Abstract
Arsenic is an environmental contaminant, and accumulating evidence has indicated that exposure to arsenic can cause various diseases, especially cardiotoxicity. Selenium (Se) exerts a vital role in the regulation of multiple physiological activities. Recently, several studies highlighted that Se treatment can effectively antagonize the toxic effects induced by arsenic. However, the exact underlying effect and mechanism of Se on Arsenic-induced cardiotoxicity has not been explored. In the current study, the arsenic trioxide (ATO)-triggered heart damage mice model was used to explore whether Se exerts protective roles in ATO-related cardiotoxicity and its potential mechanism. Our data showed that Se treatment significantly alleviated ATO-mediated cardiotoxicity evidenced by increased weight, decreased myocardial damage markers, and improved heart functions in mice. Furthermore, we demonstrated that Se remarkably inhibited ATO-mediated oxidative stress and inflammatory responses in heart tissues. Mechanistically, we showed that Se upregulated the levels of NAD+ in cardiomyocytes of the mice challenged by ATO, and this effect involved in the activation of the NAD+ biosynthesis through the salvage pathway. Collectively, our findings demonstrated that Se protected against ATO-mediated cardiotoxicity by antioxidant and anti-inflammatory effects via increasing the NAD+ pool in mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arsenic, one of the natural contaminants, is widely used in industry, agriculture, and medicine in the forms of trivalent, pentavalent, and organic compounds, and many people are exposed to excess levels globally [1]. Inorganic arsenic showed more toxic than the organic states [2]. Accumulating evidence has indicated that exposure to arsenic causes various diseases, especially cardiovascular disorders. Epidemiologic studies have shown that the incidence of coronary heart disease was closely associated with chronic low arsenic exposure in drinking water(10–100 μg/L) [3]. Nigra et al. found that even low levels of urinary arsenic (the median of total arsenic were 4.42 μg/L) was also related to increased heart disease mortality [4]. Besides, it has also been suggested that occupational exposures to inorganic arsenic accelerate myocardial injury [5]. Experimental studies have highlighted that excessive induction of oxidative stress and inflammatory response is defined as two primary causative mechanisms in arsenic-mediated heart injury. The metabolism of arsenic in the body could induce the generation of a large number of superoxide anion free radicals (O2−), subsequently activate transcription factors that regulate the release of pro-inflammatory cytokines which induce DNA damage, mitochondrial dysfunction, and finally result in cardiomyocyte death [6]. Therefore, inhibition of arsenic-triggered reactive oxygen species (ROS) production and inflammatory response might be a promising therapeutic strategy to alleviate arsenic-mediated cardiotoxicity.
Selenium (Se) as a critical micronutrient is found in all body organs. Se possesses multiple physiological activities including antioxidant, anti-inflammatory, and autophagic properties [7, 8]. The association between Se and cardiac diseases has also been reported. Bomer et al. reported that there was a close association between Se deficiency and worse symptoms in patients with heart failure [9]. Wang et al. found that Se supplementation protected cardiomyocytes from inflammatory responses and improved LPS-induced cardiac injury [10]. Previously, we verified that Se administration significantly suppressed ROS generation, inhibited inflammatory response, and improved doxorubicin-related myocardial dysfunctions [11]. Recent reports suggested that Se supplementation could antagonize arsenic-triggered immunotoxicity, liver injury, and behavioral impairments in animal models [12,13,14]. However, whether Se exerts protective roles in arsenic-related heart injury and its potential mechanism is still unknown.
Nicotinamide adenine dinucleotide (NAD+), a critical modulator of intracellular signaling, plays key roles in several aspects of cell biology, including energy hemostasis, cell survival, and DNA repair [15]. Increasing the intracellular NAD+ pool was identified as a promising way in the treatment of obesity, diabetes, brain ischemic injury, non-alcoholic fatty liver disease, kidney disease, and aging [16, 17]. Meanwhile, the essential role of NAD+ in cardiac diseases was also reported. Zhou et al. found that boosting NAD+ levels attenuated inflammatory responses and improved heart failure [18]. Increasing NAD+ pool protected cardiomyocytes from oxidative stress-mediated impairment [19]. In addition, replenishment of NAD+ also protected the myocardium against doxorubicin-induced cardiotoxicity [20]. Intriguingly, Wang et al. observed that arsenic trioxide significantly inhibited the intracellular NAD+ levels in oral squamous carcinoma cells [21]. Therefore, we speculated that increasing the NAD+ pool might be a new strategy to improve arsenic-induced cardiac injury.
