Abstract
Valsartan has significant blood pressure lowering effect via modulating renin-angiotensin system although its mechanism of action in isoproterenol (ISO)-induced myocardial injury is largely unknown. We therefore evaluated the effect of valsartan in ISO-induced oxidative stress and cardiotoxicity during β-adrenergic receptor stimulation in rats. ISO (85 mg/kg, s.c.) was administered on thirteenth and fourteenth day for induction of cardiotoxicity. ISO-treated rats showed significant decrease (P < 0.01) in mean arterial pressure (70.2 ± 9.11 vs. 104.86 ± 8.93), maximal positive (1601.3 ± 338.87 vs. 2789.16 ± 301.76), and negative (1495.76 ± 151.78 vs. 2039.6 ± 279.1) rate of developed left ventricular pressure and increase in left ventricular end-diastolic pressure (5.81 ± 0.51 vs. 2.37 ± 0.43) as compared to the sham group. Similarly, significant reduction in CK-MB (91.42 ± 5.88 vs. 142.63 ± 6.9), LDH (50.52 ± 5.18 vs. 73.28 ± 4.29) levels, and anti-oxidant enzymes activities were observed. Valsartan (15, 30, and 60 mg/kg/day, p.o.) pretreatment for 14 days favorably modulated these altered parameters. However, valsartan (60 mg/kg) only showed significant improvement (P < 0.01) in cardiac dysfunction, myocardial injury markers, and anti-oxidant status of myocardium in ISO-induced cardiotoxicity. Histopathology and ultrastructural studies further validated the protective effect of valsartan (60 mg/kg). Altogether, these results suggest that cardioprotective effect of valsartan is mediated through augmenting endogenous anti-oxidant defense system, preserving hemodynamic function and structural integrity of myocardium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The renin-angiotensin system (RAS) contributes significantly in the pathophysiology of various cardiovascular diseases viz. hypertension, myocardial infarction (MI), and congestive heart failure [1]. This realization led to the development of either angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) for the treatment of various cardiovascular diseases. Acute MI being a cardiovascular disorder is an immense health problem globally causing mortality and morbidity across the developing and industrialized countries [2]. Several mechanisms have been proposed to explain the myocardial injury and hemodynamic dysfunction during MI. It occurs due to imbalance between myocardial blood supply and demand resulting in development of ischemia and induction of necrosis in myocardium. Moreover, generation of free radicals and calcium overload has been incriminated as the major culprit in its pathogenesis [3]. The current management for MI includes beta blockers, ACE inhibitors/ARBs, nitrates, and anti-thrombotic agents. Treatment with ACE inhibitors/ARBs or both in various clinical trials had not only ameliorated the disease status but also drastically improved the clinical outcome [4, 5]. However, despite the improvement and availability of these therapies, the early mortality rate from acute MI is about 30%, with more than half of these deaths occurring before the stricken individual reaches the hospital [4].
Isoproterenol (ISO), a synthetic non-selective β-adrenoceptor agonist, owing to its positive inotropic and chronotropic effects has been reported to produce ischemia-like condition. Further, weakening of endogenous anti-oxidant defense system, lipid peroxidation, myocyte damage, and contractile dysfunction by ISO treatment contribute to the development of acute MI. Since pathophysiological changes following ISO administration in rats is similar to those in human MI, so drugs reversing the ISO damage could be useful in treating acute MI [3, 6].
Effects of ARBs on ischemic myocardium have been the subject of considerable research. Preclinical and clinical studies have established the therapeutic benefits of ARBs in treating hypertension, congestive heart failure, and diabetic nephropathy [7, 8]. Valsartan, which is one of the widely used ARBs has been found to reduce morbidity and mortality and improves quality of life in patients with heart failure (NYHA, II-IV) [9]. Recently, as demonstrated in large prospective double-blind, randomized clinical trial, valsartan has shown significant reduction in the incidence of diabetes and metabolic syndrome [10]. Unlike ACE inhibitors, valsartan does not exhibit dose limiting adverse effects such as persistent cough and angioedema [5].
Hitherto, the effect of valsartan on hemodynamic dysfunction, oxidative stress, antioxidant enzymes status, cardiac injury markers, histopathological, and ultrastructural changes in ISO-induced MI, has not been studied. Accordingly, this study was designed to investigate (1) whether the isoproterenol-induced myocardial damage can be salvaged by valsartan through attenuating oxidative stress and (2) whether this anti-oxidant activity of valsartan preserves myocardium morphology and functioning.
