Abstract
Background
Studies have established the association between blood β-amyloid (Aβ) levels and Alzheimer’s disease, but population-based studies concerning the association between selenium (Se) and Aβ levels in blood samples are very limited. Therefore, we explored the association in an elderly population with Se status and serum Aβ measures.
Methods
A cross-sectional study on 469 elderly individuals from four rural counties with diverse soil Se levels was carried out. Fasting blood Se, serum selenoprotein P (SELENOP), and glutathione peroxidase (GPX), serum Aβ42, and Aβ40 were measured. Quantile regression models were used to determine the associations of blood Se, serum GPX, and SELENOP with Aβ levels.
Results
Significant negative associations were observed between blood Se and serum Aβ42 and Aβ40 levels at all percentiles (P < 0.05). The associations were generally stronger at higher Aβ42 and Aβ40 percentiles than lower Aβ42 and Aβ40 percentiles. Blood Se was positively associated with serum Aβ42/Aβ40 ratio at 25th, 50th, and 75th percentiles. Significant positive associations were observed between serum GPX and Aβ42 and Aβ40 levels at all percentiles (P < 0.05). The positive associations were generally stronger at higher Aβ42 and Aβ40 percentiles than at lower percentiles. Serum GPX was negatively associated with Aβ42/Aβ40 ratio at 25th, 50th, 75th, and 95th percentiles. No associations with serum SELENOP and Aβ levels were observed.
Conclusions
Our results suggest that higher Se levels are associated with lower serum Aβ42 and Aβ40 levels and with higher Aβ42/Aβ40 ratio, and the results are specific for different selenoproteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a global public health threat in the context of population aging [1]. Identifying risk or protective factors associated with AD is highly critical. The deposition of neurotoxic β-amyloid (Aβ) in the brain is one of the key neuropathologic feature of AD development [2]. Aβ42 and Aβ40 are two predominant isoforms of Aβ [3], which have been examined in an increasing number of research studies for AD pathogenesis and prognosis. Abnormal Aβ status in cerebrospinal fluid (CSF) analysis has been incorporated in the diagnostic criteria for AD [4]. However, CSF acquisition may not be feasible in large observational studies of older adults. Previous studies have demonstrated that peripheral blood Aβ levels were positively correlated with CSF Aβ levels and could be regarded as predictive biomarkers for brain amyloidosis [5]. Moreover, higher Aβ42 and Aβ40 levels in peripheral blood were associated with higher risk of AD [6,7,8], and higher Aβ42/Aβ40 ratio was typically associated with lower AD risk [9].
Selenium (Se) is an essential trace element for cerebral functions and the brain is the last Se-deficient organ in Se deficiency [10]. Changes in Se concentration and selenoprotein activity in blood and brain have been reported in AD and other brain diseases [11, 12]. Se had been found to inhibit the activity of γ-secretase, attenuate the alterations in APP expression and Aβ production, and have a protective effect on the neurotoxicity of Aβ [13, 14]. Selenoproteins are the major forms for Se to perform numerous biological functions [15]. Glutathione peroxidase (GPX) plays a unique role in protecting cells from free radical-induced oxidative damage [16], and GPX gene knockout mice has been reported to exhibit higher sensitivity to Aβ-induced oxidative stress [17]. Selenoprotein P (SELENOP) is an important Se transporter [18], which was found to be co-localized with Aβ plaques in the postmortem tissue from individuals with hallmark AD lesions [19]. Reduction of SELENOP gene expression rendered the N2A cell more sensitive to the toxicity of Aβ [20]. However, the function and mechanism of Se in AD development remain elusive, and there is a paucity of research in the association between Se and Aβ levels in bio-samples from the elderly population.
Considering the difference in Se nutrition status due to geologically uneven soil Se distribution [21] and the increasing concern on Se and AD [12], this study is focused on exploring the association between Se and Aβ levels in older adults with an extensive range of Se exposure.
