Abstract
The effects of sublethal cadmium concentrations on oxidative stress parameters were evaluated in Rhamdia quelen. The fish were exposed to 0.44, 236, and 414 μg l−1 cadmium for 7 and 14 days, followed by the same time periods for recovery. Enzymes, such as catalase (CAT), superoxide dismutase (SOD), and glutathione S-transferase (GST), and indicators of oxidative stress, such as thiobarbituric acid-reactive species (TBARS) and protein carbonyl, were verified in fish tissues. In addition, the accumulation of cadmium was evaluated in these tissues. Our results indicate that CAT and GST levels decreased in gills after exposure periods associated with increased TBARS levels. In hepatic tissue, CAT, GST, TBARS, and protein carbonyl levels increased after 7 days of exposure, whereas SOD activity decreased after exposure for 14 days. In the kidney, TBARS levels decreased after exposure for 7 days and increased after exposure for 14 days. During the recovery periods, some variations persisted in gills, liver, and kidney. Cadmium accumulation was most significant in liver, followed by kidney and gills. These results indicate that cadmium concentrations studied invoke a stress response in silver catfish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Cadmium is one of the most toxic metals in the environment, does not serve a beneficial biologic function in higher organisms, and is extremely toxic even at very low concentrations (Soares et al. 2008). Cadmium is also a cumulative pollutant found in aquatic ecosystems, making fish particularly vulnerable to cadmium exposure (Bhakta and Munekage 2008). Fish metal accumulation appears to occur predominantly in metabolically active tissues, such as kidney, liver, gills, and throughout the digestive tract (Cinier et al. 1999; Soares et al. 2008). Studies exploring bioaccumulation and biochemical parameters in fish tissues can help elucidate the toxic effects of metals on aquatic organisms.
Several studies have associated heavy metals with the ability to produce reactive oxygen species (ROS), resulting in lipid peroxidation and variations in the level of antioxidant enzymes culminating in oxidative stress (Roméo et al. 2000; Sanchez et al. 2005). Biomarkers that indicate oxidative stress in various organisms have been proposed to serve an important role in the biomonitoring of toxic metal pollution in aquatic ecosystems (Sies 1993; Monserrat et al. 2007). Lipid peroxidation and protein carbonyl formation are two perturbations that are a consequence of oxidative stress. Lipid peroxidation results in the production of lipid radicals and in the subsequent formation of a complex mixture of lipid degradation products (Almroth et al. 2005; Gioda et al. 2007). Protein carbonyl content is the most general indicator and the most commonly used marker of protein oxidation (Dalle-Donne et al. 2003). However, this biomarker has been used most commonly in research on oxidative stress in humans (Almroth et al. 2005; Parvez and Raisuddin, 2005), and few reports are available regarding its use in fish exposed to toxic chemicals, such as metals.
All cells in eukaryotic organisms contain powerful antioxidant enzymes. The three major classes of antioxidant enzymes are superoxide dismutases (SOD), catalases (CAT), and glutathione peroxidases (GPx). The activities of CAT, SOD, and GPx are parameters that are frequently studied when fish are exposed to metals. These enzymes belong to the antioxidant defense system, which has evolved to protect the organism by directly scavenging oxygen intermediates as superoxide radicals and hydrogen peroxide and converting them to less reactive species (Sampaio et al. 2008). Glutathione S-transferase (GST) and GPx belong to the glutathione (GSH) system, which is one of the most important antioxidant systems in fish (Zirong and Shijun 2007). GST catalyzes the conjugation of GSH with a variety of electrophilic metabolites involved in the detoxification of both reactive intermediates and oxygen radicals (Monteiro et al. 2006).
Silver catfish (Rhamdia quelen) can be found in waters from Southern Mexico to Central Argentina, and their husbandry is spreading across Southern Brazil (Gomes et al. 2000) because these fish can survive cold winters and grow rapidly in the summer (Barcellos et al. 2003). This species shows good productivity and it is acceptable to consumers; however, there exist only data about cadmium effects from R. quelen larvae (Benaduce et al. 2008). Therefore, the objective of this study was to investigate possible consequences of cadmium exposure and the degree to which it accumulates in tissues. Another point was to verify possible cadmium alterations on some oxidative stress parameters in tissues of silver catfish.
Materials and Methods
Chemicals
All reagent-grade chemicals were purchased from Sigma (St. Louis, MO).