Materials and Methods
Male Wistar albino rats weighing 180–200 g (7–8 weeks) were obtained from the Central Animal House Facility of All India Institute of Medical Sciences, New Delhi, India. The study protocol was reviewed and approved by the Institutional Animal Ethics Committee and all study related activities conformed to the Indian National Science Academy Guidelines for the use and care of experimental animals in research. Animals were kept in the departmental animal house under controlled conditions of temperature at 25 ± 2°C, relative humidity of 60 ± 5% and light–dark cycle of 12:12 h. They were fed with food pellets (Ashirwad Industries Ltd, Chandigarh, India) and water ad libitum.
Reagents
Valsartan was obtained as a gift sample from Torrent pharmaceuticals Ltd., Ahmedabad, India and all other chemicals used in the study were of analytical grade and were procured from Sigma Chemicals (St. Louis, MO, USA). Valsartan was suspended in 0.5% hydroxyethyl cellulose whereas ISO was suspended in normal saline solution.
Experimental Protocol
Animals were divided into six groups of 14 rats each. Group 1 rats (Sham) received oral administration of 0.5% hydroxyethyl cellulose for 14 days, and on thirteenth and fourteenth day, normal saline (0.3 ml, s.c.) was given at an interval of 24 h. Group 2 rats (Vehicle + ISO) received oral administration of 0.5% hydroxyethyl cellulose for 14 days along with concurrent administration of ISO (85 mg/kg, s.c. at 24 h interval) on thirteenth and fourteenth day. Groups 3–5 rats (Valsartan + ISO) received oral administration of valsartan (15, 30, and 60 mg/kg, respectively) suspended in 0.5% hydroxyethyl cellulose for 14 days, and on thirteenth and fourteenth day, isoproterenol (85 mg/kg, s.c.) was given at an interval of 24 h. Group 6 rats (Valsartan only) received valsartan (60 mg/kg) orally for 14 days, and on thirteenth and fourteenth day, normal saline (0.3 ml, s.c.) was administered at an interval of 24 h. The dose of the valsartan was selected based on the previous literature [11, 12].
Surgical Procedures for Evaluating Hemodynamic Parameters
The animals were anesthetized with pentobarbitone sodium (60 mg/kg, i.p.), and atropine (0.6 mg/kg, i.p.) was injected to maintain heart rate and reduce tracheobronchial secretions during the surgical procedure. Neck was opened with a ventral midline incision and tracheostomy was performed. The rats were ventilated with room air from a positive pressure ventilator (Inco, Ambala, India) at the rate of 90 strokes/min and a tidal volume of 10 ml/kg. The right carotid artery was cannulated with a cannula filled with heparinized saline and connected via pressure transducer to CARDIOSYSCO-101 (Experirnentria, Hungary) for the measurement of blood pressure and heart rate. The animals were then allowed to stabilize for 10 min before recording the basal hemodynamic variables (systolic arterial pressure, diastolic arterial pressure, mean arterial pressure, and heart rate). A left thoracotomy was performed through the fourth intercostal space and the heart was exposed. A wide bore (1.5 rnrn) sterile metal cannula connected to a pressure transducer (Gould Statham P231D, USA) was inserted into the cavity of left ventricle from the posterior apical region of heart for recording left ventricular pressure dynamics (LVEDP and ±LVdP/dt) on polygraph (Grass 70, USA). After recording the hemodynamic parameters, animals of all groups were killed with an overdose of anesthesia (sodium pentobarbitone 100 mg/kg, i.v.) and their hearts were excised and processed for biochemical, histopathological, and ultrastructural studies [13].
Biochemical Estimation
Processing of heart tissue was carried out and 10% homogenate of myocardial tissue was prepared in ice-chilled phosphate buffer (50 mM, pH 7.4) and an aliquot was used to estimate malondialdehyde (MDA) [14] and reduced glutathione (GSH) levels [15]. Furthermore, the homogenate was centrifuged at 5,000 rpm for 20 min at 4°C and the supernatant was assayed for catalase [16], superoxide dismutase (SOD) activity [17], and protein content [18]. Creatine kinase-MB (CK-MB) and Lactate dehydrogenase (LDH) isoenzyme were estimated spectrophotometrically using a kit from Logotech, India.