Methods
Study Design and Participants
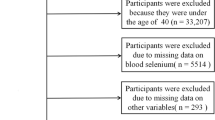
A cross-sectional study of 469 elderly people aged 60 and older from four rural counties in Enshi prefecture with diverse soil Se levels was conducted. Participants were enrolled between 2016 and 2017; biomarker measurements were conducted between 2018 and 2019. Data from 433 participants with serum Se status and Aβ measures was used for statistically analysis in this study. The four sites were from extremely high Se area (> 3.00 mg/kg), high Se area (0.40–3.00 mg/kg), Se-sufficient area (0.18–0.40 mg/kg), and Se-deficient area (< 0.18 mg/kg), respectively [21]. Residents were asked to enroll in the study if they met the following eligibility criteria: (a) had lived in the area for at least 30 years; (b) consumed local food and with no dietary supplement; (c) had no language communication problem; (d) agreed to complete a face-to-face interview and provide blood samples. All participants signed written informed consents before each interview. The study was approved by the Institute for Environmental Health and Related Safety, Chinese Center for Disease Control and Prevention.
Blood Se Measures
Fasting blood samples were collected in the morning using 5-mL purple top (EDTA) Vacutainer® tubes. All samples were stored in polypropylene tubes at – 80 °C before laboratory analysis. Blood samples were digested with concentrated nitric acid for 1 h and then placed in a boiling water bath for 4 h until the samples were clear.
Se concentrations in whole blood were measured by inductively coupled plasma mass spectrometry (ICP-MS) (Agilent 7700x, USA). The instrument parameters were as follows: RF power, 1550 W; collision mode, helium flow 4 mL/min; carrier gas flow, 1.0 L/min; plasma gas flow, 15 L/min; and auxiliary gas flow, 0.15 L/min. External calibration curves were applied for quantification. And, two quality control samples (Seronorm™ Trace Elements Whole Blood L-1 and L-2, Norway) were used to ensure the accuracy of the Se measurement. The detection limit for Se was 0.54 μg/L. The relative standard deviation (RSD) of replicate analysis of samples was less than 10%. In order to monitor the detection procedure, blank samples were analyzed for every 20 samples in all batches; 10% duplicated samples were also included; the samples were reanalyzed if the two results differed by > 10%.
Serum Selenoproteins Measures
Selenoproteins were measured by the enzyme-linked immunoassay (ELISA) method. Commercially available kits were selected according to pretest results. Serum GPX kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and SELENOP kits (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China) were used to determine the concentration according to the manufacturer’s instructions. The absorbance of each well was read on ELISA reader using 412 nm as primary wavelength for serum GPX and 450 nm as primary wavelength for serum SELENOP. As per manufacturer, for GPX, the intra- and inter-assay CV were 3.56% and 6.80%. For SELENOP, the reported intra- and inter-assay CVs were all less than 10%. In addition to quality control samples, 10% parallel samples were measured in each batch for quality assurance.
Serum Aβ Peptides Measures
Serum Aβ levels were determined using ELISA kits (Wako Pure Chemical Industries, Ltd., Osaka, Japan) according to manufacturer’s instructions. The absorbance was measured at a wavelength of 450 nm with a microplate reader (Bio-Tek, USA). In addition to quality control samples, 10% parallel samples were measured in each batch for quality assurance. Sensitivities were 0.12 pmol/L (assay range 1.0–100 pmol/L) in the Aβ40 assay and 0.08 pmol/L (assay range 1.0–100 pmol/L) in the Aβ42 assay. Intra- and inter-assay coefficients of variation were less than 10% in both assay systems.
Covariates
Information on socio-demographic characteristics (age, gender, and education) and lifestyle factors (alcohol consumption and smoking) were collected by standardized questionnaire from face-to-face interviews. Height and weight were also measured during the interview. The height-measuring instrument and electronic weighing scale were calibrated before measurement. The precision was 0.1 cm for height and 0.1 kg for weight. Body mass index (BMI, defined as body weight in kilograms divided by height in meters squared) was derived from height and weight measurements. The above variables will be used as covariates in the statistical analysis according to previous study [22].