We hypothesized that Se supplementation could increase the levels of NAD+, prevent excessive inflammatory responses and oxidative stress, and then suppress arsenic-induced cardiotoxicity. Our study might provide new insight into Se for the treatment of arsenic-related heart damage.
Methods
Materials and Agents
Arsenic trioxide (ATO, purity > 98%, the oxidation state of arsenic trioxide is 3 +) and sodium selenite (Se, the oxidation state of selenite is 4 +) were obtained from Meilunbio (Dalian, China). The standard diet was purchased from SJA laboratory animal Co., Ltd. (Hunan, China), and the compositions of the diet are shown in Supplementary Table 1. The kits including creatine kinase isoenzymes (CK), lactate dehydrogenase (LDH), glutathione (GSH), the cardiac isoform of Troponin T (cTnT), and malondialdehyde (MDA) were purchased from Jiancheng, Inc. (Nanjing, China). ELISA kit for NAD+ was obtained from BioAssay Systems (Hayward, CA). Kits including interleukin 1β (IL-1β), IL-6, superoxide dismutase (SOD), catalase (CAT), and tumor necrosis factor-α (TNF-α) were obtained from Elabscience, Inc. (Wuhan, China). Antibodies for β-actin and NAMPT were purchased from Cell Signaling Technology (Beverly, USA).
Experimental Protocol
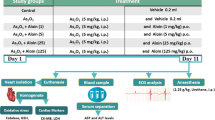
All protocols and experiments for animals were conducted following the Guide of the Shanxi Medical University Animal Care and Use Committee. In this study, male Kunming mice (22 ± 3 g) were from Shanxi Medical University and kept at 23–26 °C with a 12-h light/dark cycle. At the same time, all mice could obtain water and standard diet freely. After acclimation for 1 week, the administration was as follows: Control group (CTRL): NS (vehicle of Se, oral) + NS (vehicle of ATO group, i.p.); ATO group (ATO): NS (vehicle of Se, oral) + ATO (5 mg/kg/day, i.p.) [22]; ATO + low sodium selenite (Se) group (L-Se): 0.5 μg/g Se (oral) + ATO (5 mg/kg/day, i.p.); ATO + high Se group (H-Se): 2 μg/g Se (oral) + ATO (5 mg/kg/day, i.p.); FK866 + ATO + high Se group (FK866 + H-Se): FK866 (30 mg/kg/day, i.p.) + 2 μg/g Se (oral) + ATO (5 mg/kg/day, i.p.). Se was received by gavage for 2 weeks before ATO intraperitoneal treatment, and FK866 was treated 1 h before ATO exposure for 7 days. One week after the treatment of ATO, animals were weighed and then euthanized by sodium pentobarbital (50 mg/kg, i.p.). The heart tissues and blood samples were collected for further studies. The timeline on experimental procedures is shown in Fig. 1A.
Echocardiography
The cardiac functions were detected using the Vevo 770 high-resolution small animal system with an RMV 707-B scan head (frequency 30 MHz, focal length 12.7 mm) (VisualSonics, Canada) and the PowerLab system (AD Instruments, UK) as we previously described [11].
Measurement of Heart Injury Markers and Oxidative Stress Markers
The contents of heart injury markers in serum including CK-MB, LDH, and cTnT as well as oxidative stress markers in heart tissues including MDA, SOD, CAT, and GSH were detected following the manufacturer’s recommendations.
Measurements of Inflammatory Cytokine
The levels of myocardial inflammatory cytokine in heart tissues containing IL-1β, IL-6, and TNF-α were assessed by ELISA kits according to the manufacturer’s recommendations.
Measurements of Cellular NAD+ Levels
The cellular NAD+ levels were measured by ELISA kits in accordance with the manufacturer’s recommendations.
Hematoxylin–Eosin (H&E) Staining
The heart tissues were collected and fixed with 4% paraformaldehyde. Subsequently, the fixed heart tissues were embedded with paraffin and then sectioned into pieces. To conduct HE staining, a HE staining kit (Solarbio, Beijing, China) was used in accordance with instructions of the manufacturer. The images were capture under a light microscope (Olympus, Japan).
Immunohistochemistry Staining
The cardiac samples were preserved in 4% paraformaldehyde and then embedded by paraffin. Subsequently, the specimens were sectioned at 5 µM, and then, the sections were stained with antibodies against TNF-α and IL-6 to evaluate pro-inflammatory cytokine expression. The slices were examined using microscopy (Olympus, Japan).