Light Microscopic Study
Buffer formalin (10%) fixed tissues of heart was embedded in paraffin and serial sections (3 μm thick) were cut using microtome (Leica RM 2125, Germany). Section was stained with hematoxylin and eosin (H&E) and examined under light microscope (Nikon, Tokyo, Japan). A minimum of 12 fields per slide were examined and graded for myonecrosis, inflammatory cells, and edema on a scale of severe (+++), moderate (++), mild (+), and nil (−). The pathologist performing histopathological evaluation was blinded to the treatment protocol [13].
Transmission Electron Microscopic Study
The Karnovsky’s fixed tissues were washed in phosphate buffer (0.1 M, pH 7.4, 6°C) and post fixed for 2 h in 1% osmium tetroxide in the same buffer at 4°C. The specimens were then washed in phosphate buffer, dehydrated with graded acetone, and then embedded in araldite CY212 to make tissue blocks. Sections (70–80 nm) were cut by ultramicrotome (Ultracut E, Reichert, Austria) and stained with uranyl acetate and lead acetate, and examined under transmission electron microscope (Morgagni 268 D, Fei Co., The Netherlands) by a morphologist blinded to the treatment protocol [13].
Statistical Analysis
All data were presented as mean ± SD. One-way ANOVA followed by Bonferroni multiple range post hoc test was applied using SPSS version 11.5. Value of P < 0.05 was considered as statistically significant.
Results
Effect of Valsartan Only
Effect of orally administered valsartan (60 mg/kg) for a period of 14 days to normal rats did not exhibit any significant changes as revealed by hemodynamic, biochemical, histopathological, and ultrastructural parameters as compared with sham group.
Hemodynamic Parameters
Table 1 depicts the effect of valsartan on arterial pressures and heart rate in ISO-induced MI in rats. Administration of ISO (85 mg/kg) resulted in significant (P < 0.01) decrease in systolic arterial pressure from 124.34 ± 9.81 to 80.14 ± 9.12 mm Hg, diastolic arterial pressure from 95.12 ± 8.06 to 61.23 ± 9.11 mm Hg, and mean arterial pressure from 104.86 ± 8.93 to 70.2 ± 9.11 mm Hg, respectively, as compared to sham group. Pretreatment with valsartan at doses of 15, 30, and 60 mg/kg decreased systolic, diastolic, and mean arterial pressure as compared to sham group. Moreover, no significant change in heart rate was observed in all the experimental groups.
Figures 1 and 2 show restitution of ventricular dysfunction by valsartan in dose-dependent manner in ISO group. ISO treatment resulted in ventricular dysfunction as indicated by significant (P < 0.01) increase in LVEDP from 2.37 ± 0.43 to 5.81 ± 0.51 mm Hg, decrease in +LVdP/dtmax from 2789.16 ± 301.76 to 1601.3 ± 268.96 mm Hg/Sec and -LVdP/dtmax from 2039.6 ± 381.31 to 1495.76 ± 151.78 mm Hg/s, respectively, as compared to sham group.
Effect of valsartan on left ventricular end-diastolic pressure in ISO-induced myocardial infarction in rats. LVEDP: left ventricular end-diastolic pressure, ISO: isoproterenol. All values are expressed as mean ± SD for each group (n = 14/group). Significance was determined by one-way ANOVA followed by Bonferroni Post hoc test: ** P < 0.01 vs. sham group, * P < 0.01 vs. ISO-control group
Effect of valsartan on maximum positive and negative rates of left ventricular pressure development in ISO-induced myocardial infarction in rats. +LVdP/dt max: left ventricular maximum rate of positive pressure development. −LVdP/dt max: left ventricular maximum rate of negative pressure development, ISO: isoproterenol. All values are expressed as mean ± SD for each group (n = 14/group). Significance was determined by one-way ANOVA followed by Bonferroni Post hoc test: ** P < 0.01 vs. sham group, * P < 0.01 vs. ISO-control group
Valsartan dose dependently abrogated rise in left ventricular end-diastolic pressure (LVEDP) and improved maximal positive and negative rate of developed left ventricular pressure (±LVdP/dtmax) in comparison with ISO-control group. Valsartan at a dose of 15, 30, and 60 mg/kg decreased LVEDP in ISO group from 5.81 ± 0.51 to a range of 4.79 ± 0.39–2.74 ± 0.29 mm Hg. However, only 60 mg/kg dose showed significant (P < 0.01) decrease in LVEDP when compared with ISO-control group. Moreover, valsartan treatment (15, 30, and 60 mg/kg) increased +LVdp/dtmax and −LVdp/dtmax in ISO group from 1601.3 ± 388.87 to a range of 1783.81 ± 268.96–2654.39 ± 276.9 mm Hg/Sec and from 1495.76 ± 151.78 to a range of 1539.3 ± 178.94–1950.4 ± 229.71 mm Hg/s, respectively. However, only the dose of 60 mg/kg was found out to be statistically significant (P < 0.01) in improving ± LVdp/dtmax.