Statistical Analysis
The normality of the data was assessed by the Shapiro–Wilk test. Except for age and BMI, the distribution of other parameters was skewed in our study. The continuous variables of the normal distribution were presented as mean ± standard deviation (SD), and continuous variables of skewed distribution were presented as median (interquartile range, IQR). Categorical variables were expressed as proportion (percentage). Comparisons of demographic characteristics among Se quintiles were performed by analysis of variance or Kruskal–Wallis rank-sum test for continuous variables and chi-square tests for categorical variables. Spearman rank correlation analysis was used to estimate associations among Se, SELENOP, and GPX.
Quantile regression models were used to examine the association between Se and Aβ levels. Quantile regression is a distribution-free method overcoming the limitations of traditional linear regression when the outcome variables fail to satisfy the normal distribution assumption [23, 24]. Conditional serum Aβ percentiles as functions of Se levels were estimated at the 5th, 25th, 50th, 75th, and 95th percentiles. The models were adjusted for age, gender, education, smoking, alcohol consumption, and BMI. Quantile regression coefficient graphs were used to present the changing trend of the association between Se levels and serum Aβ levels.
Statistical analyses were performed using STATA 16.0. All tests were two-tailed, and a P-value of less than 0.05 was considered statistically significant.
Results
Description of Participants
For the 433 participants included in this analysis, mean age was 72.38 ± 5.25 years, and 50.23% were females. Median and interquartile range (IQR) of blood Se, serum SELENOP, and GPX concentrations were 0.11 (0.08, 0.18) mg/L, 4.59 (3.62, 6.75) μg/mL, and 0.20 (0.16, 0.25) mmol/L, respectively. Median and IQR serum of Aβ42 and Aβ40 concentrations were 2.48 (1.18, 4.08) pmol/L and 25.15 (8.56, 49.30) pmol/L, respectively.
Demographic characteristics of study participants by blood Se quintiles were presented in Table 1. There were significant differences in age, SELENOP, GPX, Aβ42, Aβ40, and Aβ42/Aβ40 ratio, while no significant difference was observed in BMI, gender, education, smoking, and alcohol consumption among five Se quintile groups. The blood Se level was negatively correlated with age (rs = − 0.098, P = 0.044). Non-smokers had higher SELENOP and GPX levels than that in smokers (P < 0.001). Participants who do not consume alcohol had higher serum SELENOP and GPX levels and lower Se levels than drinkers (P < 0.05). Blood Se was positively correlated with serum SELENOP (rs = 0.26, P < 0.001) and GPX (rs = 0.11, P = 0.03).
Blood Se and Serum Aβ
Regression coefficients using quantile regression were presented for the 5th, 25th, 50th, 75th, and 95th percentiles of Aβ distribution (Table 2). Significant negative associations were observed between blood Se and serum Aβ42 and Aβ40 levels at all percentiles (P < 0.05). There was an overall increasing trend of the absolute value of β-coefficients between blood Se and Aβ42 and Aβ40 percentiles, indicating that these associations were generally stronger at the higher Aβ42 and Aβ40 percentiles than lower Aβ42 and Aβ40 percentiles (Fig. 1A and B). For example, for every 1 mg/L increase in blood Se level, Aβ42 decreased by 1.58 pg/mL at the lowest Aβ42 percentile, and Aβ42 decreased by 3.71 pg/mL at the highest Aβ42 percentile. Importantly, Se was positively correlated with Aβ42/Aβ40 ratio at the 25th (β: 0.03, 95%CI: 0.01, 0.05), 50th (β: 0.06, 95%CI: 0.02, 0.10), and 75th (β: 0.07, 95%CI: 0.01, 0.14) percentiles. Interestingly, only at the highest and lowest percentiles for Aβ42/Aβ40 ratio, the association between blood Se and Aβ42/Aβ40 ratio was not significant.