Fish
Silver catfish juveniles from both sexes were obtained from a commercial fish farm near Santa Maria (Rio Grande do Sul, Brazil). Fish (weight 23.9 ± 3.1 g, length 13.4 ± 0.5 cm) were acclimated in 25-l tanks under laboratory conditions for 15 days. The fish were subsequently placed in continuously aerated tap water with a static system under a natural photoperiod (12 h light and 12 h dark) and received feed once a day (commercial fish pellets with 42% crude protein; Supra, Brazil). Water parameters were measured every day during the acclimation and experimental period and were as follows: temperature 23.7 ± 1.0°C, pH 7.6 ± 0.2, dissolved oxygen concentration 6.8 ± 0.5 mg l−1, nonionized ammonia 1.23 ± 0.05 μg l−1, nitrite 0.04 ± 0.01 mg l−1, hardness 12.0 ± 1.15 mg l−1 CaCO3, and alkalinity 39.0 ± 3.2 mg l−1 CaCO3. The experimental protocol was approved by Animal Health Committee of the Federal University of Santa Maria, RS, Brazil, by number 23081.010370/2007-82.
Exposure to Cadmium Concentrations
A previous experiment obtained a 96-h LC50 96 of 1.7 mg l−1 cadmium for silver catfish. The LC50 value for cadmium used in this experiment was obtained in a similar experiment with the same metal (Pretto et al. 2010). Fish were transferred to 45-l plastic aquaria with controlled aeration and temperature. The experiments were conducted as a random-blocks design with two replications in a factorial scheme. Factor A were the cadmium concentrations, and factor B were the exposure and recovery periods. Groups of 12 fish/aquarium were exposed to initial cadmium concentrations of 236 or 414 μg l−1 (approximately 10 and 20% of the LC50) for 7 and 14 days, and the control groups were kept in water in the absence of the cadmium. The recovery periods also were 7 and 14 days. In each aquarium were placed 12 individuals (duplicate). Six individuals were used to evaluate the effect of exposure, and six individuals were transferred to cadmium-free water to study the recovery responses in silver catfish. From each aquarium two fish were collected for metal analysis and four fish for biochemical analysis during the exposure and recovery periods. The total number for treatment was 8 for biochemical analysis and 4 for metal accumulation.
Stock solutions were prepared by dissolving cadmium chloride (CdCl2·10H2O) in water and added it to the experimental aquarium. During the exposure period of 7 days, the water was not changed during the experiment, and the metal was added to the water only at the beginning. The actual concentrations for cadmium at day 1 were 0.44 ± 0.02, 236 ± 4, and 414 ± 6 μg l−1, respectively. At day 7, cadmium concentrations were 0.42 ± 0.01, 70 ± 4, and 132 ± 14 μg l−1, respectively. However, for the exposure period of 14 days, water was changed in middle of exposure (day 7), and metal was added again to replace those removed during change of water. The water cadmium concentrations verified in the exposure period of 14 days (at days 1 and 14) were similar those observed in the 7-day period. In this study, a biofilter was used in each aquarium to maintain water quality, and uneaten food and feces were siphoned daily. Fish were fed in excess once a day with commercial fish pellets. There were no mortalities throughout the experimental periods (7 and 14 days). Physicochemical characteristics of the water were as follows: temperature 23.8 ± 0.2°C, pH 7.9 ± 0.1, dissolved oxygen 6.9 ± 0.3 mg l−1, nonionized ammonia 3.42 ± 0.4 μg l−1, nitrite 0.04 ± 0.01 mg l−1, hardness 15.0 ± 2.1 mg l−1 CaCO3, and alkalinity 45.5 ± 2.8 mg l−1 CaCO3.
After each exposure period, the fish were killed by cutting the spinal cord behind the operculum, and the tissues to be assayed (gills, liver, and kidney) were removed and quickly placed on ice and frozen at −70°C for analysis of oxidative stress parameters and accumulation in tissues. The remaining fish were maintained in cadmium-free water for 7 and 14 days to recover (after 7 and 14 days of exposure, respectively). Control fish were also sampled for each experimental period.
Water and Tissue Ion Content Analysis
Two fish were sampled from each aquarium (replicate) after the exposure and recovery periods (four samples for treatment) to perform analysis of cadmium accumulation in various fish tissues. The gills, liver, and kidney were removed, washed in distilled water, dried with filter article, weighed, and stored in plastic vials at −70 °C for subsequent analysis. Tissue samples were digested (3 times for 1 min) in a microwave oven, after which 2 ml 65% HNO3 were added and the samples stored overnight. The resulting solutions were subsequently diluted to 10 ml with ultra-pure water. The total content of cadmium in the liver, kidney, gills and water samples was measured by graphite furnace–atomic absorption spectrophotometry (GF-AAS) and expressed in units μg g−1 tissue and μg l−1 water, respectively. Water samples were measured in all periods and concentrations tested according to information cited previously. The accuracy of the results was assessed by the analysis of certified reference material (dogfish liver DOLT-3 and fish protein DORM-3 from National Research Council Canada and trace elements in water 1643d from National Institute of Technology and Standards). Sample blanks were obtained using only the reagents for sample treatment. They were submitted to the same treatment as the samples. To check possible variations in the results, for every 10 determinations of Cd in the samples, a certified reference material was analyzed. If necessary, the calibration curve was adjusted accordingly.
