Abstract
Selenium is an essential trace element for human health, playing a key role in regulating cellular oxidative stress, immune response, and inflammation. In recent years, the association between selenium and Parkinson's disease (PD) has aroused people's attention. The objective of this study is to investigate the relationship between blood selenium concentrations and PD risk in a sample of U.S. adults. A cross-sectional study was conducted using the National Health and Nutrition Examination Survey (NHANES) data from 2011–2020 and included 15,660 adults aged over 40 years old. Univariate logistic regression and multivariate logistic regression models were utilized to analyze the association between blood selenium concentrations and PD prevalence. Additionally, the restricted cubic spline (RCS) model was applied to investigate the dose–response relationships between blood selenium and PD. The findings indicated a link between elevated blood selenium levels and a reduced occurrence of Parkinson's disease (PD). Notably, this association between blood selenium and PD exhibited a non-linear pattern, wherein the decline in PD risk was more pronounced at higher selenium concentrations than at lower levels. An inflection point emerged at approximately 2.4 μmol/L, beyond which the rate of decline in risk significantly diminished with increasing selenium levels. A potential association between blood selenium concentrations and PD has been observed, with PD patients having lower blood selenium levels compared to non-PD patients. Higher levels of blood selenium may have a protective effect against PD. However, further prospective studies are needed to investigate the effect of blood selenium in PD patients and to determine causality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium is an essential trace element for human health, with its biological effects primarily mediated through selenoproteins, which play a key role in regulating cellular oxidative stress, immune response, and inflammation, as well as in regulating enzyme activity by being located in the active site of the enzyme (Schweizer and Fradejas-Villar 2016; Hossain et al. 2021; Raj et al. 2021). Chronic selenium deficiency has been linked to reduced immunity, impaired nervous system function, and increased risk of myocardial infarction. In addition, chronic selenium deficiency is also linked to Keshan disease, Kashin-beck disease, Alzheimer's disease, diabetes, stroke, and other diseases. However, selenium poisoning may cause hair loss, brittle nails, skin rashes, gastrointestinal problems, and a higher risk of cancer and even lead to acute liver failure when severe (Goldhaber 2003; Navarro-Alarcon and Cabrera-Vique 2008; Hossain et al. 2021; Raj et al. 2021). It is important to note that the blood selenium range may vary in different populations and geographic regions in blood selenium. For instance, a study in the United States showed that most participants (99%) had serum selenium levels greater than 95 µg/L with median serum selenium was 124 µg/L, while in British adults the mean plasma selenium level was 86 µg/L(Monsen 2000; Stranges et al. 2010). Also, it has been observed that selenium levels in Spanish populations are significantly lower compared to those in the United States and other European countries (Millán Adame et al. 2012).WHO does not have a specific standard permissible limit for selenium in drinking water or soil. WHO suggests that human beings must consume around 55 µg of Se per day but no more than 400 µg per day (Arnaud et al. 1988). And recommends a daily average intake of 55 µg of Se, and the recommendation should be varied with age, gender, diet, and geographic location (Post et al. 2018). The International Food and Nutrition Board recommends an average daily intake of 40–70 µg of Se for men, 45–55 µg of Se for women, and 25 µg of Se for children (Galan-Chilet et al. 2014).
PD is the second most common degenerative disease of the central nervous system after Alzheimer's disease which is characterized by bradykinesia, resting tremors, myotonia, and abnormal postures and gaits. (Hauser et al. 2013; Raj et al. 2021). Elderly individuals with PD experience significant difficulties with motor function and have a poor prognosis, which can negatively impact their quality of life (Chen 2010; Bock et al. 2022). The substantia nigra is lost of dopaminergic neurons when PD occurs as a result of abnormal accumulation of α-synuclein and Lewy bodies (Hauser et al. 2013; Solovyev 2015; Raj et al. 2021). Although how PD develops has not been fully explained, oxidative stress has a central role in its pathogenesis. Selenium proteins can also be anti-inflammatory and antioxidant properties, which increases antioxidant levels and reduce dopamine neuron loss (Hauser et al. 2013; Solovyev 2015; Raj et al. 2021). People have begun to pay more attention in recent years to the association between selenium and PD, given its importance as an important element. Selenium levels in serum plasma or cerebrospinal fluid have been evaluated in several studies in PD patients with inconsistent results. The blood levels of selenium in PD patients are higher than those in controls, according to several studies (Qureshi et al. 2006; Zhao et al. 2013), while some studies showed regular levels of selenium or even decreased (Nikam et al. 2009). Given the inconsistent findings and unclear mechanisms, it is necessary to further investigate the relationship between blood selenium and PD. Consequently, this study aimed to investigate the potential association between blood selenium concentrations and PD, which could have significant implications for the prevention and treatment of the disease.