a–i Graphics of quantile regression coefficient between serum Aβ and blood Se, serum GPX and SELENOP levels. The green solid line represented the quantile regression estimated coefficients and the gray area represented 95%CI of the corresponding parameters. The black dashed line represented the ordinary least squares (OLS) estimate regression of the corresponding explanatory variable, and the area between the two black dashed lines represented the 95%CI of the OLS regression value. All estimations were adjusted for age, gender, education, BMI, tobacco smoking, and alcohol consumption.
Serum GPX and Aβ
Significant positive associations were observed between serum GPX, serum Aβ42, and Aβ40 levels at all percentiles (Table 3). There was an overall increasing trend of the β-coefficients between serum GPX and Aβ42 and Aβ40 percentiles, indicating that the positive associations were generally stronger at the higher Aβ42 and Aβ40 percentiles than lower percentiles (Fig. 1D and E). For example, for every 1 mmol/L increase in serum GPX level, Aβ42 increased by 11.21 pg/mL in the lowest Aβ42 percentile, and Aβ42 increased by 23.51 pg/mL in the highest Aβ42 percentile. In addition, serum GPX was negatively associated with Aβ42/Aβ40 ratio at 25th (β: − 0.11, 95%CI: − 0.17, − 0.06), 50th (β: − 0.31, 95%CI: − 0.31, − 0.15), 75th (β: − 0.38, 95%CI: − 0.50,0.26), and 95th (β: − 0.88, 95%CI: − 1.75, − 0.02) percentiles, but there was no association at 5th percentile (Table 3). The negative associations were generally stronger at the higher Aβ42/Aβ40 percentiles than lower percentiles (Fig. 1F).
Serum SELENOP and Aβ
No statistically significant associations were observed between serum SELENOP, Aβ42, Aβ40, and Aβ42/Aβ40 ratio (Table 4 and Fig. 1).
Discussion
In this cross-sectional study, we observed that blood Se was positively associated with serum SELENOP and GPX levels. Higher Se levels were significantly associated with lower serum Aβ42 and Aβ40 levels and associated with higher Aβ42/Aβ40 ratio. Higher serum GPX levels were significantly associated with higher serum Aβ42 and Aβ40 levels and associated with lower Aβ42/Aβ40 ratio. No association with serum SELENOP and Aβ levels was observed.
Total Se and Aβ
Our results were consistent with a cohort study in Italy involving 71 subjects, showing that higher inorganic Se concentrations in baseline CSF samples were inversely associated with lower CSF Aβ concentration in older adults [25]. However, a case–control study involving 30 AD patients, 35 vascular dementia patients, and 40 healthy controls in Indian reported that there was no association between plasma Se and Aβ42 [26]. Nevertheless, the results of some in vitro or in vivo studies could also support our results. A cellular experiment showed that selenite could reduce Aβ40 generation in APP695 stably transfected Chinese hamster ovary cells [27]. An animal study reported that Se combined with fish oil could inhibit the activity of serum β- and γ-secretase in a mouse model of aging [28]. Another in vitro study also observed that selenite could reduce Aβ40, Aβ42, and sAPP beta production by reducing Aβ producing β-secretase and γ-secretase activities [29]. In addition, Se could attenuate lead exposure-induced changes in APP expression and Aβ production in Wistar rat hippocampus [30]. These findings suggest that blood Se may affect the APP processing by regulating the activity of β- and γ-secretase and further influence the serum Aβ levels.