Antioxidant Enzymes
All enzyme activities were measured spectrophotometrically. Samples of frozen tissue (liver, kidney, and gills) were quickly weighed and homogenized in 20 mM potassium phosphate buffer (pH 7.5) using a Potter-Elvejhem glass/Teflon homogenizer. Samples were then centrifuged at 10,000×g for 10 min at 4°C and assayed for antioxidant enzymes activity. The protein content in the liver, gills, and kidney was measured using the protocol described by Lowry et al. (1951), with bovine serum albumin serving as the protein standard.
CAT (EC 1.11.1.6) activity in liver, gill, and kidney tissues was assayed according to the work of Nelson and Kiesov (1972). The assay mixture consisted of 2.0 ml potassium phosphate buffer (50 mM [pH 7.0]), 0.05 ml H2O2 (0.3 M), and 0.05 ml of the homogenate. To determine catalase activity in the kidney, 0.1 ml H2O2 (0.3 M) was used. The change in the H2O2 absorbance was measured at 240 nm. Catalase activity was expressed in units μmol mg protein−1 min−1.
GST (EC 2.5.1.18) activity was measured in the liver and gills using a procedure described by Habig et al. (1974) that involved 1-chloro-2,4-dinitrobenzene (CDNB) as substrate. The assay mixture contained 1 mM CDNB (in ethanol), 10 mM GSH, 20 mM potassium phosphate buffer (pH 6.5), and 0.05 ml of the tissue homogenates. Enzyme activity was calculated from the changes in absorbance at 340 nm using a molar extinction coefficient of 9.6 mmol−1 cm−1. One unit GST activity was defined as the amount of enzyme required to catalyze the conjugation of 1 μmol CDNB with GSH/minute at 25°C.
Measurements of SOD activity were performed for hepatic tissue based on the inhibition of the radical superoxide reaction with adrenalin as described by McCord and Fridovich (1969). In this method, SOD present in the sample competes with the detection system for radical superoxide. A unit of SOD is defined as the amount of enzyme that inhibits the rate of oxidation of adrenalin by 50%. The oxidation of adrenalin leads to the formation of the colored product, adrenochrome, which is detected using a spectrophotometer. SOD activity is determined by measuring the rate of adrenochrome formation, observed at 480 nm, in a reaction medium containing glycine-NaOH (50 mM [pH 10]) and adrenalin (1 mM).
TBARS Determination
Tissue samples (liver, kidney, and gills) were weighed and homogenized as described previously in 20 mM potassium phosphate buffer (pH 7.5) using a Potter-Elvejhem glass/Teflon homogenizer. Samples were then centrifuged at 10,000 g for 10 minutes at 4°C and analyzed using TBARS assay. Aliquots of the homogenate aliquots were added to a solution containing 10% trichloroacetic acid and 0.67% TBA and were adjusted to a final volume of 1.0 mL. The reaction mixture was placed in a microcentrifuge tube and incubated for 15 minutes at 95°C. After cooling, the reaction was centrifuged at 5000×g for 10 min, after which the optical density at 532 nm was determined. The TBARS level was expressed in units of nmol MDA mg protein−1 according to Buege and Aust (1978).
Carbonyl Assay
The liver tissue was homogenized in 10 volumes (w/v) of 10 mM Tris-HCl buffer (pH 7.4) using a glass homogenizer. Protein carbonyl content was assayed by a procedure described previously by Yan et al. (1995) with slight modifications. Soluble protein (1.0 ml) was reacted with 10 mM DNPH in 2 N hydrochloric acid and incubated for 1 h in darkness at room temperature. A 0.5-ml aliquot of a denaturing buffer (150 mM sodium phosphate buffer [pH 6.8] containing SDS 3.0%), 2.0 ml heptane (99.5%) and 2.0 ml ethanol (99.8%) were then added sequentially to the mixture, which was vortexed for 40 s and centrifuged 3000×g for 15 min. The protein isolated from the interface of the aforementioned reaction was washed twice by resuspension in ethanol/ethyl acetate (1:1) and then suspended in 1 ml denaturing buffer. The carbonyl content was then measured spectrophotometrically (Femto Scan spectrophotometer) at 370 nm. This assay was performed in duplicate, and two blank tubes incubated with 2 N HCl in the absence of DNPH were included for each sample. The total carbonylation was calculated using a molar extinction coefficient of 22.000 M−1 cm−1.