Western Blot Analysis
The total heart proteins were collected by commercial RIPA buffer obtained from Beyotime, China. The protein contents of samples were measured with BCA kits. The samples were separated by 10% SDS-PAGE followed by transfer to nitrocellulose membranes. At the end of transfer, the membranes were blocked with 5% BSA. Subsequently, the membranes were incubated with primary antibodies against NAMPT or β-actin at 4 ℃ overnight followed by incubation with secondary antibodies. Ultimately, chemiluminescent kit was used to detect the bands.
Real-Time PCR
To extract the total RNA of heart tissues, TRIZOL was used following manufacturer’s instructions. cDNA synthesize and quantitative PCR analysis was conducted as previously reported. The levels of target genes were normalized to β-actin and analyzed with the 2−ΔΔCt method. All the primer sequences are presented in Table 1.
Statistical Analysis
All data in this study were presented as mean ± SEM and analyzed with GraphPad Prism software, while one-way ANOVA with Tukey’s post hoc test was used during the analysis. P < 0.05 was considered significant.
Results
Se Attenuated ATO-Triggered Body Weight Loss and Cardiac Dysfunction
Firstly, to detect the possible effects of Se on the cardiotoxicity challenged by ATO, the body weight and cardiac functions were assessed. As shown in Fig. 1B, the mice exposed to ATO significantly reduce body weight; however, Se supplementation remarkably reverses this change in mice exposed to ATO. Furthermore, the effects of Se on ATO-triggered dyscardiac functions were also assessed. As shown in Fig. 1C–E, the cardiac function indexes, such as ejection fraction (EF), maximal slope of systolic pressure increment (+ dP/dt), and diastolic pressure decrement (− dP/dt) of the ATO-treated mice, are significantly lower in comparison with those of the CTRL group. However, the decrease of EF and ± dp/dt was significantly reversed by Se treatment in a dose-dependent manner.
Se Treatment Alleviated ATO-Induced Myocardial Enzyme Elevation
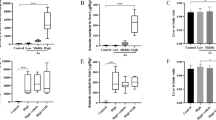
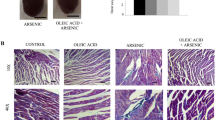
Next, the cardiac injury markers including cTnI, CK, and LDH were also detected. As shown in Fig. 2A, H&E staining show that cardiomyocytes in the CTRL group lined up regularly; however, myocardial cells in the ATO-treated group are hypertrophied and irregularly arranged. Nevertheless, these changes were alleviated by Se treatment. Meanwhile, ATO markedly elevated serum CK (Fig. 2B), LDH (Fig. 2C), and cTnI (Fig. 2D) concentrations compared to the CTRL group. However, Se treatment were significantly decreased the levels of CK, cTnI, and LDH in a dose-dependent manner.
Se Treatment Ameliorated ATO-Triggered Myocardial Oxidative Stress
As shown in Fig. 3A, the levels of lipid peroxidation MDA are detected. Supplementation of Se effectively decreased ATO-triggered MDA overproduction in heart tissues of the mice. Besides, ATO markedly downregulated SOD activities and GSH and CAT contents in the heart of mice, which were markedly elevated by Se administration (Fig. 3B–D).
Se Treatment Decreased ATO-Induced Pro-Inflammatory Cytokine Expression
The inflammatory response is greatly related to ATO-mediated cardiotoxicity. Therefore, we further detected whether Se attenuated ATO-induced pro-inflammatory cytokine expression. The expression of TNF-α, IL-6, and IL-1β in heart tissues was significantly upregulated in the ATO group, whereas a significant inhibition of TNF-α, IL-6, and IL-1β was observed in the Se-treated group (Fig. 4A–C). Furthermore, IL-6 and TNF-α staining also showed that Se treatment substantially downregulated the expression of these pro-inflammatory cytokines caused by ATO (Fig. 4D and E).
Se decreased the pro-inflammatory cytokine expression caused by ATO. A–C The concentration of TNF-α, IL-6, and IL-1β in the heart tissues of the mice. D Representative images of IL-6 staining in mice heart tissues. E Representative images of TNF-α staining in mice heart tissues. N = 5, **P < 0.01 vs. CRTL; #P < 0.05, ##P < 0.01 vs. ATO
Effects of Se on ATO-Induced Changes in NAD+ Levels
NAD+ deficiency has been identified among the primary causes in many cardiac diseases. Therefore, we investigated NAD+ levels in heart tissues in four groups. As shown in Fig. 5, the levels of NAD+ in heart tissues are remarkably decreased in ATO group. However, Se treatments effectively elevated NAD+ levels induced by the ATO, especially in the H-Se group.
