Abstract
Chloral hydrate has been long used as a safe sedative and hypnotic drug in humans. However, reports on its cardiovascular adverse effects have been published from time to time. The present study was undertaken to use Rhesus monkeys as a model to define the dose regiment of chloral hydrate at which cardiac arrhythmias can be induced and the consequences of the cardiac events. Male Rhesus monkeys of 2–3 years old were intravenously infused with chloral hydrate starting at 50 mg/kg with an increasing increment of 25 mg/kg until the occurrence of cardiac arrhythmias. In addition, a traditional up-and-down dosing procedure was applied to define a single dose level at which cardiac arrhythmias can be induced. The data obtained showed that when the sequentially escaladed dose reached 125 mg/kg, cardiac arrhythmias occurred in all monkeys tested. The single effective dose to cause cardiac arrhythmias calculated from the crossover analysis was 143 ± 4 mg/kg. This value would be equivalent to 68.6 ± 1.9 mg/kg for children and 46.4 ± 1.3 mg/kg for adults in humans. Under either multiple or single dose condition, cardiac arrhythmias did not occur before 40 min after the onset of anesthesia induced by chloral hydrate. Cardiac arrhythmias were recovered without help at the end of the anesthesia in most cases, but also continued after the regain of consciousness in some cases. The cardiac arrhythmias were accompanied with compromised cardiac function including suppressed fractional shortening and ejection fraction. This study thus suggests that cautions need to be taken when chloral hydrate is used above certain levels and beyond a certain period of anesthesia, and cardiac arrhythmias induced by chloral hydrate need to be closely monitored because compromised cardiac function may occur simultaneously. In addition, patients with cardiac arrhythmias induced by chloral hydrate should be monitored even after they are recovered from the anesthesia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chloral hydrate, acting as a sedative and hypnotic drug, has been used for more than a century. It is currently considered as a relatively nontoxic hypnotic drug at the recommended therapeutic doses ranging from 25 to 125 mg/kg orally [1–4], or 89 ± 13.3 mg/kg rectally [5, 6]. It continues to be a commonly accepted sedative for pediatric patients undergoing surgical procedures despite the availability of newer agents [7–10]. However, several cases of cardiovascular events from over dosage of chloral hydrate have been reported in the past. Marshall [11] reported two cases: a 29-year-old woman swallowed 15 g of chloral hydrate and a 64-year-old woman took 10 to 20 g of chloral hydrate with alcohol; both developed serious cardiac arrhythmias. Gustafson and colleagues [12] also reported three cases: a 39-year-old woman, a 51-year-old man, and a 21-year-old woman ingested 30, 25, and 20 g of chloral hydrate, respectively, showed abnormal electrocardiograph (ECG), including supraventricular and ventricular tachyarrhythmias. Bowyer and Glasser [13] reported that a 17-year-old white man ingested 28 chloral hydrate (Noctec) capsules (each contained 50 mg of chloral hydrate) and a 67-year-old white woman with no known heart disease ingested 60 chloral hydrate tablets (500 mg each, total dose of 30 g); both developed cardiac arrhythmias. Sing et al. [14] reported two cases that ingestion of 219 mg/kg of chloral hydrate resulted in transient bigeminy and ingestion of up to 960 mg/kg caused torsades de pointes and ventricular fibrillation.
It thus becomes a clinically practical concern related to the safety issue of the use of chloral hydrate. What is the dose regiment for chloral hydrate that can produce cardiovascular events? Are the cardiovascular events reversible upon cessation of the drug usage? These two questions are critical for the understanding and clinical management of the cardiovascular events induced by chloral hydrate.
In animal studies, chloral hydrate has also been widely used as a sedative and hypnotic agent [15]. The Rhesus monkeys, because of their close similarity to human beings in various physiological parameters, serve as a good substitute in variety of biological experiments. Using the monkey model to understand the cardiovascular effects of chloral hydrate would provide important insights into cautions and preventive measures in the clinical application of this drug. In this context, the present study was undertaken to specifically address the cardiac effect of chloral hydrate in the Rhesus monkey model, focusing on defining the minimum intravenous dose level of chloral hydrate to produce cardiac arrhythmias by a traditional up-and-down method for small sample size [16, 17]. In addition, cardiac functional alterations accompanying with cardiac arrhythmias were also monitored to evaluate the severity of the cardiac events induced by chloral hydrate.