Methods
Study population
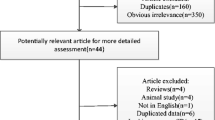
Access to all survey data can be obtained through the National Health and Nutrition Examination Survey (NHANES) website. NHANES is a large, nationally representative study of the United States population conducted on a two-year cycle, utilizing a multi-stage and stratified sampling design (Gong et al. 2022; Huang 2022). For this study, we selected 54,674 subjects from five consecutive NHANES survey cycles that spanned 2011–2020. To ensure uniformity in age, we excluded 2,111 participants who were under the age of 40. Additionally, 5,514 participants who lacked data on blood selenium were excluded. Lastly, study participants were excluded for missing data on education level, body mass index (BMI), stroke, and other variables, resulting in a final study population of 15,660. The process of recruiting is shown in Fig. 1.
Measurement of blood selenium
After collection, the whole blood specimen is processed, stored, and shipped to the National Center for Environmental Health and the Centers for Disease Control and Prevention for analysis. For selenium concentration measurements, the NHANES Laboratory utilizes inductively coupled plasma mass spectrometry. The applied methodology yields a detection limit of 0.381μmol/L for blood selenium. Detailed instructions from the NHANES Laboratory are provided for collecting and processing the specimens. In this study, blood selenium concentrations were divided into four quartiles based on their distribution range using the interquartile range method. The quartiles were defined as follows: Q1 (< 2.21), Q2 (≥ 2.21 and < 2.39), Q3 (≥ 2.39 and < = 2.61), and Q4 (> 2.61). In the sensitivity analysis, blood selenium was stratified into five levels using the quintile method, namely Q1 (< 2.16), Q2 (≥ 2.16 and < 2.32), Q3 (≥ 2.32 and < 2.48), Q4 (≥ 2.48 and < 2.67), and Q5 (≥ 2.67).
Diagnosis of PD
So to determine whether a participant has PD, the NHANES study relied on participants reporting the use of medication specifically indicated for treating PD (DeMarco et al. 2021). Since this is dependent on the medications and codes included in the NHANES, individuals had to be actively receiving treatment for PD to be classified as having it. If a participant did not report taking any anti-parkinsonian medication, they were classified as not having PD. This method of identification has its limitations, but it was the best available method to classify participants in the study as having PD or not (Fig. 2).
Multivariate logistic regression analysis of the associations of blood selenium levels with PD. Legends: The horizontal axis represents the blood selenium level (μmol/L), and the vertical axis represents the relative probability of developing PD. (A) Shows the association between blood selenium level and PD with unadjusted. (B) Shows the association between blood selenium level and PD with adjusted for gender, age, and race. (C) Shows the association between blood selenium level and PD with age, gender, race, education, BMI, DM, and stroke
Measurements of other covariates
NHANES collects some sociodemographic information through structured data. Participants self-reported their age, gender, race, and education, which were considered demographic covariates. Weight and height were measured by trained health technologists following the anthropometry procedure manual. BMI was then calculated as weight in kilograms divided by height squared in meters. Diabetes mellitus was diagnosed as hemoglobin A1c greater than 6.5% either in a doctor's office or by self-report as defined by the American Diabetes Association (Dai et al. 2022). Stroke was defined as having either been diagnosed by the doctor or by a self-report questionnaire. The study investigated hypertension status and defined it using multiple criteria. Participants were first asked if they had ever been told they had high blood pressure, which represented the self-reported status of hypertension. Mean diastolic pressure greater than 90 mmHg and mean systolic pressure greater than 140 mmHg were then determined four times. Finally, participants with hypertension were identified based on their responses to the question "Are you taking prescribed medication for hypertension?". Viral hepatitis has been linked to the onset of PD in previous studies (Choi et al. 2020), so we also include it as a confounding factor of interest in the analysis.