Effects of Se on ATO-Induced Changes on NAMPT/NAD+ Axis
The relative expression of four major enzyme regulation NAD+ biosynthesis including NAMPT, indoleamine 2,3-dioxygenases (IDO), nicotinamide adenine dinucleotide synthase (NADS), and nicotinamide/nicotinic acid mononucleotide adenylyltransferase (NMNAT) were investigated. The qPCR results indicated that there was no difference on the mRNA expression of NMNAT, NADS, and IDO in four groups (Fig. 6A–C). However, ATO exposure significantly decreased the mRNA levels of NAMPT compared with CTRL group, whereas Se pretreatment significantly upregulated the expression of NAMPT mRNA in a dose-dependent manner (Fig. 6D). Besides, western blot also showed that the expression of NAMPT was significantly decreased in the ATO group, while Se treatment remarkably reversed this change in heart tissues (Fig. 6E).
Se upregulated NAMPT/NAD+ axis in mice. A–C The relative mRNA levels of NMNAT, NADS, and IDO in heart tissues of the mice. D The relative mRNA levels of NAMPT in heart tissues of the mice. E Representative images and quantitative analysis of NAMPT protein in heart tissues. NS means no significant, N = 4–5, **P < 0.01 vs. CRTL; #P < 0.05, ##P < 0.01 vs. ATO
FK866 Treatment Abolished the Protectively Effects of Se on ATO-Induced Cardiotoxicity
To further verify the essential role of NAMPT/NAD+ axis in the protective effects of Se in ATO-induced cardiotoxicity, we pretreated the animals with the specific NAMPT inhibitor FK866 and/or Se before the exposure to ATO. As presented in Fig. 7A, Se treatment largely increases ATO-caused downregulation of levels of NAD + in heart tissues, whereas this effect is significantly blocked by FK866. Furthermore, Se treatment remarkably improved histological changes and downregulated ATO-triggered myocardial enzyme elevation; however, co-treatment with FK866 significantly reversed these changes (Fig. 7B–E). The levels of MDA and contents of SOD assay also revealed that Se-induced reduction of intracellular MDA and elevation of SOD were abolished by FK866 (Fig. 7F and G). In addition, co-treatment with FK866 significantly reversed the downregulation of Se on the expression of IL-6 and TNF-α in heart tissues (Fig. 7H and I). These data demonstrated that Se ameliorated ATO-triggered oxidative stress and inflammation in heart tissues partly via NAMPT/NAD+ axis.
FK866 reversed the cardioprotective effects of Se. The animals were pretreated with Se (2 μg/g) for 2 weeks, and then, FK866 (30 mg/kg/day, i.p.) was treated 1 h before ATO exposure for 7 days. A The levels of NAD+ in heart tissues were determined. B Representative images of H&E staining in mice heart tissues. C–E The levels of CK, LDH, and CTnl in four groups. F–G The levels of MDA and contents of SOD were assayed. H–I Representative images of IL-6 and TNF-α staining in mice heart tissues. N = 6, **P < 0.01 vs. ATO; ##P < 0.01 vs. H-Se + ATO
Discussion
In the current study, the ATO-induced heart damage mice model was used to explore whether Se exerts protective roles in arsenic-related cardiotoxicity and its potential mechanism. Our data showed that Se supplementation significantly alleviated ATO-mediated cardiotoxicity reflected by increased body weight, decreased myocardial damage markers, and improved heart functions in mice. Furthermore, we demonstrated that Se remarkably inhibited ATO-mediated oxidative stress and inflammatory responses in heart tissues. Mechanistically, we showed that Se upregulated the levels of NAD+ in heart tissues of the mice challenged by ATO, and this effect involved in the activation of the NAD+ biosynthesis through the salvage pathway. Collectively, our findings showed that Se protected against ATO-mediated cardiotoxicity by antioxidant and anti-inflammatory effects via increasing the NAD+ pool in mice.
