Abstract
Iodine is a micronutrient essential for maintaining normal body functioning, and the consumption depends on the distribution in the environment, and insufficient or excessive intake results in thyroid dysfunction. The purpose of this review was to evaluate the correlation between iodine concentration in drinking water and the iodine status of the population. The systematic review was conducted following the PRISMA guidelines and was registered at the International Prospective Register of Ongoing Systematic Reviews (CRD42019128308). A literature search was conducted using MEDLINE/PUBMED (National Library of Medicine), LILACS (Latin-American and Caribbean Literature on Health Sciences), and Cochrane Library, June 2021. The quality of the studies was assessed by a checklist for cross-sectional studies developed by Joanna Briggs Institute. The initial search identified 121 articles, out of which ten were included in this systematic review, and five were included in the meta-analysis. Among the articles listed, six adopted cutoff points to classify the iodine content in the drinking water. The study identified median iodine concentration in drinking water from 2.2 to 617.8 μg/L and the correlation between iodine concentration in drinking water and urinary iodine concentration was 0.92, according to meta-analysis. Furthermore, the iodine status was correlated to the iodine content in water. The determination of a cutoff point can contribute to the implementation of iodine consumption control measures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iodine is an essential micronutrient required to maintain the normal functioning of the thyroid [1]. Insufficient consumption of this micronutrient results in the occurrence of iodine deficiency disorders (IDD), of which the most severe are goiter and child cretinism [1]. Also, mild or moderate levels of deficiency can lead to milder damage, such as complications during pregnancy and delayed development of children [2].
As a strategy for IDD control, food fortification was implemented in many countries, particularly salt iodization, resulting in a significant reduction of IDD in countries with good iodized salt coverage [3]. According to Global Fortification Data Exchange, as of 2020, 129 countries had mandatory fortification of salt [4].
On the other hand, excessive iodine intake is associated with a higher risk of autoimmune thyroiditis, hypothyroidism, and goiter [5,6,7]. Moreover, iodine excess is a risk factor for the development of thyroid cancer, as evidenced in a retrospective analysis of 1170 patients with thyroid nodules [8]. Thus, excessive iodine intake also needs to be monitored.
Iodine status depends on ecological conditions since the environment is a determinant factor of intake [1, 9]. In regions with a low concentration of iodine in water, the adoption of the salt iodination policy can prevent IDDs. However, there is a lack of strategies to control excessive consumption in places where the micronutrient is abundant in nature [6, 7].
Considering that excessive consumption constitutes a risk as well as low consumption and that iodine content in drinking water can be a determinant of the iodine status, this review aims to assess the correlation between iodine concentration in drinking water and iodine status of the population.
Methods
Search Strategy
This systematic review was conducted following the recommendations of the Preferred Reporting Items for Systematic Reviews (PRISMA) [10]. The record was registered with the open-access database International Prospective Register of Ongoing Systematic Reviews (PROSPERO) with the identification of CRD42019128308. The review aimed to answer the following research question: “Is the concentration of iodine in drinking water correlated with the iodine status of the population?” To define this question, the PECOS criteria were used, as shown in Table 1 [11].
We identified the articles in the databases Publisher Medline (PubMed), Latin American and Caribbean Literature in Health Sciences (LILACS), and Cochrane Library in June 2021, without date delimitation. The search terms used were determined by the system of Medical Subject Headings (MeSH) and used in the English language, in the following combination: Iodine AND Nutritional Status AND Drinking Water. The “humans” filter was selected in PubMed and LILACS. In addition, in the reverse search, we examined the bibliographies of articles included.
Search Results Screening and Data Extraction
The identified studies were exported to Microsoft Excel to remove duplicates and select by reading titles and abstracts. The selection of the articles, conducted through the reading of titles and abstracts and after the full text, was done by two independent researchers and, in case of disagreement, a third researcher was consulted. The studies were systematized according to year, authorship, place of origin, target population, sample size, iodine content in water, urinary iodine content, and/or goiter prevalence, as well as the possible association between variables.