Statistical analyses
Data analysis for this study was conducted using version 4.2.2 of the open-source statistical software R. To adjust for oversampling and non-response in the study sample, weights were applied to the analysis. Weighted means and 95% confidence intervals (CIs) were used to describe continuous variables, while weighted percentages and 95% CIs were used to describe categorical variables (Gong et al. 2022; Huang 2022). To investigate the independent association between blood selenium concentrations and PD, a multivariate logistic regression model along with univariate logistic analysis was performe. The formulas for multivariate logistic regression: log(p/(1-p)) = β0 + β1X1 + β2X2 + … + βnXn (Where: p = probability of Parkinson's disease, β0 = intercept, β1 to βn = regression coefficients for each predictor variable, X1 to Xn = predictor variables (age, gender, race, etc.). Four models were created: an unadjusted model, a minimally adjusted model adjusted for age, gender, and race, a third model adjusted for the minimally adjusted model and additional variables of BMI, smoking, and alcohol drinking, and a fully adjusted model adjusted for age, gender, race, education level, BMI, stroke, diabetes, hypertension, and viral hepatitis. RCS is a statistical analysis method used to model the relationship between two continuous variables. It creates a smooth curve that visually describes the nonlinear relationship between the two variables. In this study, RCS was used to visually describe the nonlinear association between blood selenium and PD (Gong et al. 2022). Statistical significance was determined when P < 0.05. Specific sample weights were used in the data analysis for this survey.
Results
Baseline characteristics of participants
Table 1 displays the baseline characteristics of 15,660 participants in a cross-sectional survey conducted between 2011 to 2020, categorized by blood selenium concentration quartile. Of these participants, 229 (1.462%) were diagnosed with PD. The average age of all participants was 58.408 years, with 48.876% male and 51.124% female participants. Baseline characteristics were based on blood selenium concentration quartiles (Q1, < 2.21 mmol/L; Q2, ≥ 2.21 mmol/L and < 2.39 mmol/L; Q3, ≥ 2.39 mmol/L and ≤ 2.61 mmol/L; and Q4, > 2.61 mmol/L). As blood selenium concentration quartiles increased, the proportion of participants tended to be younger (P < 0.001) and included more males (P < 0.0001), while the proportion of Non-Hispanic Black people decreased (P < 0.0001). Additionally, the proportion of participants who reported drinking alcohol increased (P < 0.0001), while those who reported having had a stroke (P < 0.0001) or viral hepatitis (P = 0.003) and with below-high school education level (P < 0.0001) decreased. Furthermore, the baseline table reveals that the incidence of PD gradually decreased with increasing blood selenium concentration (P = 0.021), with the incidence of PD decreasing from 2.247% in Q1 to 1.032% in Q4.
Univariate logistics analysis of PD-related variable
Weighted univariate logistic regression analysis was conducted to observe the associations between age, gender, race, education level, smoking status, alcohol intake, BMI, stroke, diabetes, hypertension, blood selenium, and PD in the US population. Age was found to be positively associated with the occurrence of PD, with an odds ratio (OR) of 1.037 (95% confidence interval [CI]: 1.020, 1.055). Non-Hispanic Whites had a higher incidence of PD compared to Mexican Americans, with an OR of 2.006 (95% CI: 1.165, 3.453). Participants with a history of stroke or hypertension were more likely to develop PD, with ORs of 4.939 (95% CI: 2.999, 8.135) and 1.852 (95% CI: 1.197, 2.866), respectively. In addition, blood selenium concentration was found to be negatively associated with the occurrence of PD, with an OR of 0.380 (95% CI: 0.170, 0.847) (P = 0.019). Participants in blood selenium concentration quartiles Q3 and Q4 were less likely to develop PD than those in Q1, with ORs of 0.602 (95% CI: 0.381, 0.952) and 0.454 (95% CI: 0.255, 0.807), respectively. However, gender, BMI, education level, smoking, alcohol intake, diabetes, and viral hepatitis were not found to be associated with PD occurrence in this study (P > 0.05) (Table 2).