Arsenic is an environmental contaminant and humans may be exposed to arsenic in a variety of routes, including drinking water and food. Accumulating evidence has indicated that exposure to arsenic causes various diseases, especially cardiotoxicity. Recently, several studies highlighted that Se treatment can effectively antagonize the toxic effects induced by arsenic. For example, Se treatment exerted hepatoprotective roles through antioxidant capacities and alleviated arsenic-induced liver damages [23]. Ren et al. found that moderate Se added to daily feed could ameliorate the immune toxicity of arsenic in chickens [12]. Neuroprotective effects of Se against arsenic-induced behavioral impairments were also observed [13]. However, whether Se exerts protective roles in arsenic-related heart injury and its potential mechanism is still unknown. Our study demonstrated that mice exposed to ATO significantly decreased body weight and impaired cardiac dysfunction. However, Se treatment significantly reversed ATO-induced weight loss, alleviated histopathological injury, and improved cardiac functions. In addition, the levels of myocardial markers in serum including CK, LDH, and cTnI, which reflected the degree of myocardial damage and cardiac dysfunction, were also assessed. Consistent with previous studies, we found that ATO exposure presented a significant increase in the CK, LDH, and cTnI levels in mice, which were also remarkably alleviated by Se treatment. These data indicated that Se treatment effectively alleviated ATO-induced cardiotoxicity.
Oxidative stress and inflammatory response were considered the two major factors in the progression of ATO-mediated cardiotoxicity. Arsenic could bind to the SH-group of glutathione, an effective cellular antioxidant, and result in the accumulation of ROS [24]. Arsenic exposure can also significantly upregulate the expression of pro-inflammatory cytokines in heart tissues [25, 26]. Therefore, inhibition of ROS production and inflammatory response might be a possible approach to alleviate ATO-induced cardiomyopathy. In this work, we showed that treatment with Se significantly inhibited cardiomyocyte oxidative stress, as evidenced by decreased MDA contents and elevated SOD, GSH, and CAT activities in heart tissues. Furthermore, we found that Se also significantly decreased cardiomyocyte pro-inflammatory expression, as evidenced by decreased IL-1β, IL-6, and TNF-α levels in cardiomyocytes challenged by ATO. All these results demonstrated that Se alleviated ATO-mediated heart injury via attenuating cardiomyocyte oxidative stress and inflammatory response.
Given the high-energy demand of the cardiac system, the cardiomyocyte is particularly susceptible to dysregulation of NAD+ metabolism. Numerous studies have demonstrated that increasing the cellular NAD+ pool might be a promising therapeutic in cardiac diseases. For example, Breton et al. found that blood NAD+ levels were significantly decreased in old patients with heart failure [27]. Besides, booting NAD+ pool effectively reversed ischemic, hypertrophic, diabetic cardiomyopathy, heart failure, and doxorubicin-induced cardiotoxicity in animal models [28,29,30,31]. Recently, arsenic trioxide treatment significantly decreased the intracellular NAD+ levels in oral squamous carcinoma cells, indicating that decreasing NAD+ may be involved in ATO-induced cardiotoxicity [21]. Our study showed that the levels of NAD+ in heart tissues were significantly decreased in the ATO-exposed group, and this decrease was remarkably reversed after Se supplementation, suggesting that Se treatment might improve ATO-induced cardiotoxicity through increasing NAD+ pool.
As we know, in mammals, there are two ways to maintain intracellular NAD+ levels, including de novo biosynthesis and the salvage pathway. In the de novo biosynthesis pathway, NAD+ is converted through dietary tryptophan, and IDO, NMNAT, and NADS act as three important enzymes. In addition, NAD+ can also be synthesized from nicotinamide, and NAMPT is the rate-limiting enzyme in the salvage pathway [16]. However, which pathway plays an essential role in ATO-related cardiotoxicity remains unclear. Intriguingly, our study showed that there were no differences in the expression of IDO, NMNAT, and NADS in four groups. However, ATO exposure significantly decreased the expression of NAMPT when compared to the CTRL group. While after Se treatment, the expression of NAMPT was remarkably elevated in a dose-dependent manner, suggesting that NMAPT/NAD + axis significantly involved in the protective effects of Se on ATO-mediated cardiotoxicity. Consistent with our results, Wang et al. found that ATO treatment decreased the levels of NAMPT and increased cellular death in an ATO dose-dependent manner in oral squamous carcinoma cells [21]. Meanwhile, Feng et al. reported that NAMPT/NAD+ axis inhibition significantly aggravated atrial fibrillation [32]. Besides, overexpression of NAMPT effectively upregulated the NAD+ pool and increased antioxidant defense in diabetic cardiomyopathy [33]. To further elucidate the role of the NAMPT/NAD+ axis in ATO-triggered cardiotoxicity, the specific NAMPT inhibitor FK866 was used. As expected, we found that the downregulated myocardial enzymes and reduced ROS production and pro-inflammation expression as well as increased NAD+ pool induced by Se supplementation in ATO-treated animal models significantly abolished by co-treatment with FK866. Collectively, our results suggested that the NAMPT/NAD+ axis might contribute to the protective effects of Se in ATO-triggered cardiotoxicity.