Inclusion and Exclusion Criteria
Original articles that evaluated the relationship between the nutritional status of iodine according to urinary iodine or the presence of goiter and the iodine content in water were included. Articles about supplementation, biofortification, or fortification of food, soil, or water were excluded. Equally, study protocols, editorials, review articles, and those published in languages other than English were excluded.
Assessment of Study Quality
Two reviewers independently graded the methodological quality of the selected studies by the checklist for cross-sectional studies developed by Joanna Briggs Institute (JBI) and collaborators [12]. This tool consists of eight items that assess the presence of the inclusion criteria, description of the sample, adequate measure of exposure, use of objective and standardized criteria to measure the condition, identification of confounding factors and their statistical treatment, adequate measure of the results, and use of appropriate statistics. The results of this appraisal were used to inform the synthesis and interpretation of the results of the studies.
The cutoff point suggested by Costa et al. (2014) [13] was adopted for the risk of bias classification, where the percentage of affirmative (“yes”) responses ≥ 70% is considered low risk of bias, between 50 and 69% moderate, and < 50% high. The risk of bias was not used as an inclusion criterion.
Meta-analysis
Articles that presented the correlation coefficients between iodine concentration in drinking water and urinary iodine concentration (UIC) were included in the meta-analysis. The generic inverse-variance pooling method was used to combine correlations from different studies into one pooled correlation estimate [14]. The correlations were systematized and the meta-analysis was conducted in software RStudio (IDE) version 4.0.4 with the metacor function included in the meta package. In addition, the information was plotted on a graph with the function forest [15]. To detect studies that contribute to heterogeneity and the overall result, the Baujat plot was evaluated with the InfluenceAnalysis function of the dmetar package [16]. The plot’s horizontal axis illustrates study heterogeneity whereas the vertical axis illustrates the influence of a study on the overall result. Studies that fall to the top right quadrant of the plot contribute most to both these factors [16].
Results
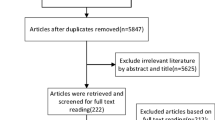
The search strategy identified 121 studies and the reverse search included two studies. Fifty-seven duplicates were excluded. We excluded 21 studies after screening the titles and abstracts. A total of 43 studies remained for full-text screening, after which 33 studies were excluded leaving ten studies to be included in the systematic review. Five studies that showed the correlation between iodine concentration in drinking water and urinary iodine concentration were included in the meta-analysis (Fig. 1).
The included studies date from the year 2005 [17] to 2020 [18], of which nine have Asian and one European origin. We included articles that assessed the iodine content of water and its effect on population in school-age, women of reproductive age, pregnant, and lactating women. The number of individuals assessed ranged from 310 [18] to 4656 [17]. The median concentration of iodine in drinking water ranged from 2.2 μg/L [19] to 617.8 μg/L [20], both in China (Table 2). Among the ten studies, six had cutoff points to classify the iodine content in drinking water, while the others worked with continuous data (Table 3).
Chandra et al. (2005) [17] investigated the total goiter rate, urinary iodine, and thiocyanate excretion pattern of 4656 school children (6–12 years), iodine content in edible salt and drinking water in West Bengal (India). Iodine content in drinking water was 22–119 μg/L, and 55.6% of salt samples had iodine levels above the recommended (15 mg/kg). The same authors published an article the following year [21], which evaluated a total of 2050 school children (6–12 years). The authors observed adequate iodine consumption (UIC 200 μg/L) and a high prevalence of goiter (33.1%). The main conclusion was that this prevalence may be for the consumption of dietary goitrogens.
Lv and colleagues [20] evaluated iodine nutrition and goiter status of a total of 1259 children (8–10 years) that live areas with mildly or excessive iodine in drinking water (> 150 μg/L) in Hebei Province of China. Children’s UIC was excessive (418.8 μg/L). In 2013, the author published another study [22] that evaluated the contributions of drinking water and iodized salt on children’s iodine nutrition to refine strategies and correct the excessive iodine intake in these areas. The UIC also was excessive (518.1 μg/L). In 2015 [19], the authors assessed the effectiveness of removing iodized salt on reducing the iodine excess in populations living in high-iodine areas. The authors suggested a cutoff point of 110 g/L of iodine in drinking water to maintain a safe level of iodine in the diet. In areas above the cutoff point, it is necessary to search for other sources of alternative drinking water supplies.
