (p < 0.05). By exposing cells to diabetic sera, a significant increase in the level of all genes CD63, Alix, Rab27a, Rab27b, and Rab8b was observed (p < 0.05). Flow cytometry analysis and immunofluorescence imaging confirmed increasing CD63 protein content upon treatment with diabetic sera (p < 0.05). We found an enhanced acetylcholinesterase activity in a diabetic condition which coincided with the increasing size of exosomes and decrease in zeta potential (p < 0.05). The fatty acid profile was not significantly affected by diabetic sera. Ultrastructural examination detected more accumulated cytoplasmic lipid vacuoles in diabetic cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exosomes, nano-sized cell-derived membrane vesicles, are secreted by many cell types (Rezaie et al. 2017). It was recently demonstrated that exosomes represent an additional mechanism of cell-to-cell communication by transfer of bio-molecules. These vesicles are easily distributed through various fluids inside the body (Record et al. 2011; Rezaie et al. 2017). Exosomes potentially participate in physiological and pathological conditions. For instance, normal cell-derived exosomes cause positive effects on tissues while exosomes originated from tumor cells could generate health deleterious effects (Record et al. 2011; Zeelenberg et al. 2008). Accumulating evidence suggests that these small vesicles, ranging from 30 to 100 nm, are released via exocytosis from multivesicular bodies (MVBs) of the late endosome (Rezaie et al. 2017). In most cell types, exosomes correspond to secreted intraluminal vesicles (ILVs) of MVBs. ILVs are formed by inward budding of the limiting membrane of MVEs into the lumen of MVB/late endosomes (Record et al. 2011). Since MVBs fuse with the plasma membrane, ILVs are released as exosomes into the extracellular matrix. At this moment, the released exosomes could bind to cells in proximity or to the extracellular matrix, or traffic through bio-fluids (Record et al. 2011; Rezaie et al. 2017). Various molecules participate in exosome biogenesis, cargo loading, intracellular trafficking, and fusion with the plasma membrane (PM). The tetraspanin CD63 and Alix (ESCRT accessory protein) are highly enriched in exosomes and considered to be important in exosome biogenesis pathway and subsequent sorting cargo (Stoorvogel 2015; Urbanelli et al. 2013; Van Niel et al. 2011). Most subcellular transport pathways are controlled by conserved families of the Rab proteins of small GTPase. Due to their restricted distribution to intracellular-specific vesicles/organelles, they regulate specific membrane trafficking (Hutagalung and Novick 2011). In this context, the key role of Rab27a, Rab27b, and Rab8b proteins in controlling MVB translocation and abscission and fusion to target membranes such as PM have been recently proven (Choi et al. 2007; Ostrowski et al. 2010; Robbins and Morelli 2014).
Human bone marrow mesenchymal stem cells (hMSCs) with self-renewal activity, trans-differentiation into different lineages, and paracrine activity contribute to the reconstitution of injured tissues by augmentation of angiogenesis. Though multiple mechanisms are engaged by hMSCs, exosomes play an important role in paracrine activity (Rahbarghazi et al. 2014). Increasing data suggested that hMSCs were recruited to the site of neovascularization in response to cytokine gradients and promoted tissue regeneration through releasing both growth factors and transferring biomaterial by exosomes (Ardestani et al. 2014; Phinney and Pittenger 2017). Recent studies confirmed the implication of MSC-derived exosomes in promoting angiogenesis (Ardestani et al. 2014).
Diabetes type 2 (DM2) is a chronic metabolic disease and causes a high rate of mortalities globally. It was reported that in patients with DM2, deficient and abnormal angiogenesis is most common (Cade 2008). Previous studies described adverse effects of DM2 on stem cell function (Rezaie et al. 2018; Yan et al. 2012). Our previous study showed that DM2 inhibited the angiogenic ability of hMSCs through the downregulation of pro-angiogenic factors, contributing to impaired differentiation capacity and inhibited paracrine secretion.
To our knowledge, there are few reports related to the effect of diabetic condition on exosome biogenesis in hMSCs and the underlying mechanisms must be addressed. In the present work, we are curious about the evaluated dynamic of exosome signaling pathway in hMSCs following exposure to DM2 sera over a period of 7 days. It seems that the results of the present experiment may help to collect a detailed knowledge about intracellular mechanisms controlling exosome biogenesis in diabetic cells, providing a guideline for future studies of exosome-based therapeutics.