Multivariable logistics regression analysis of the association between blood selenium and PD
Four weighted logistic regression models were used to analyze the relationship between blood selenium and PD in American adults, as presented in Table 3. Model 1 had no covariate adjustment, model 2 adjusted for age, gender, and race, model 3 adjusted for age, gender, race, education level, BMI, smoking, and alcohol intake, and model 4 was the full model that adjusted for age, gender, race, education level, BMI, smoking, alcohol intake, as well as disease factors including stroke, diabetes, hypertension, and viral hepatitis.
All four logistic regression models showed a significant negative association between blood selenium concentration and PD, as evidenced by ORs and their 95% CIs: 0.380 (0.170, 0.847), 0.418 (0.195, 0.895), 0.441 (0.210, 0.930), and 0.482 (0.240, 0.969) (P < 0.05). Furthermore, when blood selenium concentration was stratified, models 1 and 2 showed that participants in quartiles Q3 and Q4 had a lower risk of PD compared to those in Q1, with ORs and CIs of 0.602 (0.381, 0.952) and 0.454 (0.255, 0.807) for model 1, and 0.635 (0.405, 0.997) and 0.486 (0.271, 0.873) for model 2, respectively. In models 3 and 4, only quartile Q4 was significantly associated with a lower risk of PD, with ORs and CIs of 0.505 (0.281, 0.907) and 0.523 (0.296, 0.924), respectively. Additionally, in all models, the risk of PD gradually decreased with increasing blood selenium concentration quartile (P for trend < 0.05). Therefore, the results of the regression models support the conclusion that higher blood selenium concentrations are associated with a lower likelihood of PD occurrence, particularly at higher quartile levels.
Subgroup analysis of the association between blood selenium and PD
To further analyze the study results and identify any special subgroups in our survey, subgroup analyses were performed. As shown in Table 4, we divided the population into subgroups based on age, gender, race, education, BMI, smoking, alcohol, stroke, diabetes, hypertension, and viral hepatitis. Our findings indicate that no significant associations were observed when stratifying by age or stroke. However, we found a significant positive association with females [0.277(0.090,0.851)] (P = 0.026), while males did not show a significant association with blood selenium. Moreover, participants with higher blood selenium levels and a lower risk of PD were more likely to be non-Hispanic Whites [0.340(0.131,0.880)] (P = 0.027), highly educated (over the high school) [0.331(0.119,0.922)] (P = 0.035), and overweight (BMI > 28) [0.373(0.176,0.789)] (P = 0.010). Furthermore, participants who had a history of smoking [0.176(0.059,0.526)] (P = 0.002), alcohol consumption [0.339(0.141,0.817)] (P = 0.017), non-diabetic status [0.338(0.119,0.959)] (P = 0.042), non-viral hepatitis [0.379(0.165,0.870)] (P = 0.023), and hypertension [0.282(0.106,0.749)] (P = 0.012) were also more likely to have higher blood selenium levels and a lower risk of PD. Additionally, smoking was found to be an interaction factor affecting the relationship between blood selenium and PD (P for interaction = 0.014).
Nonlinear Associations between blood selenium and the Risk of PD
Our study included an RCS plot to visually depict the relationship between blood selenium concentration and PD. Additionally, four models were developed to adjust for different confounding factors. Results from all four nonlinear RCS models demonstrated a significant association between higher blood selenium levels and a lower risk of PD, with the association being particularly strong at levels > 2.39 µmol/L (P < 0.05).
Sensitivity analyses
To evaluate the robustness of our findings, we conducted a sensitivity analysis categorizing blood selenium into quintiles (Q1 to Q5) rather than quartiles. As shown in Table 5, the adjusted odds ratios (ORs) for the association between blood selenium and PD risk remained statistically significant across model 1 to model 3, though the strength of the association attenuated slightly in the higher quintiles. In Model 1, participants in Q4 and Q5 had ORs of 0.449 (95% CI: 0.239, 0.844; P = 0.014) and 0.519 (95% CI: 0.282, 0.955; P = 0.035) respectively compared to Q1. The P for trend was also significant at 0.009. Similarly, Models 2–4 showed significantly decreased ORs for PD risk in the upper quintiles of blood selenium, with P for trend ranging from 0.019 to 0.038. Overall, this sensitivity analysis categorizing selenium into quintiles demonstrates consistent protective associations between higher blood selenium levels and lower PD risk, further validating the robustness of our primary results.