In conclusion, we demonstrated that Se treatment significantly alleviated ATO-mediated cardiotoxicity via reducing oxidative stress and inflammation. Mechanistically, we showed that Se upregulated the levels of NAD+ in cardiomyocytes of the mice challenged by ATO through the salvage pathway. Therefore, our findings provided evidence that Se supplementation might be a potential therapeutic agent for ATO-mediated heart damages.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ATO:
-
Arsenic trioxide
- Se:
-
Selenium
- NAD + :
-
Nicotinamide adenine dinucleotide
- CK:
-
Creatine kinase isoenzymes
- LDH:
-
Lactate dehydrogenase
- GSH:
-
Glutathione
- cTnT:
-
Cardiac isoform of Troponin T
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- IL-1β :
-
Interleukin 1β
- IL-6:
-
Interleukin 6
- TNF-α :
-
Tumor necrosis factor-α
- EF:
-
Ejection fraction
- NAMPT:
-
Nicotinamide phosphoribosyltransferase
- IDO:
-
Indoleamine 2,3-dioxygenases
- NADS:
-
Nicotinamide adenine dinucleotide synthase
- NMNAT :
-
Nicotinamide/nicotinic acid mononucleotide adenylyltransferase
References
Wang J, Hu W, Yang H, Chen F, Shu Y, Zhang G, Liu J, Liu Y, Li H, Guo L (2020) Arsenic concentrations, diversity and co-occurrence patterns of bacterial and fungal communities in the feces of mice under sub-chronic arsenic exposure through food. Environ Int 138:105600. https://doi.org/10.1016/j.envint.2020.105600
Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, Rhodes CJ, Valko M (2011) Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol 31:95–107. https://doi.org/10.1002/jat.1649
James KA, Byers T, Hokanson JE, Meliker JR, Zerbe GO, Marshall JA (2015) Association between lifetime exposure to inorganic arsenic in drinking water and coronary heart disease in Colorado residents. Environ Health Perspect 123:128–134. https://doi.org/10.1289/ehp.1307839
Nigra AE, Moon KA, Jones MR, Sanchez TR, Navas-Acien A (2021) Urinary arsenic and heart disease mortality in NHANES 2003–2014. Environ Res 200:111387. https://doi.org/10.1016/j.envres.2021.111387
Alamolhodaei NS, Shirani K, Karimi G (2015) Arsenic cardiotoxicity: an overview. Environ Toxicol Pharmacol 40:1005–1014. https://doi.org/10.1016/j.etap.2015.08.030
Xu M, Rui D, Yan Y, Xu S, Niu Q, Feng G, Wang Y, Li S, Jing M (2017) Oxidative damage induced by arsenic in mice or rats: a systematic review and meta-analysis. Biol Trace Elem Res 176:154–175. https://doi.org/10.1007/s12011-016-0810-4
Ojeda ML, Nogales F, Del Carmen G-L, Carreras O (2022) Binge drinking during the adolescence period causes oxidative damage-induced cardiometabolic disorders: a possible ameliorative approach with selenium supplementation. Life Sci 301:120618. https://doi.org/10.1016/j.lfs.2022.120618
Kielczykowska M, Kocot J, Pazdzior M, Musik I (2018) Selenium - a fascinating antioxidant of protective properties. Adv Clin Exp Med 27:245–255. https://doi.org/10.17219/acem/67222
Bomer N, Grote Beverborg N, Hoes MF, Streng KW, Vermeer M, Dokter MM, IJ J, Anker SD, Cleland JGF, Hillege HL, Lang CC, Ng LL, Samani NJ, Tromp J, van Veldhuisen DJ, Touw DJ, Voors AA, van der Meer P (2020) Selenium and outcome in heart failure. Eur J Heart Fail 22:1415–1423. https://doi.org/10.1002/ejhf.1644
Wang X, Yang B, Cao HL, Wang RY, Lu ZY, Chi RF, Li B (2021) Selenium supplementation protects against lipopolysaccharide-induced heart injury via sting pathway in mice. Biol Trace Elem Res 199:1885–1892. https://doi.org/10.1007/s12011-020-02295-5