Henjum et al. [23] assessed iodine status (thyroid volume and UIC) and their determinants in 394 Saharawi refugee reproductive age women (15–45 years). Median UIC was 466 (P25-P75: 294–725) μg/L and the goiter prevalence was 22%. The UIC was positively associated with iodine in drinking water. Another study was developed by Sang and colleagues [24] with 384 pregnant Chinese pregnant women and assessed excessive iodine intake. The authors observed that living with high-water iodine content are associated risk factors for subclinical hypothyroidism in pregnant women. Liu et al. [25] developed a study with lactating women, to evaluate iodine nutrition in both lactating women and their infants and the prevalence of thyroid disease in areas with different levels of iodine in water. The main result was that the prevalence of thyroid disease in lactating women was higher in the iodine excess area.
Li and collaborators [26] assessed 1594 Chinese schoolchildren (8–10 years) and examined the urinary iodine excretion and the iodine content of drinking water and salt samples. In areas with an iodine content higher than 150 mg/L in the drinking water, the schoolchildren had more than adequate or excessive iodine intake. The last study included in this review was also carried out with Chinese schoolchildren (7–12 years) [18]. This study assessed the iodine nutrition, thyroid function, and influencing factors for thyroid abnormalities in 310 children from areas with different concentrations of water iodine. In areas with iodine water concentration ≥ 300 μg/L, the median UIC in children was higher than that in other groups, and the prevalence of thyroid nodules and the thyroid goiter rate.
The studies included in this review had a low risk of bias, with positive responses greater than 70%, indicating optimal methodological quality [13]. According to the quality analysis of the studies, five did not report the identification and treatment of confounding factors. However, the other items were attended in all studies. Therefore, the authors consider that all articles had the methodological quality to be included (Fig. 2).
The correlation between iodine concentration in drinking water and urinary iodine concentration was 0.92, according to meta-analysis considered the result of the random-effects model (Fig. 3) [17, 19, 21, 22, 26]. Despite the high heterogeneity, all studies included in the meta-analysis showed a direct and strong correlation, corroborating the systematized result. Examining the Bajaut plot, two studies [17, 26] contribute more to heterogeneity, and one study influence more the meta-analysis overall result (Fig. 4) [17]. However, the exclusion of the aforementioned studies did not reduce heterogeneity.
Discussion
Iodine concentration in drinking water was correlated with iodine nutrition status and thyroid dysfunction. The main related outcomes were goiter, hypothyroidism, and hyperthyroidism [17, 21, 22, 26]. Thus, studies evaluating the iodine concentration in drinking water have found a significant and positive correlation with urinary iodine concentration [17, 21, 22, 26]. In addition, salt available for home consumption was iodized, even in regions with a high-iodine concentration in water. Therefore, combined consumption of drinking water with excess iodine and iodized salt can lead to excessive iodine intake.
A survey conducted with children from four regions of China reported different concentrations of iodine in drinking water. The authors indicated that the consumption of water in some regions supplies the recommended iodine intake for the population. Therefore, the authors recommended stopping the supply of iodized salt in areas with a water iodine concentration of 150–300 μg/L [18]. The water iodine contributed with 58.19% of children’s iodine intake and the median urinary concentration was 209.15 μg/L, slightly higher than the appropriate level of iodine (100–199 μg/L) [1, 18].
Wang et al. [18] assessed the iodine status, thyroid function, and influencing factors for thyroid abnormalities in children from an area with concentrations of drinking water iodine above 300 μg/L, where the supply of iodized salt has been stopped and concluded that this intervention alone is not enough to avoid thyroid dysfunction in areas with excess water iodine. Therefore, the urinary iodine concentration (UIC) was excessive (≥ 300 μg/L) [1].