Cardiac Injury Markers
Treatment of rats with ISO resulted in significant (P < 0.01) decrease in the levels of CK-MB and LDH from 142.63 ± 6.9 to 91.42 ± 5.88 IU/mg protein and 73.28 ± 4.29 to 50.52 ± 5.18 IU/mg protein, respectively, as compared to sham group. Pretreatment with valsartan at a dose of 60 mg/kg resulted in significantly (P < 0.01) restoration of both myocardial CK-MB and LDH levels as shown in Fig. 3.
Effect of valsartan on CK-MB isoenzyme and LDH activities in ISO-induced myocardial infarction in rats. CK-MB: creatine kinase-MB, LDH: lactate dehydrogenase, ISO: isoproterenol. All values are expressed as mean ± S.D. for each group (n = 8/group). Significance was determined by one-way ANOVA followed by Bonferroni Post hoc test: ** P < 0.01 vs. sham group, * P < 0.01 vs. ISO-control group
Oxidative Stress Markers
As shown in Table 2, MI-induced by ISO was associated with marked (P < 0.01) decrease in the level of GSH from 1.436 ± 0.15 to 0.84 ± 0.10 μg/g tissue, activity of catalase from 17.3 ± 1.48 to 11.50 ± 1.2 U/mg protein, activity of SOD from 5.53 ± 0.7 to 2.26 ± 0.5 U/mg protein and increase in the levels of MDA from 55.81 ± 6.93 to 86.64 ± 10.19 nmol/g tissue, respectively, as compare to sham group. Prior treatment with valsartan (60 mg/kg) for 14 days significantly (P < 0.01) decreased the level of MDA and increased the content of GSH, catalase, and SOD when compared with ISO-control group.
Histopathological Examination
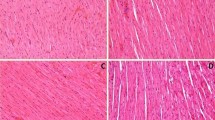
Table 3 shows effect of valsartan on histopathological changes of rat myocardium in different experimental groups. Figure 4a showed the light micrograph of vehicle-treated heart showing normal architecture. Light micrograph of ISO-control group showed focal confluent necrosis of muscle fibers with inflammatory cell infiltration, edema, and myophagocytosis along with extravasation of red blood cells (Fig. 4c). The degree of myocardial damage in valsartan (15 mg/kg) in ISO-treated rats was similar to that of ISO-control group with similar morphological changes (Fig. 4d). Valsartan (30 mg/kg)-treated group showed myonecrosis with less edema and inflammatory cells (Fig. 4e). Light micrograph of valsartan (60 mg/kg)-treated rat heart tissue showed mild edema with no infarction and normal architecture of myocardial fibers (Fig. 4f).
Light microscopic study of heart in different experimental groups (H&E, × 200). a Sham group; b valsartan only group; c isoproterenol-control group; d valsartan (15 mg/kg) plus isoproterenol group; e valsartan (30 mg/kg) plus isoproterenol group; f valsartan (60 mg/kg) plus isoproterenol group. (→): Inflammatory cells and edema, (↔): myonecrosis
Ultrastructural Examination
Figure 5 shows the effect of valsartan on ultrastructural changes in ISO-induced MI. Normal cardiac architecture was seen in electron micrographs of animals in sham group (Fig. 5a). Administration of ISO to the rats resulted in swelling of mitochondria, disruption of cristae with vacuolation, and myonecrosis as revealed by TEM (Fig. 5c). Similar ultrastructural changes were seen in valsartan (15 and 30 mg/kg)-treated groups, although the changes were milder in the 30 mg/kg group (Fig. 5d, e). Valsartan (60 mg/kg) group showed mild myonecrosis, swelling and vacuolation along with normal ultrastructure (Fig. 5f).