Yang HB, Lu ZY, Yuan W, Li WD, Mao S (2022) Selenium attenuates doxorubicin-induced cardiotoxicity through Nrf2-NLRP3 pathway. Biol Trace Elem Res 200:2848–2856. https://doi.org/10.1007/s12011-021-02891-z
Ren Z, Wu Q, Deng H, Yu Y, Tang W, Deng Y, Zhu L, Wang Y, Deng J (2021) Effects of selenium on the immunotoxicity of subacute arsenic poisoning in chickens. Biol Trace Elem Res 199:4260–4272. https://doi.org/10.1007/s12011-020-02558-1
Adedara IA, Fabunmi AT, Ayenitaju FC, Atanda OE, Adebowale AA, Ajayi BO, Owoeye O, Rocha JBT, Farombi EO (2020) Neuroprotective mechanisms of selenium against arsenic-induced behavioral impairments in rats. Neurotoxicology 76:99–110. https://doi.org/10.1016/j.neuro.2019.10.009
Ren Z, Deng H, Deng Y, Tang W, Wu Q, Zuo Z, Cui H, Hu Y, Yu S, Xu SY, Deng J (2021) Effects of selenium on arsenic-induced liver lesions in broilers. Biol Trace Elem Res 199:1080–1089. https://doi.org/10.1007/s12011-020-02222-8
Galli U, Colombo G, Travelli C, Tron GC, Genazzani AA, Grolla AA (2020) Recent advances in NAMPT inhibitors: a novel immunotherapic strategy. Front Pharmacol 11:656. https://doi.org/10.3389/fphar.2020.00656
Garten A, Schuster S, Penke M, Gorski T, de Giorgis T, Kiess W (2015) Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol 11:535–546. https://doi.org/10.1038/nrendo.2015.117
Zhou CC, Yang X, Hua X, Liu J, Fan MB, Li GQ, Song J, Xu TY, Li ZY, Guan YF, Wang P, Miao CY (2016) Hepatic NAD(+) deficiency as a therapeutic target for non-alcoholic fatty liver disease in ageing. Br J Pharmacol 173:2352–2368. https://doi.org/10.1111/bph.13513
Zhou B, Wang DD, Qiu Y, Airhart S, Liu Y, Stempien-Otero A, O’Brien KD, Tian R (2020) Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J Clin Invest 130:6054–6063. https://doi.org/10.1172/JCI138538
Li, L, Xu W, Zhang L (2021) KLF15 Regulates oxidative stress response in cardiomyocytes through NAD(). Metabolites 11 https://doi.org/10.3390/metabo11090620
Zheng D, Zhang Y, Zheng M, Cao T, Wang G, Zhang L, Ni R, Brockman J, Zhong H, Fan GC, Peng T (2019) Nicotinamide riboside promotes autolysosome clearance in preventing doxorubicin-induced cardiotoxicity. Clin Sci (Lond) 133:1505–1521. https://doi.org/10.1042/CS20181022
Wang XY, Wang JZ, Gao L, Zhang FY, Wang Q, Liu KJ, Xiang B (2017) Inhibition of nicotinamide phosphoribosyltransferase and depletion of nicotinamide adenine dinucleotide contribute to arsenic trioxide suppression of oral squamous cell carcinoma. Toxicol Appl Pharmacol 331:54–61. https://doi.org/10.1016/j.taap.2017.05.008
Zheng B, Yang Y, Li J, Li J, Zuo S, Chu X, Xu S, Ma D, Chu L (2021) Magnesium isoglycyrrhizinate alleviates arsenic trioxide-induced cardiotoxicity: contribution of Nrf2 and TLR4/NF-kappaB signaling pathway. Drug Des Devel Ther 15:543–556. https://doi.org/10.2147/DDDT.S296405
Messarah M, Klibet F, Boumendjel A, Abdennour C, Bouzerna N, Boulakoud MS, El Feki A (2012) Hepatoprotective role and antioxidant capacity of selenium on arsenic-induced liver injury in rats. Exp Toxicol Pathol 64:167–174. https://doi.org/10.1016/j.etp.2010.08.002
Chen YC, Lin-Shiau SY, Lin JK (1998) Involvement of reactive oxygen species and caspase 3 activation in arsenite-induced apoptosis. J Cell Physiol 177:324–333. https://doi.org/10.1002/(SICI)1097-4652(199811)177:2%3c324::AID-JCP14%3e3.0.CO;2-9