Metabolic disturbances of Aβ homeostasis are the initial culprit in the pathogenesis of AD [31]. Recent reports suggested that high-accuracy assays of plasma Aβ42/Aβ40 ratio strongly predicted brain amyloidosis, and higher plasma Aβ42/Aβ40 ratios were correlated with lower Aβ deposition in brain tissue [5, 32]. However, no observational studies had focused on the association between Se and Aβ42/Aβ40 ratio before. As far as we know, our study is the first report showing that higher blood Se levels were associated with higher Aβ42/Aβ40 ratio, and we could further infer that higher blood Se levels could be associated with higher cognitive performance. Actually, this inference has been confirmed by our previous observational study in China [33] and another cross-sectional study in the USA [34]. Therefore, we speculate that Se could affect cognitive function by affecting Aβ metabolism; higher Se could reduce the generation of Aβ and increase Aβ42/Aβ40 ratio, which may delay the onset of cognitive impairment.
GPX and Aβ
Higher serum GPX was associated with higher serum Aβ and lower Aβ42/Aβ40 ratio in our study, which was opposite to the findings from total blood Se. This difference could be partly explained by the feedback effect of oxidative stress. As a group of antioxidant enzymes, the main biological role of GPX is to protect the organism from oxidative damage, for example, GPX4 could protect cortical neurons from oxidative injury and amyloid toxicity [35], and neurons of GPX1 gene knockout mice exhibited higher sensitivity to Aβ-induced oxidative stress [17]. Some studies suggested that the increase in antioxidant enzymes may represent a compensatory upregulation in response to increased oxidative stress [36, 37]. Interestingly, several studies have confirmed that oxidative stress could increase the expression of APP and the intracellular and secreted Aβ levels [38], and the increase of Aβ levels can aggravate oxidative stress in turn, forming a vicious cycle [39, 40]. This feedback effect could be found in other population-based studies; higher plasma GPX activity was observed in patients with AD and in those with vascular dementia compared with healthy controls [41, 42].
In addition, other selenoproteins may be responsible for the opposite finding in our study. Except for GPX and SELENOP, there are several more selenoproteins which could be associated with AD. In vitro studies showed that selenoprotein S could prevent Aβ production by degrading C99 which could be cleaved to Aβ [43], and overexpression of selenoprotein M could inhibit the α/γ-secretase activity related to the protection of Aβ42 production [44]. The thioredoxin reductase (TrxR) protein family may also have a protective roll in AD [10]. Therefore, we hypothesized that other selenoproteins or Se compounds might have covered up the inverse association of GPX compared with total Se.
SELENOP and Aβ
In the current study, we did not find significant association between serum SELENOP and Aβ levels. However, as the main Se transport protein, SELENOP has been supposed to be responsible for maintaining Se homeostasis and be vital for proper brain development and function [11, 45]. A postmortem study did observe an association that SELENOP was co-localized with Aβ plaques after examining postmortem brain tissue from individuals with the hallmark lesions of AD and individuals without these lesions [19]. In the triple-transgenic AD (3 × Tg-AD) model of mice, overexpression of SELENOP can inhibit the aggregation of Aβ in the brain of mice [46]. Meanwhile, some studies found consistent results as ours. An animal experiment found no effect on the number of amyloid plaque nor intracellular Aβ-containing neurons [47]. And, a cross-sectional study reported that CSF SELENOP levels were not associated with CSF Aβ levels [25]. The conflicting findings suggested that SELENOP may not directly affect the formation and deposition of Aβ; the function of SELENOP in brain health needs to be verified in future studies.
Strengths and Limitations
Our study has several strengths. Firstly, this is the first cross-sectional study with a relatively large sample to explore the relationship between peripheral blood Se levels and Aβ levels in the general population. Compared with CSF sample collection in other studies, blood sample collection in our study is less invasive. Therefore, blood Aβ measures are more suitable for epidemiological studies on general population. Secondly, our study design ensures an extensive range of Se exposure as subjects came from areas with different soil Se levels. Finally, the rural elderly Chinese participants were mostly lifelong residents consuming local food and without Se supplementation; hence, the ascertained Se levels could be inferred to be lifelong Se exposure without the influence of supplements.