Electron microscopic study of myocardium in different experimental groups. a Sham group (3500X); b valsartan only group (3500X); c Isoproterenol-control group (3300X); d valsartan (15 mg/kg) plus isoproterenol group (3200X); e valsartan (30 mg/kg) plus isoproterenol group (3200X); f valsartan (60 mg/kg) plus isoproterenol group (3200X)
Discussion
The present study demonstrates the cardioprotective potential of valsartan by improving hemodynamic, biochemical, histopathological, and ultrastructural changes in ISO-induced myocardial damage. ISO-induced MI is a well-authenticated model for screening of various drugs for their cardioprotective potential and also provides insight into the concerned mechanisms [3, 6]. Furthermore, myocardial lesions produced by ISO in rats closely resembles to that of pathological state of human MI [6, 19, 25]. ISO through spontaneous auto-oxidation leads to the formation of adrenochrome which is implicated in the generation of highly toxic oxygen-derived free radicals. These radicals cause lipid peroxidation and enhanced oxidative stress in heart tissue which subsequently leads to myocardial injury and cardiac dysfunction [3, 6, 20, 25].
Blockade of RAS by ARBs has been known to have beneficial effects in hypertension, on prognosis in patients with MI and congestive heart failure [1, 5, 7]. Similarly, our study clearly demonstrates that valsartan exhibits dose dependent reduction in systolic, diastolic, and mean arterial pressure as compared to vehicle-treated group. The plausible reasons which may contribute to this effect are valsartan antagonize angiotensin II type I receptor and/or down regulation of sympathetic adrenergic activity by blocking the effects of angiotensin II on sympathetic nerve release and hence results in decrease in arterial pressures. Furthermore, ARBs also inhibit cardiac and vascular remodeling associated with chronic hypertension and MI thereby suggesting a protective role in myocardial injury [7, 8]. In consonance with our findings various experimental studies have also reported beneficial effects of AT1 antagonists in myocardial infarction in rats [21–23]. However, no significant alterations were observed in heart rate, among all groups. Likewise, Kato et al. also observed no significant changes on heart rate on ARBs administration [24]. The cardioprotective effects of valsartan is also manifested hemodynamically in terms of improvement in left ventricular functions as evidenced by amelioration of ventricular inotropic (+LVdP/dtmax) and lusitropic (−LVdP/dtmax) impairment caused by ISO-induced myocardial damage. Further, it also improved ISO-induced increase in LVEDP which also reflects restoration in ventricular function. The pharmacological mechanism is that ARBs have direct effect via dilatation of arteries and veins and thereby results in reduction in preload and afterload and hence subsequent decrease in LVEDP [1, 5, 8]. In corroboration with our findings Ohta et al. have also demonstrated that ARBs restored cardiac dysfunction induced by ISO [22].
Lipid peroxidation plays an important role in the pathogenesis of MI. A significant increase in the levels of lipid peroxidation product such as MDA in the heart indicates increased oxidative stress in ISO-induced rats [3, 6, 19, 20]. Reactive oxygen species which are highly toxic by-products of aerobic metabolism are known to react extensively with cellular membranes and macromolecules thus enhancing formation of lipid peroxides and culminates into tissue damage. The increased levels of MDA, a lipid peroxidation end-product, observed in our study following ISO administration may be due to free radical-mediated membrane damage. On the other hand, prior treatment with valsartan decreased the level of lipid peroxidation product in ISO-treated rats. Keles et al. also reported the scavenging action of ARBs on hydroxyl, superoxide, and lipid radicals [23].
ISO metabolism produces quinones, which react with oxygen to produce superoxide anions and hydrogen peroxide, leading to oxidative stress and depletion of endogenous anti-oxidant enzymes [3, 6]. In the present study, ISO administration caused oxidative stress in heart as evidenced by the reduction in activities of myocardial SOD, catalase, and GSH levels [19–21]. Interestingly, valsartan treatment significantly increased these anti-oxidant enzymes levels. Moreover, it appears that the effect of valsartan on oxidative injury is likely indirect due to receptor blockade that leads to attenuation of angiotensin II-mediated oxidative stress or due to direct antioxidant activity of valsartan which precludes the heart from oxidative damage. Consistent with our results, ARBs such as olmesartan and telmisartan have shown similar anti-oxidant effect in experimental model of myocardial infarction [13, 20].