Material and methods
Serum collection
Sera were collected from middle-aged healthy and newly diagnosed type 2 diabetic patients and subjected to biochemical analysis (Table 1). In samples, fasting glucose levels more than 120 mg/dl was considered as the diabetic patient. All volunteers were asked to fill out an informed consent. All procedures performed through the current experiment, involving human participants, were in accordance with the local ethics committee of Tabriz University of Medical Sciences (ethical code no. TBZMED.REC.1394.928) and ethical principles of Declaration of Helsinki.
Cell culture protocol
Human bone marrow MSC (hMSC) line was purchased from Royan Institute (Iran). hMSCs were plated at an initial density of 2 × 105 cells/cm2 in low-glucose Dulbecco’s modified Eagle’s medium (DMEM/LG, Gibco) with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin-streptomycin (Biosera). Culture flasks were placed into a humidified incubator at 37 °C with 5% CO2. To passage the hMSCs, 0.25% trypsin-EDTA solution (Gibco) was used. hMSCs between passages 3 and 6 were subjected to different analyses.
Treatment protocol
For in vitro assays, hMSCs were classified into two groups as follows: control: cells received sera from healthy subjects and diabetic groups treated with diabetic sera. After reaching 70–80% confluency, hMSCs were exposed to DMEM/LG containing 10% normal and diabetic sera over a period of 7 days. The exhausted medium was replenished every 3–4 days.
MTT assay
To measure hMSC viability after exposure to diabetic sera, we used MTT assay. An initial cell density of 1 × 104 was seeded on each well of 96-well plate (SPL) and incubated media supplemented with 10% normal and diabetic sera for 7 days. After the incubation period, the supernatant was discarded and replaced with 100 μl of MTT solution (dilution 5 μg/ml). Following 4-h incubation at 37 °C, 50 μl of dimethyl sulfoxide was overlaid and gently agitated for 15 min. The absorbance of each sample was measured at 570 nm (BioTek) and cytotoxicity expressed as percentage of the control hMSCs.
Detecting apoptosis by flow cytometry analysis
Seven days after incubation with diabetic sera, the rate of apoptotic hMSCs was detected by flow cytometry assay. In brief, hMSCs were washed twice with phosphate-buffered saline (PBS) and exposed to binding buffer (ref no. 00-0055-56, eBioscience) at RT for 10 min. Next, cells were incubated with the FITC-conjugated mouse anti-human Annexin-V antibody (ref no. 11-8005-74, eBioscience) at 4 °C for 40 min. For background exclusion, we used an appropriate isotype control. Flow cytometry analysis was performed by BD FACSCalibur system and FlowJo software (version 7.6.1.).
Quantitative real-time PCR analysis
Total RNA was extracted from normal and diabetic hMSCs using GeneMATRIX universal RNA purification kit (EURx, Poland). RNA integrity and purity were calculated by Picodrop spectrophotometer system (model PICOPET01, UK). We performed the reverse transcription reaction by using cDNA synthesis kit (cat no. k-2046, Bioneer). In the current experiment, the expression of Alix, CD63, Rab27a, Rab27b, and Rab8b was quantified by using SYBR Green PCR Master Mix (cat no. YT2551, Iran) and Rotor-Gene 3000 real-time system (Corbett Robotics). All gene expression was normalized to GAPDH. Primers used for real-time PCR analysis were listed in Table 2.
Exosome secretion quantification by acetylcholinesterase assay
Exosome secretion was measured in condition media (CM) by monitoring ACE activity. After treatment with normal and diabetic sera for a period of 7 days, hMSCs were gently washed with PBS three times and incubated with serum-free medium for the next 48 h. CMs were collected and centrifuged at 15,000g for 20 min at 4 °C. CM was added to a buffer solution (75 mM pyrophosphate and 2 mM potassium hexacyanoferrate) and maintained for 5 min at RT (cat no. BXC0801; Biorexfars). After the addition of s-butyrylthiocholine iodide, absorbance was read at 405 nm at three different intervals. Choline esterase activity was calculated by following the formula: Activity (U/l) = ∆Abs/ min × 65,800.
Western blotting
hMSC lysates were extracted from control and diabetic groups by using ice-cold cell lysis buffer solution containing NaCl, Nonidet, NP-40, Tris–HCl, and cocktail enzyme inhibitors. An equal amount of protein samples were resolved on 10% SDS polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane. Primary antibody against human CD63 (cat no. ab118307, Abcam) was used at a dilution of 1:1000 overnight at 4 °C. Immunoreactive bands were visualized by goat-anti-rabbit IgG antibody conjugated to HRP (Cell Signaling) and ECL plus solution (Roche). Densitometry analysis was performed with ImageJ software ver.1.44p (NIH). β-Actin (Cell Signaling) was used as a housekeeping protein.