There are also some limitations. On the one hand, we did not determine oxidative stress biomarkers to explore the relationship between Se and Aβ. On the other hand, with the cross-sectional study design, we cannot make causal inferences on the association between Se and Aβ levels. Therefore, more longitudinal studies are necessary to confirm our results.
Conclusion
Our results suggest that higher Se levels are associated with lower serum Aβ42 and Aβ40 levels and with higher serum Aβ42/Aβ40 ratio, and the results are specific for different selenoproteins. More longitudinal evidence is needed to confirm our findings, and further studies are needed to elucidate the underline mechanisms.
References
Association Alzheimer’s (2019) 2019 Alzheimer’s disease facts and figures. Alzheimer’s & dementia 15(3):321–387
Wang D, Chen F, Han Z, Yin Z, Ge X, Lei P (2021) Relationship between amyloid-beta deposition and blood-brain barrier dysfunction in Alzheimer’s disease. Front Cell Neurosci 15:695479
Benilova I, Karran E, De Strooper B (2012) The toxic Abeta oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat Neurosci 15(3):349–357
Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K et al (2014) Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol 13(6):614–629
Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Dore V et al (2018) High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature 554(7691):249–254
van Oijen M, Hofman A, Soares HD, Koudstaal PJ, Breteler MM (2006) Plasma Abeta(1–40) and Abeta(1–42) and the risk of dementia: a prospective case-cohort study. Lancet Neurol 5(8):655–660
Scharpf M, Schweizer U, Arzberger T, Roggendorf W, Schomburg L, Kohrle J (2007) Neuronal and ependymal expression of selenoprotein P in the human brain. J Neural Transm (Vienna) 114(7):877–884
Toledo JB, Vanderstichele H, Figurski M, Aisen PS, Petersen RC, Weiner MW et al (2011) Factors affecting Abeta plasma levels and their utility as biomarkers in ADNI. Acta Neuropathol 122(4):401–413
Janelidze S, Teunissen CE, Zetterberg H, Allue JA, Sarasa L, Eichenlaub U et al (2021) Head-to-head comparison of 8 plasma amyloid-beta 42/40 assays in Alzheimer disease. JAMA Neurol 78(11):1375–1382
Pillai R, Uyehara-Lock JH, Bellinger FP (2014) Selenium and selenoprotein function in brain disorders. IUBMB Life 66(4):229–239
Chen J, Berry MJ (2003) Selenium and selenoproteins in the brain and brain diseases. J Neurochem 86(1):1–12
Solovyev N, Drobyshev E, Bjorklund G, Dubrovskii Y, Lysiuk R, Rayman MP (2018) Selenium, selenoprotein P, and Alzheimer’s disease: is there a link? Free Radic Biol Med 1(127):124–133
Gwon A-R, Park J-S, Park J-H, Baik S-H, Jeong H-Y, Hyun D-H et al (2010) Selenium attenuates Aβ production and Aβ-induced neuronal death. Neurosci Lett 469(3):391–395
Godoi GL, de Oliveira PL, Schulz JF, Kaufmann FN, da Rocha JB, de Souza DO et al (2013) Selenium compounds prevent amyloid beta-peptide neurotoxicity in rat primary hippocampal neurons. Neurochem Res 38(11):2359–63
Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo R et al (2003) Characterization of mammalian selenoproteomes. Science 300(5624):1439–1443
Brigelius-Flohé R (1999) Tissue-specific functions of individual glutathione peroxidases. Free Radical Biol Med 27(9–10):951–965
Crack PJ, Cimdins K, Ali U, Hertzog PJ, Iannello RC (2006) Lack of glutathione peroxidase-1 exacerbates Abeta-mediated neurotoxicity in cortical neurons. J Neural Transm (Vienna) 113(5):645–657