Liang Y, Zheng B, Li J, Shi J, Chu L, Han X, Chu X, Zhang X, Zhang J (2020) Crocin ameliorates arsenic trioxideinduced cardiotoxicity via Keap1-Nrf2/HO-1 pathway: reducing oxidative stress, inflammation, and apoptosis. Biomed Pharmacother 131:110713. https://doi.org/10.1016/j.biopha.2020.110713
Sun TL, Liu Z, Qi ZJ, Huang YP, Gao XQ, Zhang YY (2016) (-)-Epigallocatechin-3-gallate (EGCG) attenuates arsenic-induced cardiotoxicity in rats. Food Chem Toxicol 93:102–110. https://doi.org/10.1016/j.fct.2016.05.004
Breton M, Costemale-Lacoste JF, Li Z, Lafuente-Lafuente C, Belmin J, Mericskay M (2020) Blood NAD levels are reduced in very old patients hospitalized for heart failure. Exp Gerontol 139:111051. https://doi.org/10.1016/j.exger.2020.111051
Abdellatif M, Sedej S, Kroemer G (2021) NAD(+) Metabolism in cardiac health, Aging, and Disease. Circulation 144:1795–1817. https://doi.org/10.1161/CIRCULATIONAHA.121.056589
Chiao YA, Chakraborty AD, Light CM, Tian R, Sadoshima J, Shi X, Gu H, Lee CF (2021) NAD(+) redox imbalance in the heart exacerbates diabetic cardiomyopathy. Circ Heart Fail 14:e008170. https://doi.org/10.1161/CIRCHEARTFAILURE.120.008170
Khadka D, Kim HJ, Oh GS, Shen A, Lee S, Lee SB, Sharma S, Kim SY, Pandit A, Choe SK, Kwak TH, Yang SH, Sim H, Eom GH, Park R, So HS (2018) Augmentation of NAD(+) levels by enzymatic action of NAD(P)H quinone oxidoreductase 1 attenuates adriamycin-induced cardiac dysfunction in mice. J Mol Cell Cardiol 124:45–57. https://doi.org/10.1016/j.yjmcc.2018.10.001
Tong D, Schiattarella GG, Jiang N, Altamirano F, Szweda PA, Elnwasany A, Lee DI, Yoo H, Kass DA, Szweda LI, Lavandero S, Verdin E, Gillette TG, Hill JA (2021) NAD(+) repletion reverses heart failure with preserved ejection fraction. Circ Res 128:1629–1641. https://doi.org/10.1161/CIRCRESAHA.120.317046
Feng, D, Xu D, Murakoshi N, Tajiri K, Qin R, Yonebayashi S, Okabe Y, Li S, Yuan Z, Aonuma K, Ieda M (2020) Nicotinamide phosphoribosyltransferase (Nampt)/nicotinamide adenine dinucleotide (NAD) axis suppresses atrial fibrillation by modulating the calcium handling pathway. Int J Mol Sci 21 https://doi.org/10.3390/ijms21134655
Oka SI, Byun J, Huang CY, Imai N, Ralda G, Zhai P, Xu X, Kashyap S, Warren JS, Alan Maschek J, Tippetts TS, Tong M, Venkatesh S, Ikeda Y, Mizushima W, Kashihara T, Sadoshima J (2021) Nampt potentiates antioxidant defense in diabetic cardiomyopathy. Circ Res 129:114–130. https://doi.org/10.1161/CIRCRESAHA.120.317943
Funding
This work was supported by The Program of Yingshang ChengDong Hospital (201903127).
Author information
Authors and Affiliations
Contributions
Hai-Bing Yang provided funding and designed research; Hai-Bing Yang and Wei Yuan performed experiments; Hai-Bing Yang and Wei Yuan analyzed data and wrote the manuscript; Shang Mao checked the manuscript. All authors contributed with productive discussions and knowledge to the final version of this manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, HB., Yuan, W., Li, WD. et al. Selenium Supplementation Protects Against Arsenic-Trioxide-Induced Cardiotoxicity Via Reducing Oxidative Stress and Inflammation Through Increasing NAD+ Pool. Biol Trace Elem Res 201, 3941–3950 (2023). https://doi.org/10.1007/s12011-022-03478-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03478-y