Statistical Procedures
The data for each of these biochemical parameters were compared using two-way analysis of variance followed by Duncan’s test (p ≤ 0.05). Results obtained for biochemical parameters (n = 8) and bioaccumulation (n = 4) were expressed as mean ± SD. Statistical tests were performed using GraphPad PRISM software (San Diego, CA).
Results
The mean values of cadmium concentrations in water samples collected from the first day of exposure (7 and 14 days) were 0.44 ± 0.02, 236 ± 4, and 414 ± 6 μg l−1, respectively. During recovery periods, metal concentration means were 0.40 ± 0.02, 0.40 ± 0.02, and 0.55 ± 0.06 μg l−1, respectively. According to data showed in Table 1 an increase of cadmium occurred in liver, kidney, and gills of silver catfish after exposure periods of 7 and 14 days. In kidney, the accumulation pattern was similar to that found in liver, which demonstrated a significant increase after exposure periods. The cadmium concentration in gills was similar for both exposure periods, with a significant increase observed with a concomitant enhancement in the waterborne cadmium concentration (Table 1). After a recovery period of 14 days, we observed elimination of cadmium from the liver in the control and the 236 μg l−1 concentration, but this was not found in the 414 μg l−1 concentration. However, we did observe a decrease of cadmium in kidney of approximately 38%. In the groups of fish exposed to cadmium and subsequently allowed to recover for periods of 7 or 14 days, we observed an approximate 46% and 50% elimination of cadmium, respectively, from gill tissues.
Fish exposed to cadmium exhibited increased CAT activity in liver at both cadmium concentrations tested (236 and 414 μg l−1) after 7 days (70–56%) and 14 days (45–49%) (Table 2). However, after a 7-day period of recovery, CAT activity in this tissue decreased by approximately 38% in fish exposed to 414 μg l−1 cadmium compared with the unexposed control fish. There were no changes in CAT activity in hepatic tissue after a recovery period of 14 days. CAT activity decreased in gill after 7- and 14-day periods of exposure to cadmium. This inhibition reached 68% at 236 μg l−1 and 83% at 414 μg l−1 cadmium after exposure for 7 days and approximately 63% after 14 days (Table 2). After a recovery period of 7 days, CAT inhibition in gills remained (41–63%), but the activity of this enzyme was not altered compared with the control values after 14 days of recovery. In kidney, we observed that CAT activity did not change between the assays performed during the exposure and the recovery periods.
Silver catfish maintained at 236 and 414 μg l−1 cadmium for 7 days demonstrated a significant increase in liver GST activity (69.8 and 31.5%, respectively) (Table 3). In addition, when these fish that had been exposed to 414 μg l−1 cadmium were transferred to cadmium-free water for 7 days, we observed a 34% decrease in GST activity. After 14 days, occur alterations in the GST activity only after exposure to 236 μg l−1 cadmium. After the recovery period, liver GST activity returned to levels observed in the control fish liver tissues. In gill, GST activity significantly decreased approximately 63% after 7 and 45 to 52% after 14 days of exposure to 236 and 414 μg l−1 cadmium. Even after the recovery period of 7 days, GST activity in gills remained inhibited (approximately 44%), but these values recovered after 14 days in metal-free water (Table 3).
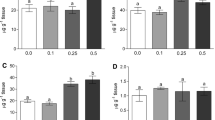
In the present study, changes in the liver SOD activity were also observed. After 7 days in water with cadmium (414 μg l−1), silver catfish exhibited a 22% decrease in SOD activity, and this decrease remained constant even after a recovery period of 7 days (Fig. 1A). After an exposure period of 14 days, SOD was inhibited at both metal concentrations (approximately 34% at 236 μg l−1 and 29% at 414 μg l−1 cadmium). However, SOD values returned to values observed with the control fish after a 14-day recovery period (Fig. 1B).