World Health Organization classifies as appropriate the 100–199 μg/L urinary iodine ranges for adults and school-age children, 150–249 μg/L for pregnant women, ≥ 100 for lactating women, and children aged less than 2 years [1]. A study conducted in Spain with women of reproductive age identified that the increase of 1 μg/L of iodine in drinking water is able to increase the urinary concentration of iodine by 1.32 μg/L, thus, consumption between 1.5 and 2.5 L of water for children and pregnant women would be able to supply the need for iodine [23]. Considering that water consumption can exceed two liters per day, adequacy levels can easily be exceeded, depending on the region of residence [27].
Regarding the place assessed, the studies were mostly conducted in China, this is due to the fact that the country presents a continental dimension and has variations of mineral composition of water and that many regions have a high content of iodine in the water, a determinant factor of the iodine status in the population [19].
Lv et al. [19] also showed that after the interruption of the use of iodized salt, iodine levels in the urine of the population remained high, exceeding the cutoff point of 300 μg/L [1, 19]. This study was conducted in 12 villages of five cities in China and assessed whether the decrease in iodized salt consumption would be able to reduce excessive iodine in the population, but no effect was observed. Only regions with iodine content in drinking water less than 110 μg/L presented adequate iodine intake [19]. According to the National Criteria for Classifying High Iodine Regions of China, this value is safe (< 150 μg/L) and provides adequate iodine intake [28].
In China, an iodine concentration of 150 μg/L in water is used to define areas at risk of excessive iodine intake. According to National Criteria for Classifying High Iodine Regions, a town with median water iodine (MWI) between 150 and 300 μg/L in drinking water is classified as a high-iodine town. A town with MWI above 300 μg/L is classified as a high-iodine endemic town [28]. This reference is applied to groundwater and drinking water and the iodine intake for populations in these areas is likely to be excessive and endemic goiter may occur.
In addition, some studies have found a relationship between iodine concentration and the presence of goitrogenic substances in water, such as thiocyanate, that was responsible for increasing the prevalence of goiter. Studies by Chandra et al. [17, 21] in 2005 and 2006 found a prevalence of goiter in the population of 38.2% and 33.1%, respectively, classifying it as a severe public health problem (total goiter rate ≥ 30%) [1]. The authors attributed the high prevalence of goiter to the presence of organic matter of sedimentary rocks present in the water, especially in places close to rivers and the sea [17, 21]. These goitrogens are natural or synthetic compounds present in water or food, industrial products, and pesticides that are able to interfere in the functioning of the thyroid and are called thyroid disruptors or endocrine disruptors [29].
Likewise, the prevalence of goiter was assessed by Henjum et al. [23]. The authors identified that 22% of the assessed population presented with goiter, which was considered a moderate problem of public health (total goiter rate of 20.0–29.9%) [1]. Increased thyroid volume was attributed to excessive consumption of iodine in the water. Two other studies have found a low prevalence of goiter and are considered mild public health problems (total goiter rate of 5.0–19.9%) [1, 20, 26]. A more detailed assessment is necessary, as the studies cited evaluated children and this group does not have specific parameters for the assessment of goiter.
Only two studies compared the groups according to the classification of iodine content in drinking water [19, 20]. Lv et al. [20] assessed schoolchildren and found 11% of goiter prevalence in twenty villages, with median iodine in drinking water > 150 μg/L, whereas for villages with a median content of < 150 μg/L, the prevalence was 10.8% without differences between the two groups.
However, a study that evaluated lactating women and infants in areas considered deficient, sufficient, and excessive iodine concentrations found UIC values of 51.30 μg/L, 282.42 μg/L, and 822.51 μg/L, respectively [19]. Compared with the region group sufficient in iodine, UIC values were significantly lower in the iodine-deficient group in drinking water and higher in the iodine excessive group; likewise, differences were observed in infants [19].