Immunofluorescence imaging
The intracellular distribution of exosomes in diabetic hMSCs was investigated by immunofluorescence (IF) assay. Expression of CD63 has been conceived as a reliable factor to monitor the dynamic of exosomes inside the cell. In short, 5 × 104 hMSCs were plated on each well of 8-well tissue culture chamber (SPL) and treated as above-mentioned. For fixation, hMSCs were incubated with pre-chilled 4% paraformaldehyde (PFA) solution, permeabilized with 0.1% Triton 100-X (Sigma) solution, and blocked with 1% bovine serum albumin (BSA, Sigma) for 30 min. Thereafter, cells were incubated with the primary anti-human CD63 antibody (cat no. ab118307, Abcam) overnight at 4 °C. After washing twice with PBS, hMSCs were incubated with FITC-conjugated donkey anti-rabbit IgG (BioLegend) for 30 min at 37 °C in the dark. For nuclear counterstaining, 1 μg/ml DAPI solution (Sigma) was used. Cells were imaged using a fluorescent microscope (model BX51, Olympus) and processed with cellSens imaging software.
Flow cytometric detection of surface and cytoplasmic CD63
Cell membrane (surface) and cytoplasmic levels of CD63 were measured by flow cytometry. Different staining protocols were used as follows: protocol I: hMSCs were fixed with 4% PFA solution and permeabilized with 0.1% Triton X-100 solution, and protocol II, hMSCs were only fixed with 4% PFA solution. Cells prepared by two protocols were subjected to incubation with the anti-human CD63-PE antibody (cat no. 12-0639-42, eBioscience). Flow cytometry analysis was performed by BD FACSCalibur system and FlowJo software version 7.6.1. Finally, data were expressed as the ratio of the surface to the cytoplasmic CD63 in control and diabetic hMSCs.
Exosome purification
To isolate exosome, after 7-day incubation with different sera, cells were cultivated with 2% exosome-depleted FBS (cat no. A25904DG, Invitrogen) over a period of 48 h. Exosomes were collected from 10 ml of hMSC-CMs according to the total exosome isolation kit instructions (publication no. MAN0006949, Invitrogen). In brief, The CMs were collected on ice and centrifuged at 2000×g for 30 min to eliminate the cells and debris. Thereafter, the supernatant was collected and mixed with 0.5 volumes of the kit reagent and kept overnight at 4 °C. The next day, samples were centrifuged at 10,000×g for 1 h at 4 °C. The supernatant was discarded and the exosome pellet was suspended in 50 μl of PBS and subjected to analysis.
Characterizing of exosomes
To confirm exosome isolation, we used flow cytometry and scanning electron microscopy (SEM). For flow cytometry monitoring, 50 μl of isolated exosome fraction was incubated with the primary anti-CD63 antibody (cat no. ab118307, Abcam) for 2 h at 4 °C. Then, exosomes were incubated with the anti-human CD63-PE secondary antibody (cat no. 12-0639-42, eBioscience) for 1 h at 37 °C and to BD FACSCalibur system. Data were analyzed using FlowJo software version 7.6.1. For SEM imaging, 25 μl of exosome fraction was fixed directly in 2.5% phosphate-buffered glutaraldehyde for 1 h and loaded on grids and frozen-dried. Grids were then coated with Au and photos were obtained at 30 kV by SEM system (MIRA3 FEG-SEM, Tescan). To further confirm, isolated exosomes were monitored by SEM.
Determining the exosome size and zeta potential
To investigate the possible effect of diabetic sera on the exosome size and zeta potential (ZP), we used dynamic light scattering (DLS) technique. Briefly, 25 μl of exosome fraction was suspended in a final volume of 1 ml PBS and subjected to Zetasizer Nano Z system (Nano ZS ZEN 3600, Malvern). Three replicates were measured from each sample.
Transmission electron microscopy
To gain further insight into the effect of diabetic sera on hMSCs, any changes in the ultrastructure of cells were investigated using transmission electron microscopy (TEM). The cells were fixed in 2.5% glutaraldehyde solution (Sigma) for 2 h. The cells were then rinsed in 1% osmium tetroxide and 0.05 M cacodylate buffer (pH = 7.4) for 30 min. Samples were dehydrated in a graded ethanol series and permeated with Epoxy resin. Fifty- to 80-nm ultrathin thick sections were obtained by using an ultramicrotome (JEOL USA Inc.). Afterwards, the grids were incubated with 2% uranyl acetate as a contrast dye and examined using a Phillips (FEI Company, Eindhoven, Netherlands) electron microscope operated at 80 kV.