Materials and Methods
Animals and Drugs
Male Rhesus (Macaca mulatta) monkeys, aged 2–3 years and weighed 4.5–5.5 kg, were obtained from Chengdu Ping—an experimental animal breeding and research base, a Chinese government accredited non-human primate center in Sichuan province. The animals were housed in individual cages and acclimatized to the laboratory condition for a period of at least 1 month. They received conventional laboratory diet with free access to drinking water as approved by the Laboratory Animal Management Committee of Sichuan province. All animal procedures were approved by the Institutional Animal Care and Use Committee at the Sichuan University West China Hospital, following the guideline of the US National Institutes of Health. Chloral hydrate (10%) was obtained from the West China Hospital of Sichuan University.
Experimental Procedure
Prior to the drug treatment and measurement, all subjects received an intramuscular injection of 5 mg/kg ketamine and 0.2 mg/kg midazolam to induce sedation while they were maintained in the supine position on the operating table. Each subject was prepared surgically with a chronic indwelling venous catheter under the sterile condition.
During the experiment, each monkey was continuously monitored by electrocardiogram (ECG, GE MAC800, USA) and pulse oxygen saturation (SpO2) after the monkey laid on the operating table. The ECGs were displayed and recorded at 25 mm/s with a frequency of 50 Hz. The standard bipolar and unipolar limb leads were recorded. The cardiac arrhythmias were diagnosed according to the ECG, which were defined by conspicuous and significant rhythm abnormalities of the ECG wave forms.
The persisting time of anesthesia by each dose of chloral hydrate was recorded by a timer. Echocardiography (Siemens ACUSON Antares System, German) was performed to detect the change in cardiac function. Internal diastolic and systolic diameter (IDd and IDs), end-diastolic (EDV) and end-systolic (ESV) volumes, and stroke volumes (SV) of left ventricle (LV) were recorded and calculated, and ejection fraction (EF) was calculated as EF = (EDV − ESV)/EDV × 100%. Fractional shortening (FS) was calculated as FS = [(LVIDd–LVIDs)/LVIDd] × 100%. Data were analyzed using repeated-measures ANOVA (SPSS 11.0 for Windows, SPSS Inc).
This study was divided into two parts. In the first part, three monkeys were administered intravenously with the starting dose of 50 mg/kg, followed by 25 mg/kg increments for the succeeding doses. After the monkeys regained consciousness after each ascending dose, the subsequent dose was administered by infusion until cardiac arrhythmias were detected.
The second part consisted of a single dose progression in which monkeys were dosed in increments or decrements depending upon the outcome of the dose previously received. The first animal received the starting dose of 120 mg/kg, which would be below the dose of chloral hydrate to produce cardiac arrhythmias based on the result obtained from experiment part 1. If the cardiac arrhythmias did not occur in the first monkey, the dose for the next monkey was increased with 10 mg/kg increments. If cardiac arrhythmias occurred, the dose for the next monkey was decreased with 10 mg/kg decrements. This up-and-down method provided the crossover analysis and helped calculating the minimum intravenous dose for chloral hydrate to cause cardiac arrhythmias (Fig. 1). The testing was concluded when 5 reversals occurred in 6 consecutive animals tested, at which time an estimate of the minimum intravenous dose of chloral hydrate to produce cardiac arrhythmias was calculated using a Statistical Software for Social Sciences (SPSS 11.0 for Windows, SPSS Inc). Data were expressed as means ± S.D.
The up-and-down procedure to determine the effective dose that causes cardiac arrhythmias and the crossover check. Each point represents a single defined dose infused to an individual monkey. Heavy line (crossover) connects the two monkeys who were consecutively infused with a different dose of chloral hydrate: one caused cardiac arrhythmias and the other one did not
Results
In the first part of the study, a dose of chloral hydrate that can produce adverse effect in the form of cardiac arrhythmias in the monkey model was defined. Each of the 3 monkeys was infused sequentially with 50, 75, 100, and 125 mg/kg of chloral hydrate. At the doses below 100 mg/kg, no cardiac arrhythmias were detected. But the persistent time of anesthesia was prolonged corresponding to the dose increase (Table 1). At the dose of 125 mg/kg, cardiac arrhythmias were observed in all of the three monkeys. Cardiac arrhythmias occurred during the last 1/3 period of anesthesia and recovered after the regain of consciousness in one monkey, but continued in the other two monkeys even after they have become conscious (Table 1).