Burk RF, Hill KE, Motley AK (2001) Plasma selenium in specific and non-specific forms. BioFactors 14(1–4):107–114
Bellinger FPHQ, Bellinger MT, Lin Y, Raman AV, White LR, Berry MJ (2008) Association of selenoprotein p with Alzheimer’s pathology in human cortex. J Alzheimers Dis 15(3):465–472
Takemoto ASBM, Bellinger FP (2010) Role of selenoprotein P in Alzheimer’s disease. Ethn Dis 20(Suppl 1):92–95
Dinh QT, Cui Z, Huang J, Tran TAT, Wang D, Yang W et al (2018) Selenium distribution in the Chinese environment and its relationship with human health: a review. Environ Int 112:294–309
Xu W, Tan L, Wang HF, Jiang T, Tan MS, Tan L et al (2015) Meta-analysis of modifiable risk factors for Alzheimer’s disease. J Neurol Neurosurg Psychiatry 86(12):1299–1306
Koenker R, Hallock KF (2001) Quantile regression. J Econ Perspect 15(4):143–156
Staffa SJ, Kohane DS, Zurakowski D (2019) Quantile regression and its applications: a primer for anesthesiologists. Anesth Analg 128(4):820–830
Marco V, Annalisa C, Marcel E, J RK, Tommaso F, Carlotta M, et al (2017) A selenium species in cerebrospinal fluid predicts conversion to Alzheimer’s dementia in persons with mild cognitive impairment. Alzheimer’s Res Ther 9(1):100
Krishnan S, Rani P (2014) Evaluation of selenium, redox status and their association with plasma amyloid/tau in Alzheimer’s disease. Biol Trace Elem Res 158(2):158–165
Li GZ, Liu F, Xu C, Li JY, Xu YJ (2018) Selenium and zinc against Abeta25-35-induced cytotoxicity and Tau phosphorylation in PC12 cells and inhibits gamma-cleavage of APP. Biol Trace Elem Res 184(2):442–449
Fu CXDL, Yuan XY, Xu YJ (2021) Effects of fish oil combined with selenium and zinc on learning and memory impairment in aging mice and amyloid precursor protein processing. Biol Trace Elem Res 199(5):1855–1863
Du X, Wang C, Liu Q (2016) Potential roles of selenium and selenoproteins in the prevention of Alzheimer’s disease. Curr Top Med Chem 16(8):835–848
Deng Z, Fu H, Xiao Y, Zhang B, Sun G, Wei Q et al (2015) Effects of selenium on lead-induced alterations in Abeta production and Bcl-2 family proteins. Environ Toxicol Pharmacol 39(1):221–228
Pankiewicz JE, Diaz JR, Marta-Ariza M, Lizinczyk AM, Franco LA, Sadowski MJ (2020) Peroxiredoxin 6 mediates protective function of astrocytes in Abeta proteostasis. Mol Neurodegener 15(1):50
Schindler SE, Bollinger JG, Ovod V, Mawuenyega KG, Li Y, Gordon BA et al (2019) High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 93(17):e1647–e1659
Gao S, Jin Y, Hall KS, Liang C, Unverzagt FW, Ji R et al (2007) Selenium level and cognitive function in rural elderly Chinese. Am J Epidemiol 165(8):955–965
Yan X, Liu K, Sun X, Qin S, Wu M, Qin L et al (2020) A cross-sectional study of blood selenium concentration and cognitive function in elderly Americans: National Health and Nutrition Examination Survey 2011–2014. Ann Hum Biol 47(7–8):610–619
Ran Q, Gu M, Van Remmen H, Strong R, Roberts JL, Richardson A (2006) Glutathione peroxidase 4 protects cortical neurons from oxidative injury and amyloid toxicity. J Neurosci Res 84(1):202–208
Turk HM, Sevinc A, Camci C, Cigli A, Buyukberber S, Savli H et al (2002) Plasma lipid peroxidation products and antioxidant enzyme activities in patients with type 2 diabetes mellitus. Acta Diabetol 39(3):117–122