Evaluating total fatty acid profile by gas chromatography
To investigate whether sera from diabetic subjects were able to exert any changes in the total fatty acid profile, we measured total fatty acid profiles by the direct trans-esterification method as was previously described by protocols (Rezabakhsh et al. 2017b). Methyl esters were detected by gas chromatography normalized to an internal standard by using a PeakSimple software version 3.59. The percentage of fatty acids—including saturated (SFAs; palmitate, stearate, and myristate), monounsaturated (MUFAs; palmitoleate and oleate), and polyunsaturated fatty acids (PUFAs; arachidonate and linoleate)—was expressed as the percentage of total fatty acids.
Statistical analysis
Data are expressed as mean ± SD. Student’s t test was used to calculate the significance of differences between groups. We performed data analysis with SPSS 20. Values of p < 0.05 were considered statistically significant. In histograms, statistical differences between groups are indicated by brackets with *p < 0.05, **p < 0.01, and ***p < 0.001.
Results
DM2 sera decreased hMSC survival and induced apoptosis
To investigate whether diabetic sera could affect hMSC survival rate and promote apoptosis, we performed MTT assay and flow cytometry analysis, respectively. Our results showed that the number of viable cells was reduced in hMSCs receiving diabetic sera as compared to cell treated with normal sera (Fig. 1(a)). We observed a slight and non-significant decrease in hMSC viability after being exposed to diabetic sera (pcontrol versus diabetic > 0.05; Fig. 1(b)). These data indicated that diabetic condition had potential to decrease hMSC viability although at a low rate. We next went on to detect the possible effect of DM2 on the diabetic hMSC apoptotic rate. Compared to cells given normal sera, a statistically significant difference was observed in the percent of apoptotic hMSCs exposed to diabetic sera (Fig. 1(c), (d)). We showed that the percentage of normal hMSCs undergoing apoptosis was 11.98 ± 1.34% while this value increased to 33.62 ± 2.82% in diabetic condition (pcontrol versus diabetic > 0.001). These data suggest that diabetic condition potentially induces cytotoxicity in stem cells possibly by engaging apoptosis.
The cell viability rate (%) of each subject in control and diabetic sera groups after 7 days (a). Percentage of cell viability rate in diabetic sera group was decreased (b). Flow cytometric analysis of Annexin-V-positive cells in the groups (c). Percentage of apoptotic cells was increased in diabetic sera group (d). ***p < 0.001 versus between two groups, using Student’s t test. All values are means ± SD; n = 6 per group
DM2 sera upregulated the expression of genes involved in exosome biogenesis
To assess the effect of DM2 sera on exosome biogenesis, we used quantitative real-time PCR analysis to measure the transcription level of Alix, CD63, Rab27a, Rab27b, and Rab8b 7 days post-treatment with diabetic sera. Data showed that DM2 significantly upregulated the expression of genes Alix, CD63, Rab27a, Rab27b, and Rab8b over a period of 7 days (pcontrol versus diabetic > 0.05; Fig. 2(a), (a’), (a”), (a”’), (a””)). Compared to the control, the mRNA level of Alix, CD63, Rab27a, and Rab8b genes was significantly increased in the diabetic group (Alix, pcontrol versus diabetic < 0.01; CD63, pcontrol versus diabetic < 0.01; Rab27a, pcontrol versus diabetic < 0.01; Rab27b, pcontrol versus diabetic < 0.05; Rab8b, pcontrol versus diabetic < 0.01; Fig. 2(a), (a’), (a”), (a”’), (a””)). These results indicated the enhancement of exosome biogenesis by upregulating key genes upon hMSC treatment with a diabetic condition.
The level of expression of CD63-, Alix-, Rab27a-, Rab27b-, and Rab8b-specific mRNA in cells was analyzed by qPCR (a, a’, a”, a”’ and a””). The released exosomes were quantified by measuring exosome-associated acetylcholinesterase (b). Quantitation of CD63 protein level by western blot and densitometric quantitation of the bands is depicted (c and c’). *p < 0.05, **p < 0.01, ***p < 0.001 versus between two groups, using Student’s t test. All values are means ± SD; n = 6 per group
hMSC AChE activity was increased in diabetic condition
We used AChE activity assay to evaluate the rate of EV release to CMs after cell treatment with diabetic sera. Based on the data, we found an increased AChE activity in supernatants from the diabetic cells (pcontrol versus diabetic < 0.001; Fig. 2(b)). Along with enhanced expression of Alix, CD63, Rab27a, Rab27b, and Rab8b genes, diabetic hMSCs were found to show a high rate of AChE activity, indicating a promoted exosome release to extracellular niche.