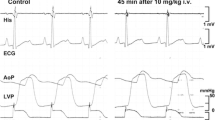
In the second part of the study, a single dose of 120 mg/kg, 130 mg/kg, or 140 mg/kg was administered, respectively, to each of three monkeys. Cardiac arrhythmias were not detected in any of the first three monkeys. However, when the fourth monkey received the dose of 150 mg/kg, cardiac arrhythmias developed (Figs. 2, 3). Therefore, the dose for the fifth monkey was decreased to 140 mg/kg, and the up-and-down procedure was continued according to the outcome of previous monkey received. When the dose regimen was completed, there were ten monkeys included in this part of experiment. With the dose increased from 120 mg/kg to 150 mg/kg, persisting time of anesthesia was prolonged from 38 min to 84 min. Four of the ten monkeys developed cardiac arrhythmias at the single dose of 150, 150, 150, or 140 mg/kg, respectively. The occurrence of cardiac arrhythmias was also at the last 1/3 period of anesthesia, and the recovery of cardiac arrhythmias occurred at the end of the anesthesia period in all cases with one exception, in whom cardiac arrhythmias continued after the animal regained consciousness (Table 2). The minimum intravenous dose for chloral hydrate to cause cardiac arrhythmias, determined from the crossover analysis, was 143 ± 4 mg/kg.
Electrocardiograph (ECG) obtained from Lead II recording. a The ECG obtained from a monkey without drug treatment. b Heart rate increased transiently after the drug treatment in a monkey. c Ventricular premature beats induced by chloral hydrate. d Conspicuous sinus arrest in one monkey treated with chloral hydrate
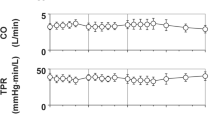
In addition, the results from echocardiography analysis showed that when cardiac arrhythmias occurred, compromised cardiac function was noticed (Table 3). Importantly, the fractional shortening and rejection fraction significantly reduced during the period of cardiac arrhythmias. However, all monkeys regained consciousness and showed no signs of other adverse effects after cessation of the experiment. Most of monkeys recovered from cardiac arrhythmias without help and were discharged after complete recovery. Only one monkey exhibited a sinus arrest after chloral hydrate treatment. After infusion with atropine and dobutamine, the monkey successfully recovered.
Discussion
The results obtained from this study demonstrate that chloral hydrate at a single intravenous dose above 140 mg/kg induces cardiac arrhythmias in the monkey model. In experiment part 1, all monkeys developed cardiac arrhythmias at the cumulative dose reached to 125 mg/kg. In part 2, one monkey developed cardiac arrhythmias at the single dose of 140 mg/kg and three developed cardiac arrhythmias at the single dose of 150 mg/kg. It appeared that the sequentially escalading dose regiment used in part one would cause an accumulation of the residues of previous doses, leading to the eventual dose level causing cardiac arrhythmias much less than the one for the single effective dose observed in part 2. Therefore, the lingering effects resulting from multiple infusions of chloral hydrate increased the risk of cardiac arrhythmias, but a single effective dose level to cause cardiac arrhythmias was much higher due to the elimination of the sedimentation effects generated from multiple infusions.
We also observed that when the dose increased from 120 mg/kg to 150 mg/kg, the persisting time for anesthesia was prolonged from 38 min to 84 min. However, the increase in the persistent time of anesthesia was associated with the increased risk of cardiac arrhythmias. The probability for the occurrence of cardiac arrhythmias was increased when the dose increased above 140 mg/kg. When the monkey developed cardiac arrhythmias, the cardiac function was also compromised, demonstrating the severity of the arrhythmias. The severity was further reflected by the appearance of the ventricular arrhythmia, such as ventricular premature beats, bigeminal rhythm, and trigeminy. However, in most of the cases, the monkeys recovered from the cardiac arrhythmias without further noticeable adverse effects. But there were three monkeys in whom the cardiac arrhythmias persisted after the regain of consciousness, and one monkey in whom the sinus arrest was observed but recovered after infusion with atropine and dobutamine.
It is interesting to note that chloral hydrate-induced cardiac arrhythmias at the single dose above 140 mg/kg or the cumulative dose reached 125 mg/kg did not occur until more than 40 min after the onset of anesthesia induced by the drug. We cannot provide a defined explanation to this phenomenon, but would present two alternative speculations. One is that the late onset of cardiac arrhythmias may be related to metabolic process of chloral hydrate in the body and only until the accumulation of some critical metabolites generated from chloral hydrate reaches a threshold level do cardiac arrhythmias occur. This of course needs further investigation to confirm. The other is that the anesthesia induced by chloral hydrate presets the cardiac tissue vulnerable to the arrhythmic effect of the drug itself, so that until the anesthesia has undergone through a period of time long enough do the arrhythmias occur. This would provide a clinical caution when chloral hydrate is used, the sedation induced by this drug should be closely monitored for the occurrence of cardiac arrhythmias after a certain period of time.