Discussion
This study investigated the association between blood selenium concentration and PD using data collected from five cycles of the NHANES (2011–2020). Our results demonstrated that the prevalence of PD decreased with increasing blood selenium concentration, irrespective of age, body mass index (BMI), or stroke history. Participants with higher blood selenium levels and a lower risk of PD were more likely to be women, non-Hispanic Whites, highly educated (over high school), and overweight (BMI > 28), as well as have a history of smoking, alcohol consumption, non-diabetic status, non-viral hepatitis, and hypertension. Blood selenium levels were associated with a lower risk of PD, with the association being particularly remarkable at levels > 2.39 µmol/L.
Previous studies have reported a controversial relationship between blood selenium levels and PD. Some studies have found that selenium levels in PD patients' blood are higher than those in normal individuals, suggesting that selenium may be a risk factor for the disease or may increase blood selenium levels to enhance antioxidant ability (Qureshi et al. 2006; Zhao et al. 2013). Conversely, other studies have shown that PD patients have lower blood selenium levels compared to normal individuals, indirectly attributing selenium deficiency to neuronal loss and potentially accelerating PD pathogenesis (Nikam et al. 2009). However, a meta-analysis and a prospective case–control study found no significant difference in serum selenium levels between PD cases and controls (Gellein et al. 2008; Ahmed and Santosh 2010; Zhang et al. 2021). Furthermore, studies have found that the level of selenium in cerebrospinal fluid of PD patients who have not received anti-PD drugs is significantly higher than that of patients who have received dopaminergic drugs (Aguilar et al. 1998). These results suggest that selenium may play a role in the occurrence and progression of PD. Overall, these findings point to the need for further research to better understand the relationship between selenium and PD pathogenesis.
During periods of heightened brain metabolic activity, the production of reactive oxygen species (ROS) and free radicals can increase, leading to oxidative stress, which can harm normal neuron function if not properly eliminated (Chen and Berry 2003; Novoselov et al. 2010; Ramos et al. 2015). Selenium is bound to proteins in the body, such as glutathione peroxidase (GPx), which can co-localize with Lewy bodies—a biomarker of PD pathogenesis—and exert antioxidant effects (Power and Blumbergs 2009). Oxidative stress is known to contribute to the development and progression of PD. Therefore, selenium's antioxidant properties may help reduce oxidative damage to neurons and protect against Parkinson's pathology. Also, GPx4 is a subtype of GPx that is located on the inner mitochondrial membrane to prevent oxidation of membrane phospholipids, stabilize the complexes of the electron transport chain, maintain mitochondrial function, and preserve mitochondrial enzyme activity (Houtkooper and Vaz 2008; Hauser et al. 2013). Selenium's role in keeping mitochondria functioning may help mitigate neuronal damage associated with PD. Dopamine can spontaneously oxidize to form dopamine quinone (DAQ), which is a reactive metabolite of dopamine that can cause dopaminergic neuron death. The selenol group of GPx4 can form covalent bonds with DAQ, leading to the aggregation and degradation of DAQ, thereby reducing its damage to dopaminergic neurons (Hauser et al. 2013). Selenium effects on protein folding and clearance pathways may influence the aggregation and clearance of misfolded proteins which may potentially impact the progression of Parkinson's pathology. In addition, basic research has confirmed selenium as a protective factor for PD. For example, sodium selenite can dose-dependently reverse the decrease in dopamine and its metabolites induced by MPTP (Khan 2010). A selenium-deficient diet can enhance the tyrosine hydroxylase-like immunoreactivity (TH-IR) in the substantia nigra induced by methamphetamine (MA), reduce dopamine and its metabolite levels, exacerbate the loss of substantia nigra dopaminergic neurons, while a selenium-rich diet can significantly block the toxicity of MA-induced neurotoxicity in dopamine neurons (Kim et al. 2000). Ebselen is an organic selenium compound with glutathione peroxidase-like activity that can inhibit dopamine-induced oxidative DNA damage in the presence of copper ions. It can prevent neuron loss and clinical symptoms in the MPTP-induced PD model in primates and can prevent the toxic effects of MPTP in cellular PD models (Moussaoui et al. 2000; Li and Cao 2002). Therefore, the link between selenium and Parkinson's disease has the potential to influence clinical practice by guiding dietary recommendations, patient monitoring, and treatment strategies. It can also impact public health policy by shaping guidelines for diet, supplementation, and research funding priorities. As our understanding of this relationship evolves, it can lead to more effective strategies for PD prevention and management.