The protein level of CD63 was increased in hMSCs treated with DM2 sera
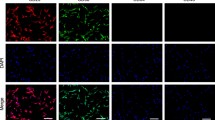
To measure the protein level of CD63, we performed western blotting and immunofluorescence assays. Densitometry analysis showed a ~ 2-fold increase in the protein level of CD63 in the diabetic hMSCs as compared to non-treated control (pcontrol versus diabetic < 0.01) (Fig. 2(c), (c’)). The cellular distribution of CD63 was also studied by immunofluorescence microscopy. According to data from this panel, the number of FITC-CD63+ was increased following the 7-day incubation with diabetic sera (Fig. 3(a), (a’)). These data support a notion that diabetic condition could possibly stimulate the exosome signaling pathway in the level of gene expression and protein synthesis.
Representative images of CD63-PE distribution in hMSCs of control and diabetic sera groups, detected by fluorescence microscopy. Arrows show the distribution of CD63. Scale bar: 50 μm (a and a’). Cells were stained with a CD63-PE antibody with (total) or without (surface) permeabilization, and analyzed by flow cytometry; diagram corresponds to ratio of surface CD63/total CD63 (b and c) a significant increase was shown in panel B. *p < 0.05 versus between two groups, using Student’s t test. All values are means ± SD; n = 6 per group
The subcellular distribution of CD63 was altered in diabetic hMSC
For further insight into the mechanisms involved in the enhanced secretion of exosomes during the diabetic change, the subcellular distribution of CD63 was monitored by using a flow cytometry analysis. First, total and surface distribution of CD63 was investigated using permeabilized or non-permeabilized hMSCs, respectively (Fig. 3(b)). Flow cytometry analysis showed that the surface/total CD63 ratio was significantly increased in the diabetic group as compared to the control hMSCs (pcontrol versus diabetic < 0.05; Fig. 3(c)). Based on data, the surface CD63 was 39.02 ± 4.43% while this level increased to 42.28 ± 4.97% after hMSCs exposed to a diabetic condition. These results showed the cell-surface accumulation of CD63 in diabetic hMSCs.
Characterization of isolated exosomes by flow cytometry and SEM
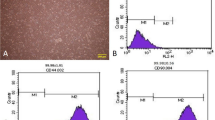
Characterization of exosomes released from hMSCs was performed by flow cytometry analysis and SEM. SEM imaging revealed round shape and nano-scale-sized exosomes, showing the efficiency of kit in isolating exosomes from the supernatant medium (Fig. 4(a), (a’), (a”)). Immunophenotyping by flow cytometry showed that near to 87.2 ± 6.7% of isolated exosomes expressed an exosome common factor CD63 (Fig. 4(b)). Noteworthy, exosome analysis with an antibody against CD9 showed no reactivity (data not shown here). It seems that the level of CD9 is possibly low in hMSC-derived exosomes.
Scanning electron micrographs of isolated exosomes. Scale bar 500 nm (a, a” and a”). Flow cytometric analysis confirmed isolated exosomes by detecting CD63 as exosome marker (b). DLS plot related to the size of isolated exosomes (c and c’). DLS analysis of size and zeta potential of hMSCs released exosomes are shown (d and d’). *p < 0.05 versus between two groups, using Student’s t test. All values are means ± SD; n = 6 per group
DM2 sera altered the size and ZP of hMSC exosomes
In order to evaluate the possible effect of DM2 sera on the size and ZP of exosomes from diabetic hMSCs, we used DLS technique (Fig. 4(c), (c’), (d), (d”)). Compared to control cells, we found that the size of exosomes significantly was increased upon hMSCs treatment with diabetic sera (63.5 ± 8.7 nm vs. 178.3 ± 21.5 nm; pcontrol versus diabetic < 0.05) (Fig. 4(c), (c’)). We observed an increase in the ZP value of diabetic hMSC-derived exosomes (− 7.04 ± 1.24 vs. − 5.39 ± 1.58 mV; pcontrol versus diabetic < 0.05; Fig. 4(d), (d’)). Based on the data, the concentration of exosomes in supernatant from diabetic sera was more compared to the control (160.03 ± 17.55 vs. 133.83 ± 29.3). These results indicate that diabetic condition potentially modified both size and ZP index of hMSC exosome following incubation with sera from type 2 diabetes mellitus subjects.