It is important to emphasize that the studies used different cutoff points and besides that Liu et al. [25] used two references to classify iodine content in water, stratifying in three groups, being more sensitive to identify areas at risk for IDD [19, 20, 25]. Despite the elevated values observed, WHO has not yet defined a guideline value for iodine in drinking water [18, 24, 30].
The limitation of this review was that the cutoff values used to classify the iodine content in water are different among the included studies. In addition, the majority of the studies were conducted in China and India, limiting representativeness to other regions of the world. However, the countries cited above have a large territorial extension, which guarantees a diversity of environmental conditions. Another limiting point is the iodine intake of foods, especially processed foods because the iodine content after industrial processing has no relation to the water used to cultivate local foods. On the other hand, the studies represented different physiological groups, including pregnant women, lactating women, schoolchildren, and women of childbearing age.
Final Remarks
Iodine status is directly correlated to the iodine content in water. Future studies can use the iodine concentration in drinking water as an indicator of dietary intake. This review indicates that excessive iodine in drinking water was the key contributor to the population’s excessive iodine intake.
In addition, the determination of a cutoff point that can be reproduced to other locations around the world will contribute to the implementation of measures to control iodine consumption in different populations.
References
WHO. Assessment of the iodine deficiency disorders and monitoring their elimination. Geneva; 2007.
Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. THYROID. 2017 [cited 2018 Nov 30];27(3). Available from: https://www.liebertpub.com/doi/pdf/https://doi.org/10.1089/thy.2016.0457
Dold S, Zimmermann MB, Jukic T, Kusic Z, Jia Q, Sang Z, et al. Universal salt iodization provides sufficient dietary iodine to achieve adequate iodine nutrition during the first 1000 days: a cross-sectional multicenter study. J Nutr. 2018;148(4):587–98. Available from: https://academic.oup.com/jn/article/148/4/587/4965927
Global Fortification Data Exchange. Chart: Year when food fortification mandated.
Teng X, Shan Z, Chen Y, Lai Y, Yu J, Shan L et al (2011) More than adequate iodine intake may increase subclinical hypothyroidism and autoimmune thyroiditis: a cross-sectional study based on two Chinese communities with different iodine intake levels. Eur J Endocrinol 164(6):943–950
Leung AM, Braverman LE (2014) Consequences of excess iodine. Nat Rev Endocrinol 10(3):136–142
Katagiri R, Yuan X, Kobayashi S, Sasaki S (2017) Effect of excess iodine intake on thyroid diseases in different populations: a systematic review and meta-analyses including observational studies. PLoS One 12(3):1–24
Kim HJ, Kim NK, Park HK, Byun DW, Suh K, Yoo MH et al (2017) Strong association of relatively low and extremely excessive iodine intakes with thyroid cancer in an iodine-replete area. Eur J Nutr 56(3):965–971
Duan L, Wang W, Sun Y, Zhang C. Iodine in groundwater of the Guanzhong Basin, China: sources and hydrogeochemical controls on its distribution. Environ Earth Sci. 2016;75(11).
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M et al (2012) Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: Elaboration and explanation. BMJ 349(February 2012):1–25
Morgan RL, Whaley P, Thayer KA, Schünemann HJ. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int. 2018 Dec;121:1027–31. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0160412018302046
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Qureshi R, Mattis P, Lisy K MP-F. Systematic reviews of etiology and risk. In: JBI Manual for Evidence Synthesis. JBI; 2020.