While our study suggests a link between selenium and Parkinson's disease risk, we need further research to understand how selenium affects PD development and progression. To gain a more complete understanding, a multidisciplinary approach involving laboratory experiments, animal studies, analysis of human tissue, epidemiological investigations, clinical trials, and genetic research is essential. We believe our findings will encourage more research in this area, potentially leading to improved strategies for preventing and treating PD. Future research should focus on gathering long-term data about dietary and supplemental selenium intake's impact on blood selenium levels, conducting genome-wide association studies to identify genetic factors influencing selenium metabolism and its interaction with PD risk, and conducting longitudinal cohort studies that follow participants over extended periods, tracking selenium levels and PD onset.
The present study has several limitations that should be acknowledged. Firstly, the cross-sectional design of the study precludes the drawing of causal conclusions due to its inherent limitations. Therefore, future prospective cohort studies should be conducted to further investigate the impact of blood selenium on patients with PD. Secondly, a lack of data covariates in the study led to the exclusion of more than half of the participants, which could potentially have affected the study's results. Moreover, the development of PD is influenced by a variety of genetic and environmental factors, some of which may not have been accounted for in this study. As such, the interpretation of study results must be viewed in light of these limitations, and alternative approaches may be needed to fully understand the role of blood selenium in PD pathogenesis. Thirdly, though, NHANES uses a complex, multistage sampling design to select participants representative of the U.S. population, it may still introduce some selection biases inevitably. Therefore, the study sample may not fully represent the entire population. Ultimately, the analysis of our study results relied on the data gathered from the survey, and it's possible that certain covariates, such as dietary selenium supplementation and other dietary intake factors, were not comprehensively accounted for. This limitation could lead to an incomplete representation of participants' health status or introduce potential biases into the results.
Conclusion
In conclusion, our study suggests that there is an association between blood selenium levels and reduced PD risk. Our findings suggest that having higher levels of blood selenium may be beneficial for patients with PD. Further research is warranted to investigate the potential use of selenium as a preventative or therapeutic intervention for PD. Nevertheless, these results provide insights into the role of selenium in PD pathogenesis and offer hope for new treatment approaches to improve patient outcomes.
Data availability
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
Abbreviations
- NHANES :
-
National Health and Nutrition Examination Survey
- PD :
-
Parkinson’s disease
- U.S :
-
United States
- RCS :
-
Restricted cubic spline
- BMI :
-
Body mass index
- SD :
-
Standard deviation
- SE :
-
Standard error
- CI :
-
Confidence interval
- OR :
-
Odds ratio
- WHO :
-
World Health Organization
References
Aguilar MV, Jiménez-Jiménez FJ, Molina JA et al (1998) Cerebrospinal fluid selenium and chromium levels in patients with Parkinson’s disease. J Neural Transm (vienna) 105:1245–1251. https://doi.org/10.1007/s007020050127
Ahmed SS, Santosh W (2010) Metallomic profiling and linkage map analysis of early Parkinson’s disease: a new insight to aluminum marker for the possible diagnosis. PLoS One 5:e11252. https://doi.org/10.1371/journal.pone.0011252
Arnaud S, Mouchiroud G, Blanchet JP (1988) Erythroid colony formation by human CFU-E is stimulated by compatible human serum but impaired by blood group antibodies. Eur J Haematol 40:256–261. https://doi.org/10.1111/j.1600-0609.1988.tb00833.x