From this study, we can conclude that chloral hydrate can be safely and effectively used as a sedative in monkeys below the maximum acceptable dose of 143 ± 4 mg/kg, and the narcotization is stable without adverse events. Human equivalent doses converted from this monkey dose are 68.6 ± 1.9 mg/kg for children and 46.4 ± 1.3 mg/kg for adults [18]. However, when multiple doses are indicated for a longer period of time of anesthesia, cardiac arrhythmic effects need to be closely monitored for two reasons: one is that the dose causing cardiac arrhythmias in multiple applications is much less than a single dose. And the second is that prolonged anesthesia increased the risk of cardiac arrhythmias induced by chloral hydrate.
There is a major limitation in the present study. The data were obtained only from male monkeys and females were not included. Using female monkeys for experimental studies is prohibited, so that it was impossible to include females in this study. However, it should be noticed that the responses of females to chloral hydrate may be different. Therefore, additional cautions should be taken when the data presented here are extrapolated to humans.
References
Napoli, K. L., Ingall, C. G., & Martin, G. R. (1996). Safety and efficacy of chloral hydrate sedation in children undergoing echocardiography. The Journal of Pediatrics, 129, 287–291.
D’Agostino, J., & Terndrup, T. E. (2000). Chloral hydrate versus midazolam for sedation of children for neuroimaging: A randomized clinical trial. Pediatric Emergency Care, 16, 1–4.
Wheeler, D. S., Jensen, R. A., & Poss, W. B. (2001). A randomized, blinded comparison of chloral hydrate and midazolam sedation in children undergoing echocardiography. Clinical Pediatrics, 40, 381–387.
Mason, K. P., Sanborn, P., Zurakowski, D., Karian, V. E., Connor, L., Fontaine, P. J., et al. (2004). Superiority of pentobarbital versus chloral hydrate for sedation in infants during imaging. Radiology, 230, 537–542.
Dacher, J. N., Neuenschwander, S., Monroc, M., Vanier, A., Eurin, D., & Le Dosseur, P. (1996). Sedation with oral hydroxyzine and rectal chloral hydrate in pediatric MRI and CT. Journal de Radiologie, 77, 1189–1192.
Treluyer, J. M., Andre, C., Carp, P. F., Chalumeau, M., Tonnelier, S., Cuq, C., et al. (2004). Sedation in children undergoing CT scan or MRI: Effect of time-course and tolerance of rectal chloral hydrate. Fundamental and Clinical Pharmacology, 18, 347–350.
Krauss, B., & Green, S. M. (2000). Sedation and analgesia for procedures in children. The New England Journal of Medicine, 342, 938–945.
Heistein, L. C., Ramaciotti, C., Scott, W. A., Coursey, M., Sheeran, P. W., & Lemler, M. S. (2006). Chloral hydrate sedation for pediatric echocardiography: Physiologic responses, adverse events, and risk factors. Pediatrics, 117, 434–441.
Low, E., O’Driscoll, M., MacEneaney, P., & O’Mahony, O. (2008). Sedation with oral chloral hydrate in children undergoing MRI scanning. Irish Medical Journal, 101, 80–82.
Britton, J. W., & Kosa, S. C. (2010). The clinical value of chloral hydrate in the routine electroencephalogram. Epilepsy Research, 88, 215–220.
Marshall, A. J. (1977). Cardiac arrhythmias caused by chloral hydrate. British Medical Journal, 15, 994.
Gustafson, A., Svensson, S.-E., & Ugander, L. (1977). Cardiac arrhythmias in chloral hydrate poisoning. Acta Medica Scandinavica, 201, 227–230.
Bowyer, K., & Glasser, S. P. (1980). Chloral hydrate overdose and cardiac arrhythmias. Chest, 77, 232–235.
Sing, K., Erickson, T., Amitai, Y., & Hryhorczuk, D. (1996). Chloral hydrate toxicity from oral and intravenous administration. Journal of toxicology. Clinical toxicology, 34, 101–106.
Silverman, J., & Muir, W. W. (1993). A review of laboratory animal anesthesia with chloral hydrate and chloralose. Laboratory Animal Science, 43, 210–216.
Dixon, W. J. (1965). The up-and-down method for small samples. Journal of the American Statistical Association, 60, 967–968.
Bruce, R. D. (1985). An up-and-down procedure for acute toxicity testing. Fundamental and Applied Toxicology, 5, 151–157.
Reagan-Shaw, S., Nihal, M., & Ahmad, N. (2008). Dose translation from animal to human studies revisited. The FASEB journal, 22, 659–661.
Author information
Authors and Affiliations
Corresponding author
Additional information
Pengfei Han and Haibo Song made equal contributions to this study.
Rights and permissions
About this article
Cite this article
Han, P., Song, H., Yang, P. et al. Cardiac Arrhythmias Induced by Chloral Hydrate in Rhesus Monkeys. Cardiovasc Toxicol 11, 128–133 (2011). https://doi.org/10.1007/s12012-011-9106-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-011-9106-2