TEM revealed an ultrastructural alternation in diabetic hMSCs
To obtain further insight into diabetic hMSCs at the ultrastructural level, we performed TEM analysis (Fig. 5(a), (a’)). High-resolution imaging revealed a large number of cytoplasmic vacuoles in diabetic hMSCs in comparison with normal cell counterpart. At the ultrastructural level, we observed an increase in the number of lipid droplets and excretory vacuoles (Fig. 5(a), (a’)). Noteworthy, the size of lipid droplets in diabetic hMSCs was also reduced. A large number of lipid droplets may correlate with increased intracellular glucose and fat contents.
Transmission electron micrographs of representative hMSCs after 7 days period of treatment (a and a’). Exo, exosome; MVB, multivesicular body; M, mitochondria; V, vacuole. Scale 2000 nm. Fatty acid profile analysis of hMSCs by gas chromatography (b). Statistical analysis: Student’s t test. All values are means ± SD; n = 6 per group
DM2 did not change fatty acid profile in hMSCs
To determine whether the DM2 sera could affect dynamic of fatty acid profile, we used GC. Our results showed that cell treatment with DM2 sera resulted in a slight non-significant alternation in fatty acid profile as compared to control group (pcontrol versus diabetic > 0.05; Fig. 5(b)). According to data, the level of intracellular palmitate was increased while the total content of stearate, oleate, and linoleate decreased upon cell treatment with diabetic sera over a period of 7 days. Statistically non-significant results were observed in fatty acid content between control and diabetic groups (p > 0.05).
Discussion
It is well established that DM2 is associated with progression of different pathologies and angiogenesis complication (Cade 2008; Kolluru et al. 2012; Rezaie et al. 2018). Recent studies showed that DM2 could affect the dynamics of stem cells and various progenitors (Ostrowski et al. 2010; Rezaie et al. 2018). MSCs contribute to neovascularization in normal and pathological conditions by angiocrine activity and releasing a plethora of factors through the exosomal pathway (Ardestani et al. 2014; Shabbir et al. 2015). The molecular mechanisms involving in the exosome signaling pathway in DM2 condition are still largely unknown.
Here, we examined the relationship between DM2 and the capacity of exosome secretion at hMSCs 7 days post-treatment with sera from DM2 patients. The data from the current study and previous experiments showed that diabetic sera had potential to augment apoptosis in hMSCs and various cell types (Makino et al. 2005; Record et al. 2011; Rezabakhsh et al. 2017a). Stolzing and co-workers previously stated that apoptotic status appears to be initiated by advanced glycation end products and upregulation of AGE receptor in murine MSCs (Stolzing et al. 2010). To assess the hypothesis that DM2 may affect the dynamic of exosome signaling pathway, we evaluated the expression of key genes involved in exosome intracellular secretory pathway termed CD63, Alix, Rab27a, Rab27b, and Rab8b (Kowal et al. 2014). Interestingly, we found that all of these genes were upregulated in diabetic hMSCs. In contrary, our group previously reported a decrease in the expression of CD63, Alix, and Rab27a in human endothelial progenitor cells (EPCs) after exposure to diabetic sera in vitro (Hassanpour et al. 2016). One explanation would be that various stem cell types may respond differently to a diabetic condition. An increased intracellular CD63 protein level was further confirmed by western blotting which coincided with enhanced AChE activity in the supernatant medium. These features might confirm an accelerated exosome secretion (Baietti et al. 2012; Ostrowski et al. 2010). Consistently, Chen and colleagues reported that the incubation of EPCs with diabetic sera was associated with the elevated amount of EVs (Chen et al. 2011). Additionally, the exposure of glomerular endothelial cells to high content glucose induced the production of exosomes (Wu et al. 2016).