Bock MA, Brown EG, Zhang L, Tanner C (2022) Association of Motor and Nonmotor Symptoms With Health-Related Quality of Life in a Large Online Cohort of People With Parkinson Disease. Neurology 98:e2194–e2203. https://doi.org/10.1212/WNL.0000000000200113
Chen J, Berry MJ (2003) Selenium and selenoproteins in the brain and brain diseases. J Neurochem 86:1–12. https://doi.org/10.1046/j.1471-4159.2003.01854.x
Chen JJ (2010) Parkinson’s disease: health-related quality of life, economic cost, and implications of early treatment. Am J Manag Care 16 Suppl Implications:S87–93
Choi HY, Mai TH, Kim KA et al (2020) Association between viral hepatitis infection and Parkinson’s disease: A population-based prospective study. J Viral Hepat 27:1171–1178. https://doi.org/10.1111/jvh.13346
Dai X, Zhou G, Xu L (2022) Associations between red blood cell count and metabolic dysfunction-associated fatty liver disease(MAFLD). PLoS One 17:e0279274. https://doi.org/10.1371/journal.pone.0279274
DeMarco EC, Al-Hammadi N, Hinyard L (2021) Exploring Treatment for Depression in Parkinson's Patients: A Cross-Sectional Analysis. Int J Environ Res Public Health 18. https://doi.org/10.3390/ijerph18168596
Galan-Chilet I, Tellez-Plaza M, Guallar E et al (2014) Plasma selenium levels and oxidative stress biomarkers: a gene-environment interaction population-based study. Free Radic Biol Med 74:229–236. https://doi.org/10.1016/j.freeradbiomed.2014.07.005
Gellein K, Syversen T, Steinnes E et al (2008) Trace elements in serum from patients with Parkinson’s disease–a prospective case-control study: the Nord-Trøndelag Health Study (HUNT). Brain Res 1219:111–115. https://doi.org/10.1016/j.brainres.2008.05.002
Goldhaber SB (2003) Trace element risk assessment: essentiality vs. toxicity. Regul Toxicol Pharmacol 38:232–242. https://doi.org/10.1016/s0273-2300(02)00020-x
Gong R, Pu X, Cheng Z et al (2022) The association between serum cadmium and diabetes in the general population: A cross-sectional study from NHANES (1999–2020). Front Nutr 9:966500. https://doi.org/10.3389/fnut.2022.966500
Hauser DN, Dukes AA, Mortimer AD, Hastings TG (2013) Dopamine quinone modifies and decreases the abundance of the mitochondrial selenoprotein glutathione peroxidase 4. Free Radic Biol Med 65:419–427. https://doi.org/10.1016/j.freeradbiomed.2013.06.030
Hossain A, Skalicky M, Brestic M et al (2021) Selenium Biofortification: Roles, Mechanisms, Responses and Prospects. Molecules 26. https://doi.org/10.3390/molecules26040881
Houtkooper RH, Vaz FM (2008) Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci 65:2493–2506. https://doi.org/10.1007/s00018-008-8030-5
Huang Z (2022) Association Between Blood Lead Level With High Blood Pressure in US (NHANES 1999–2018). Front Public Health 10:836357. https://doi.org/10.3389/fpubh.2022.836357
Khan HA (2010) Selenium partially reverses the depletion of striatal dopamine and its metabolites in MPTP-treated C57BL mice. Neurochem Int 57:489–491. https://doi.org/10.1016/j.neuint.2010.06.020
Kim H, Jhoo W, Shin E, Bing G (2000) Selenium deficiency potentiates methamphetamine-induced nigral neuronal loss; comparison with MPTP model. Brain Res 862:247–252. https://doi.org/10.1016/s0006-8993(00)02085-0
Li Y, Cao Z (2002) The neuroprotectant ebselen inhibits oxidative DNA damage induced by dopamine in the presence of copper ions. Neurosci Lett 330:69–73. https://doi.org/10.1016/s0304-3940(02)00444-5
Millán Adame E, Florea D, Sáez Pérez L et al (2012) Deficient selenium status of a healthy adult Spanish population. Nutr Hosp 27:524–528. https://doi.org/10.1590/S0212-16112012000200026
Monsen ER (2000) Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc 100:637–640. https://doi.org/10.1016/S0002-8223(00)00189-9
Moussaoui S, Obinu MC, Daniel N et al (2000) The antioxidant ebselen prevents neurotoxicity and clinical symptoms in a primate model of Parkinson’s disease. Exp Neurol 166:235–245. https://doi.org/10.1006/exnr.2000.7516
Navarro-Alarcon M, Cabrera-Vique C (2008) Selenium in food and the human body: a review. Sci Total Environ 400:115–141. https://doi.org/10.1016/j.scitotenv.2008.06.024
Nikam S, Nikam P, Ahaley SK, Sontakke AV (2009) Oxidative stress in Parkinson’s disease. Indian J Clin Biochem 24:98–101. https://doi.org/10.1007/s12291-009-0017-y