Costa AB, Zoltowski APC, Koller SH, Teixeira MAP. Construção de uma escala para avaliar a qualidade metodológica de revisões sistemáticas. Cien Saude Colet. 2015 Aug;20(8):2441–52. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1413-81232015000802441&lng=pt&tlng=pt
Mathias Harrer, Pim Cuijpers, Toshi A. Furukawa, David D. Ebert. Chapter 4 Pooling effect sizes | doing meta-analysis in R. Taylor & Francis eBooks; 2021 [cited 2021 Dec 7]. 1–500 p. Available from: https://bookdown.org/MathiasHarrer/Doing_Meta_Analysis_in_R/pooling-es.html#pooling-cor
Balduzzi S, Rücker G, Schwarzer G (2019) How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 22(4):153–160
Baujat B, Mahé C, Pignon J-P, Hill C. A graphical method for exploring heterogeneity in meta-analyses: application to a meta-analysis of 65 trials. Stat Med. 2002 Sep 30;21(18):2641–52. Available from: https://onlinelibrary.wiley.com/doi/https://doi.org/10.1002/sim.1221
Chandra AK, Tripathy S, Ghosh D, Debnath A, Mukhopadhyay S (2005) Iodine nutritional status & prevalence of goitre in Sundarban delta of South 24-Parganas, West Bengal. Indian J Med Res 122(November):419–424
Wang Y, Cui Y, Chen C, Duan Y, Wu Y, Li W et al (2020) Stopping the supply of iodized salt alone is not enough to make iodine nutrition suitable for children in higher water iodine areas: a cross-sectional study in northern China. Ecotoxicol Environ Saf. 188(September):109930
Lv S, Zhao Y, Li Y, Wang Y, Liu H, Li Y et al (2015) Impact of removing iodized salt on the iodine nutrition of children living in areas with variable iodine content in drinking water. Eur J Nutr 54(6):905–912
Lv S, Chong Z, Du Y, Ma J, Rutherford S, Jia L et al (2012) An epidemiological survey of children’s iodine nutrition and goitre status in regions with mildly excessive iodine in drinking water in Hebei Province, China. Public Health Nutr 15(07):1168–1173
Chandra AK, Tripathy S, Ghosh D, Debnath A, Mukhopadhyay S (2006) Goitre prevalence and the state of iodine nutrition in sundarban delta of north 24-parganas in West Benegal. Asia Pac J Clin Nutr 15(3):357–361
Lv S, Zhao J, Rutherford S, Du Y, Xu D, Wang Y et al (2013) Drinking water contributes to excessive iodine intake among children in Hebei, China. Eur J Clin Nutr 67(9):961–965
Henjum S, Barikmo I, Strand TA, Oshaug A, Torheim LE (2012) Iodine-induced goitre and high prevalence of anaemia among Saharawi refugee women. Public Health Nutr 15(8):1512–1518
Sang ZN, Wei W, Zhao N, Zhang GQ, Chen W, Liu H et al (2012) Thyroid dysfunction during late gestation is associated with excessive iodine intake in pregnant women. J Clin Endocrinol Metab 97(8):1363–1369
Liu L, Wang D, Liu P, Meng F, Wen D, Jia Q et al (2015) The relationship between iodine nutrition and thyroid disease in lactating women with different iodine intakes. Br J Nutr 114(9):1487–1495
Li WH, Dong B Sen, Li P, Li YF. Benefits and risks from the national strategy for improvement of iodine nutrition: a community-based epidemiologic survey in Chinese schoolchildren. Nutrition. 2012;28(11–12):1142–5. Available from: https://doi.org/10.1016/j.nut.2012.04.014
WHO. Nutrients in Drinking Water. Geneva; 2005.
China Ministry of Health. Classification of areas with high iodine in water and endemic areas of goiterNo Title. Beijing;
Brucker-Davis F, Hiéronimus S, Fénichel P (2016) Thyroïde et environnement. Press Medicale 45(1):78–87
WHO. Iodine in Drinking Waters. Geneva; 2003.
Funding
We would like to thank the Coordination of Improvement of Higher Education Personnel—Brazil (CAPES)—Financing Code 001. National Council for Scientific and Technological Development (CNPq), case 408295/2017–1. Foundation of Support and Research of the State of Minas Gerais (FAPEMIG) case APQ-03336–18.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Azevedo, F.M., Machamba, A.A.L., Candido, A.C. et al. Correlation Between Drinking Water and Iodine Status: a Systematic Review and Meta-analysis. Biol Trace Elem Res 201, 129–138 (2023). https://doi.org/10.1007/s12011-022-03127-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03127-4