Commensurate with these comments, we found an increased surface CD63/total CD63 ratio in diabetic hMSCs, showing the induction of MVB fusion with the cell membrane upon treatment with diabetic sera. Overall, these data support a notion that sera from DM2 are able to induce the production and abscission of exosomes. Ostrowski and co-workers found that the inhibition of Rab proteins changes the cellular distribution of exosomes and abnormal morphology of CD63-positive compartments (Kowal et al. 2014). Considering a great body of experiments, it seems that an enhanced exosome production and release are strategies that possibly help cells to excrete the harmful compounds and obsolescent proteins (Harding et al. 1983; Lehmann et al. 2008; Pan et al. 1985). Recently, it was determined that exosomes could support cell homeostasis by expelling harmful DNA from cells (Takahashi et al. 2017). The future studies must focus to elucidate whether increased exosome secretion is a cellular strategy to eliminate cell damages or is a therapeutic compensatory manner in response to a diabetic stress condition. We also found that the size of hMSC exosomes was increased in diabetic condition while ZP value decreased. Generally, exosomes bear different saccharide groups such as polylactosamine, mannose, α-2,6 sialic acid, and complex N-linked glycans that are responsible for ZP (Akagi et al. 2016). One could hypothesize that the decrease in ZP value may correlate with an altered carbohydrate metabolism during DM2. The ultrastructural analysis also confirmed the existence of numerous lipid droplets, indicating abnormal lipid and carbohydrate metabolism in diabetic hMSCs (Chen et al. 2015; Wang et al. 2014). It was recently declared that exosomes from various cell sources show different ZP activities. In line with these comments, the changes in ZP of exosomes reflect the alternation PM, specifically in the expression of sugar chains of their parent cells (Akagi et al. 2016).
Conclusion
In conclusion, the present study showed an increased exosome secretion rate in diabetic hMSCs. Nevertheless, ongoing researchers are essential to prove the exact pivotal role of activated exosome secretory pathway to predict stem cell behavior in diabetic condition.
References
Akagi T, Ichiki T, Ohshima H (2016) Evaluation of zeta-potential of individual exosomes secreted from biological cells using a microcapillary electrophoresis chip. Encyclopedia of Biocolloid and Biointerface Science 2V Set 469–473
Ardestani A, Paroni F, Azizi Z, Kaur S, Khobragade V, Yuan T, Frogne T, Tao W, Oberholzer J, Pattou F (2014) MST1 is a key regulator of beta cell apoptosis and dysfunction in diabetes. Nat Med 20:385–397
Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E (2012) Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol 14:677–685
Cade WT (2008) Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther 88:1322
Chen J, Chen S, Chen Y, Zhang C, Wang J, Zhang W, Liu G, Zhao B, Chen Y (2011) Circulating endothelial progenitor cells and cellular membrane microparticles in db/db diabetic mouse: possible implications in cerebral ischemic damage. Am J Physiol Endocrinol Metab 301:E62–E71
Chen LN, Sun Q, Liu SQ, Hu H, Lv J, Ji WJ, Wang M, Chen MX, Zhou J (2015) Erythropoietin improves glucose metabolism and pancreatic β-cell damage in experimental diabetic rats. Mol Med Rep 12:5391–5398
Choi D-S, Lee J-M, Park GW, Lim H-W, Bang JY, Kim Y-K, Kwon K-H, Kwon HJ, Kim KP, Gho YS (2007) Proteomic analysis of microvesicles derived from human colorectal cancer cells. J Proteome Res 6:4646–4655
Harding C, Heuser J, Stahl P (1983) Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol 97:329–339
Hassanpour M, Cheraghi O, Siavashi V, Rahbarghazi R, Nouri M (2016) A reversal of age-dependent proliferative capacity of endothelial progenitor cells from different species origin in in vitro condition. J Cardiovasc Thoracic Res 8:102–106
Hutagalung AH, Novick PJ (2011) Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 91:119–149
Kolluru GK, Bir SC, Kevil CG (2012, 2012) Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med
Kowal J, Tkach M, Théry C (2014) Biogenesis and secretion of exosomes. Curr Opin Cell Biol 29:116–125
Lehmann BD, Paine MS, Brooks AM, McCubrey JA, Renegar RH, Wang R, Terrian DM (2008) Senescence-associated exosome release from human prostate cancer cells. Cancer Res 68:7864–7871
Makino N, Maeda T, Sugano M, Satoh S, Watanabe R, Abe N (2005) High serum TNF-α level in type 2 diabetic patients with microangiopathy is associated with eNOS down-regulation and apoptosis in endothelial cells. J Diabetes Complicat 19:347–355
Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP (2010) Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 12:19–30
Pan B-T, Teng K, Wu C, Adam M, Johnstone RM (1985) Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol 101:942–948
Phinney DG, Pittenger MF (2017) Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells 35:851–858
Rahbarghazi R, Nassiri SM, Ahmadi SH, Mohammadi E, Rabbani S, Araghi A, Hosseinkhani H (2014) Dynamic induction of pro-angiogenic milieu after transplantation of marrow-derived mesenchymal stem cells in experimental myocardial infarction. Int J Cardiol 173:453–466
Record M, Subra C, Silvente-Poirot S, Poirot M (2011) Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol 81:1171–1182
Rezabakhsh A, Cheraghi O, Nourazarian A, Hassanpour M, Kazemi M, Ghaderi S, Faraji E, Rahbarghazi R, Avci ÇB, Bagca BG (2017a) Type 2 diabetes inhibited human mesenchymal stem cells angiogenic response by over-activity of the autophagic pathway. J Cell Biochem 118:1518–1530
Rezabakhsh A, Nabat E, Yousefi M, Montazersaheb S, Cheraghi O, Mehdizadeh A, Fathi F, Movassaghpour AA, Maleki-Dizaji N, Rahbarghazi R (2017b) Endothelial cells’ biophysical, biochemical, and chromosomal aberrancies in high-glucose condition within the diabetic range. Cell Biochem Funct 35:83–97
Rezaie J, Ajezi S, Avci ÇB, Karimipour M, Geranmayeh MH, Nourazarian A, Sokullu E, Rezabakhsh A, Rahbarghazi R (2017) Exosomes and their application in biomedical field: difficulties and advantages. Mol Neurobiol https://doi.org/10.1007/s12035-017-0582-7
Rezaie J, Mehranjani MS, Rahbarghazi R, Shariatzadeh MA (2018) Angiogenic and restorative ability of human mesenchymal stem cells were reduced following treatment with serum from diabetes mellitus type 2 patients. J Cell Biochem 119(1):524–535
Robbins PD, Morelli AE (2014) Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 14:195–208
Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Badiavas EV (2015) Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev 24:1635–1647
Stolzing A, Sellers D, Llewelyn O, Scutt A (2010) Diabetes induced changes in rat mesenchymal stem cells. Cells Tissues Organs 191:453–465
Stoorvogel W (2015) Resolving sorting mechanisms into exosomes. Cell Res 25:531–532
Takahashi A, Okada R, Nagao K, Kawamata Y, Hanyu A, Yoshimoto S, Takasugi M, Watanabe S, Kanemaki MT, Obuse C (2017) Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun 8
Urbanelli L, Magini A, Buratta S, Brozzi A, Sagini K, Polchi A, Tancini B, Emiliani C (2013) Signaling pathways in exosomes biogenesis, secretion and fate. Genes 4:152–170
Van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G (2011) The tetraspanin CD63 regulates ESCRT-independent and-dependent endosomal sorting during melanogenesis. Dev Cell 21:708–721
Wang L, Wang Y, Liang Y, Li J, Liu Y, Zhang J, Zhang A, Fu J, Jiang G (2014) PFOS induced lipid metabolism disturbances in BALB/c mice through inhibition of low density lipoproteins excretion. Scientific reports 4:
Wu X-m, Gao Y-b, F-q C, Zhang N (2016) Exosomes from high glucose-treated glomerular endothelial cells activate mesangial cells to promote renal fibrosis. Biol Open 5:484–491
Yan J, Tie G, Wang S, Messina KE, DiDato S, Guo S, Messina LM (2012) Type 2 diabetes restricts multipotency of mesenchymal stem cells and impairs their capacity to augment postischemic neovascularization in db/db mice. J Am Heart Assoc 1:e002238
Zeelenberg IS, Ostrowski M, Krumeich S, Bobrie A, Jancic C, Boissonnas A, Delcayre A, Le Pecq J-B, Combadière B, Amigorena S (2008) Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses. Cancer Res 68:1228–1235
Acknowledgments
The authors gratefully thank the personnel of Stem Cell Research Center, Tabriz University of Medical Sciences.
Funding
This work was supported by a grant (no. TBZMED.REC.1394.928) from the Research Council, Tabriz University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All volunteers were asked to fill out an informed consent. All procedures performed through the current experiment, involving human participants, were in accordance with the local ethics committee of Tabriz University of Medical Sciences (Ethical code no. TBZMED.REC.1394.928) and ethical principles of Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Rezaie, J., Nejati, V., Khaksar, M. et al. Diabetic sera disrupted the normal exosome signaling pathway in human mesenchymal stem cells in vitro. Cell Tissue Res 374, 555–565 (2018). https://doi.org/10.1007/s00441-018-2895-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-018-2895-x