Novoselov SV, Kim HY, Hua D et al (2010) Regulation of selenoproteins and methionine sulfoxide reductases A and B1 by age, calorie restriction, and dietary selenium in mice. Antioxid Redox Signal 12:829–838. https://doi.org/10.1089/ars.2009.2895
Post M, Lubiński W, Lubiński J et al (2018) Serum selenium levels are associated with age-related cataract. Ann Agric Environ Med 25:443–448. https://doi.org/10.26444/aaem/90886
Power JH, Blumbergs PC (2009) Cellular glutathione peroxidase in human brain: cellular distribution, and its potential role in the degradation of Lewy bodies in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol 117:63–73. https://doi.org/10.1007/s00401-008-0438-3
Qureshi GA, Qureshi AA, Memon SA, Parvez SH (2006) Impact of selenium, iron, copper and zinc in on/off Parkinson's patients on L-dopa therapy. J Neural Transm Suppl 229–236. https://doi.org/10.1007/978-3-211-33328-0_24
Raj K, Kaur P, Gupta GD, Singh S (2021) Metals associated neurodegeneration in Parkinson’s disease: Insight to physiological, pathological mechanisms and management. Neurosci Lett 753:135873. https://doi.org/10.1016/j.neulet.2021.135873
Ramos P, Santos A, Pinto NR et al (2015) Anatomical regional differences in selenium levels in the human brain. Biol Trace Elem Res 163:89–96. https://doi.org/10.1007/s12011-014-0160-z
Schweizer U, Fradejas-Villar N (2016) Why 21? The significance of selenoproteins for human health revealed by inborn errors of metabolism. FASEB J 30:3669–3681. https://doi.org/10.1096/fj.201600424
Solovyev ND (2015) Importance of selenium and selenoprotein for brain function: From antioxidant protection to neuronal signalling. J Inorg Biochem 153:1–12. https://doi.org/10.1016/j.jinorgbio.2015.09.003
Stranges S, Laclaustra M, Ji C et al (2010) Higher selenium status is associated with adverse blood lipid profile in British adults. J Nutr 140:81–87. https://doi.org/10.3945/jn.109.111252
Zhang YJ, Sun HL, Wang T et al (2021) Selenium level does not differ in blood but increased in cerebrospinal fluid in Parkinson’s disease: a meta-analysis. Int J Neurosci 131:95–101. https://doi.org/10.1080/00207454.2020.1733557
Zhao HW, Lin J, Wang XB et al (2013) Assessing plasma levels of selenium, copper, iron and zinc in patients of Parkinson’s disease. PLoS One 8:e83060. https://doi.org/10.1371/journal.pone.0083060
Acknowledgements
The author thanks the staff and the participants of the NHANES study for their valuable contributions.
Funding
This work was supported by Science and Technology Planning Project of Shenzhen Municipality (KCXFZ20201221173605013).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Zhaohao Zeng. The first draft of the manuscript was written by Yanmei Cen and Zhaohao Zeng. Supervision, Editing and Funding acquisitionand were performed by Xiaoguang Luo. And all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The data used in this study were obtained from the NHANES, conducted by the National Center for Health Statistics (NCHS) with appropriate ethical approval. The studies involving human participants were reviewed and approved by NCHS and the Research Ethics Review Board (ERB). Written informed consent for participation was not required for this study following the national legislation and the institutional requirements.
Consent for publication
Not applicable.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Four different statistical models were utilized to examine the association between blood selenium and PD and demonstrate evidence of negative linear associations from different perspectives.
• RCS models were constructed and showed a nonlinear relationship between PD and blood selenium.
• Subgroup analyses to further analyze the study results and identify any special subgroups in our survey.
• High levels of blood selenium may have a protective effect against PD.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zeng, Z., Cen, Y. & Luo, X. Association between blood selenium with parkinson’s disease in the US (NHANES 2011–2020). Environ Sci Pollut Res 30, 117349–117359 (2023). https://doi.org/10.1007/s11356-023-30337-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30337-7