ISO challenge causes myocardial cell damage resulting in enhanced cell membrane permeability or rupture that leads to leakage of cardiac enzymes such as CK-MB and LDH. These cardiac injury markers were decreased in heart of ISO-induced myocardial infarcted rats. In consensus with this, we also observed decreased CK-MB and LDH levels in ISO-control group whereas administration of valsartan significantly prevented the loss of CK-MB and LDH in heart [13, 20]. Thus, valsartan prevented the leakage of these enzymes from the heart via attenuating lipid peroxidation and myocyte damage in ISO-challenged myocardium.
Hemodynamic and biochemical improvement by valsartan were further substantiated by significant prevention of morphological alterations induced by ISO. The pathological features observed in ISO-treated rat hearts confers myofibre degeneration, interstitial edema, and congestion associated with infiltration of both neutrophils and lymphocytes [3, 6, 13, 20]. Light microscopic studies of the heart pretreated with valsartan showed a well-preserved morphology of cardiac muscle with no indication of necrosis when compared to ISO-induced myocardial infracted heart. In addition, ultrastructural studies showed that valsartan decreases myonecrosis, inflammatory changes, and edema and showed normal ultrastructure in most of the myocardium except irregular disruption of myofilaments as compared to ISO group. These data further confirmed the cardioprotective action of valsartan in ISO-induced myocardial infarction.
In conclusion, our study provides strong evidence that valsartan protects the ISO-challenged myocardium not only via improving cardiac functions, attenuating oxidative stress, lipid peroxidation but also by preserving cardiomyocytes morphology. Importantly, our findings clearly demonstrates that valsartan has the potential to be used for the primary prevention in those patients who are having multiple risk factors like diabetes, hypertension, hypercholesterolemia, and family history of early coronary artery disease. Secondly, it would be beneficial for the secondary prevention of coronary artery disease in post-MI patients. Thus, our study established that ARBs especially valsartan which has been approved by USFDA for hypertension could also be recommended for prophylaxis in MI. However, further investigations to decipher the mechanism for the altered anti-oxidative enzymes by valsartan would be necessary, which might give us deeper insights into its cardioprotective potential.
References
Pfeffer, M. A. (2000). Will more complete inhibition of the RAAS with angiotensin receptor blockade improve survival following myocardial infarction? Journal of the Renin-Angiotensin-Aldosterone System, 1, S41–S43.
Lopez, A. D., & Murrau, C. C. (1998). The global burden disease, 1990–2020. Nature Medicine, 4, 1241–1243.
Guo, X. Z., Shoji, K., Akira, N., Takatomi, S., Matlubur, R., Li, Y., et al. (2005). Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovascular Research, 65, 230–238.
Heart and Stroke facts 2002. (2003). Statistical supplement. Dallas: American Heart Association.
Böhm, M., Baumhäkel, M., Mahfoud, F., & Werner, C. (2010). From evidence to rationale: Cardiovascular protection by angiotensin II receptor blockers compared with angiotensin-converting enzyme inhibitors. Cardiology, 117, 163–173.
Milei, J., Nunez, R. G., & Rapaport, M. (1978). Pathogenesis of isoprenaline-induced myocardial lesions: Its relation to human ‘coagulative myocytolysis’. Cardiology, 63, 139–151.
Verdecchia, P., Angeli, F., Repaci, S., Mazzotta, G., Gentile, G., & Reboldi, G. (2009). Comparative assessment of angiotensin receptor blockers in different clinical settings. Vascular Health and Risk Management, 5, 939–948.
Black, H. R., Bailey, J., Zappe, D., & Samuel, R. (2009). Valsartan: More than a decade of experience. Drugs, 69, 2393–2414.
Cohn, J. N., & Tognoni, G. (2001). Valsartan heart failure trial investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. New England Journal of Medicine, 345, 1667–1675.
McMurray, J. J., Holman, R. R., Haffner, S., The NAVIGATOR Study Group, et al. (2010). Effect of valsartan on the incidence of diabetes and cardiovascular events. New England Journal of Medicine, 362, 1477–1490.