Iborra M, Moret I, Rausell F, Bastida G, Aguas M, Cerrillo E et al (2011) Role of oxidative stress and antioxidant enzymes in Crohn’s disease. Biochem Soc Trans 39(4):1102–1106
Likidlilid A, Patchanans N, Peerapatdit T, Sriratanasathavorn C (2010) Lipid peroxidation and antioxidant enzyme activities in erythrocytes of type 2 diabetic patients. J Med Assoc Thai 93(6):682–693
Tong Y, Zhou W, Fung V, Christensen MA, Qing H, Sun X et al (2005) Oxidative stress potentiates BACE1 gene expression and Abeta generation. J Neural Transm (Vienna) 112(3):455–469
McCarty MF, DiNicolantonio JJ, Lerner A (2021) A fundamental role for oxidants and intracellular calcium signals in Alzheimer’s pathogenesis-and how a comprehensive antioxidant strategy may aid prevention of this disorder. Int J Mol Sci 22(4):2140
Marrocco I, Altieri F, Peluso I (2017) Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxid Med Cell Longev 2017:6501046
Saeedi M, Rashidy-Pour A (2021) Association between chronic stress and Alzheimer’s disease: therapeutic effects of Saffron. Biomed Pharmacother 133:110995
Jang JK, Park KJ, Lee JH, Ko KY, Kang S, Kim IY (2017) Selenoprotein S is required for clearance of C99 through endoplasmic reticulum-associated degradation. Biochem Biophys Res Commun 486(2):444–450
Yim SY, Chae KR, Shim SB, Hong JT, Park JY, Lee CY et al (2009) ERK activation induced by selenium treatment significantly downregulates beta/gamma-secretase activity and Tau phosphorylation in the transgenic rat overexpressing human selenoprotein M. Int J Mol Med 24(1):91–96
Solovyev N (2020) Selenoprotein P and its potential role in Alzheimer’s disease. Hormones (Athens) 19(1):73–79
Yue C, Shan Z, Tan Y, Yao C, Liu Y, Liu Q et al (2020) His-rich domain of selenoprotein P ameliorates neuropathology and cognitive deficits by regulating TrkB pathway and zinc homeostasis in an Alzheimer model of mice. ACS Chem Neurosci 11(24):4098–4110
Van der Jeugd A, Parra-Damas A, Baeta-Corral R, Soto-Faguas CM, Ahmed T, LaFerla FM et al (2018) Reversal of memory and neuropsychiatric symptoms and reduced tau pathology by selenium in 3xTg-AD mice. Sci Rep 8(1):6431
Funding
This work was supported by grants from the National Key Research and Development Program of China (2018YFC801102), the National Institutes of Health of USA (R01AG019181), and the Key Laboratory of Trace Element and Nutrition, National Health Commission of China (WLKFZ202201).
Author information
Authors and Affiliations
Contributions
Jiao Luo: data curation; software; formal analysis; writing—original draft; Liqin Su: conceptualization, funding acquisition, project administration, writing—review and editing; Xiaohong He: investigation, data curation, resources; Yegang Du: methodology, data curation, resources; Ning Xu: investigation, supervision; Rangpeng Wu: investigation, data curation; Yunfeng Zhu: investigation, data curation; Ting Wang: data curation, validation; Ranqi Shao: data curation, software; Frederick W. Unverzagt: resources, supervision; Ann M. Hake: resources, supervision; Yinlong Jin: resources, funding acquisition, supervision; Sujuan Gao: resources, funding acquisition, supervision.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, J., Su, L., He, X. et al. Blood Selenium and Serum Glutathione Peroxidase Levels Were Associated with Serum β-Amyloid in Older Adults. Biol Trace Elem Res 201, 3679–3687 (2023). https://doi.org/10.1007/s12011-022-03480-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03480-4