Tominaga, N., Robert, A., Izuhara, Y., Ohtomo, S., Dan, T., Chihara, K., et al. (2009). Very high doses of valsartan provide renoprotection independently of blood pressure in a type 2 diabetic nephropathy rat model. Nephrology (Carlton), 14, 581–587.
Wang, T., Yin, K. S., Liu, K. Y., Lu, G. J., Li, Y. H., & Chen, J. D. (2008). Effect of valsartan on the expression of angiotensin II receptors in the lung of chronic antigen exposure rats. Chinese Medical Journal (England), 121, 2312–2319.
Goyal, S., Arora, S., Mittal, R., Joshi, S., Nag, T. C., Ray, R., et al. (2009). Myocardial salvaging effect of telmisartan in experimental model of myocardial infarction. European Journal of Pharmacology, 619, 75–84.
Ohkawa, H., Ohishi, N., & Yagi, K. (1979). Assay of lipid peroxide in animal tissue by thiobarbituric acid reaction. Analytical Biochemistry, 95, 351–358.
Moron, M. S., Depierre, J. W., & Manmerik, B. (1979). Level of glutathione, glutathione reductase and glutathione S transferase activity in rat lung and liver. Biochimica et Biophysica Acta, 582, 67–78.
Aebi, H. (1974). Catalase. In H. U. Bergmeyer (Ed.), Methods of enzymatic analysis (pp. 673–685). London: Academic Press.
Marklund, S., & Marklund, G. (1974). Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. European Journal of Biochemistry, 47, 469–474.
Bradford, M. M. (1976). A rapid and sensitive method for quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Albayrak, F., Bayir, Y., Halici, Z., Kabalar, E., Bayram, E., Ozturk, C., et al. (2009). Preventive effect of amiodarone during acute period in isoproterenol-induced myocardial injury in Wistar rats. Cardiovascular Toxicology, 9, 161–168.
Zhang, G. X., Ohmori, K., Nagai, Y., Fujisawa, Y., Nishiyama, A., Abe, Y., et al. (2007). Role of AT1 receptor in isoproterenol-induced cardiac hypertrophy and oxidative stress in mice. Journal of Molecular and Cellular Cardiology, 42, 804–811.
Jalowy, A., Schulz, R., & Heusch, G. (1999). AT1 receptor blockade in experimental myocardial ischemia/reperfusion. Journal of the American Society of Nephrology, Suppl, 11, S129–S136.
Ohta, T., Hasebe, N., Tsuji, S., Izawa, K., Jin, Y. T., Kido, S., et al. (2004). Unequal effects of renin-angiotensin system inhibitors in acute cardiac dysfunction induced by isoproterenol. American Journal of Physiology. Heart and Circulatory Physiology, 287, H2914–H2921.
Keles, M. S., Bayir, Y., Suleyman, H., & Halici, Z. (2009). Investigation of effects of Lacidipine, Ramipril and Valsartan on DNA damage and oxidative stress occurred in acute and chronic periods following isoproterenol-induced myocardial infarct in rats. Molecular and Cellular Biochemistry, 328, 109–117.
Kato, M., Sada, T., Mizuno, M., Kitayama, K., Inaba, T., & Koike, H. (2005). Effect of combined treatment with an angiotensin II receptor antagonist and an HMG-CoA reductase inhibitor on atherosclerosis in genetically hyperlipidemic rabbits. Journal of Cardiovascular Pharmacology, 46, 556–562.
Akinmoladun, A. C., Obuotor, E. M., Barthwal, M. K., Dikshit, M., & Farombi, E. O. (2010). Ramipril-like activity of Spondias mombin linn against no-flow ischemia and isoproterenol-induced cardiotoxicity in rat heart. Cardiovascular Toxicology, 10, 295–305.
Acknowledgments
The electron microscopic work was conducted at SAIF (Department of Science and Technology), Department of Anatomy, AIIMS, New Delhi, India.
Conflict of interest
All authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors Sameer Goyal and Saurabh Bharti contributed equally to the work.
Rights and permissions
About this article
Cite this article
Goyal, S., Bharti, S., Sahoo, K.C. et al. Valsartan, an Angiotensin II Receptor Blocker, Attenuates Cardiac Dysfunction and Oxidative Stress in Isoproterenol-Induced Cardiotoxicity. Cardiovasc Toxicol 11, 148–156 (2011). https://doi.org/10.1007/s12012-011-9108-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-011-9108-0