A SOD activity in liver of R. quelen after 7-day exposure to cadmium and recovery. Data are reported as means ± SDs (n = 8). Letters indicate significant differences among concentrations within the same exposure and recovery time. * Differences between exposure and recovery period by Duncan’s test (p ≤ 0.05). B SOD activity in liver of R. quelen after 14-day exposure to cadmium and recovery. Data are reported as means ± SDs (n = 8). Letters indicate significant differences among concentrations within the same exposure and recovery time. * Differences between exposure and recovery period by Duncan’s test (p ≤ 0.05)
Exposure to cadmium (236 and 414 μg l−1) altered TBARS levels in different tissues of silver catfish. Liver did not show any changes in TBARS levels after a 7-day exposure period (Table 4). However, a significant increase in TBARS levels was observed after 14 days of exposure, with an increase of TBARS of 29 and 62% in fish from the two test groups exposed to 236 and 414 μg l−1 cadmium, respectively. After 7 days in water without cadmium, the increase was approximately 37%, and after 14 days the TBARS levels returned to values observed in control fish. In fish exposed to 236 and 414 μg l−1 cadmium, after 7 days we not observed alteration in the level of TBARS in the kidney, but interestingly, a significant increase (127–150%) was verified after an exposure period of 14 days at both concentrations tested (Table 4). After both recovery periods, we observed that TBARS levels returned to those observed for the control experiments for this tissue. The higher values of gill TBARS levels were obtained when silver catfish were exposed for 7 days to waterborne cadmium (increases of 296 and 273% at 236 and 414 μg l−1, respectively). After a recovery period of 7 days, TBARS levels returned to values observed in fish maintained in water without the addition of cadmium. After 14 days of cadmium exposure, there was a twofold increase in TBARS levels in fish exposed to 236 μg l−1 cadmium. This increase persisted even after the 14-day recovery period (Table 4).
Protein carbonyl formation was observed in liver, and few changes in these levels were observed in this study. After fish were exposed to cadmium (414 μg l−1) for 7 days, we discovered a 47% increase in protein carbonyl formation. In addition, a decrease in protein carbonyl levels in fish previously exposed to 236 μg l−1 cadmium was observed after the 7-day recovery period (Fig. 2A). However, exposure and a 14-day recovery period did not alter protein carbonyl levels in the liver of silver catfish (Fig. 2B).
A Protein carbonyl levels in liver of R. quelen after 7-day exposure to cadmium and recovery. Data are reported as means ± SDs (n = 8). Letters indicate significant differences among concentrations within the same exposure and recovery time. * Differences between exposure and recovery period by Duncan’s test (p ≤ 0.05). B Protein carbonyl levels in liver of R. quelen after 14-day exposure to cadmium and recovery. Data are reported as means ± SDs (n = 8). Letters indicate significant differences among concentrations within the same exposure and recovery time. * Differences between exposure and recovery period by Duncan’s test (p ≤ 0.05)
Discussion
The results obtained from tissue analysis of the certified reference materials (DOLT-3, DORM-3, and NIST 1643d) were in good agreement (recovery of Cd 96 to 102%) with the certified values. In general, the precision was better than 5% (expressed as the relative SD of five consecutive replicate measurements of Cd in the sample).
Data from the present study indicated that cadmium exposure produces a significant accumulation of cadmium in tissues, such as kidney, liver, and gills. Pollutants rarely distribute uniformly in animal body tissues, but they can accumulate to varying degrees in particular target organs, such as liver, gill, or kidney (Cinier et al. 1999). At the end of the 7- and 14-day exposure periods, cadmium had accumulated most significantly in kidney, followed by liver and gill. These results are similar to those reported by Cattani et al. (1996) in Dicentrarchus labrax that were exposed to 0.5 and 5 μg l−1 cadmium in seawater for 7 days. In studies performed in Clarias gariepinus that were exposed for 21 days to cadmium (0.1, 0.2, and 0.4 mg l−1), cadmium accumulated preferentially in kidney, followed by gill, liver, and, finally, muscle tissue (Asagba et al. 2008). Generally, the uptake of cadmium is tissue-specific, and the concentration of cadmium in the tissues is influenced by waterborne metal concentration. In addition, gill, liver, and kidney are the main sites of metallothionein production and metal retention. In these tissues, increased cadmium concentration possibly increased the presence of these metallothionein proteins that are able to bind to the metal (Asagba et al. 2008). An increase in liver and kidney cadmium concentrations was observed in silver catfish after a 7-day recovery period in cadmium-free water. Cinier et al. (1999) also observed, interestingly, that after a 42-day elimination period, the cadmium concentration in kidney of Cyprinus carpio had increased. According to Cinier et al. (1999), this unexpected increase may occur due to a redistribution of cadmium among the tissues before its ultimate excretion. For example, the metal may be transferred from muscle to liver and kidney for excretion during the detoxification process. In our study, the uptake of cadmium was influenced by water cadmium concentrations and certainly appeared to be tissue specific.
Gills are known as a temporary target organ for cadmium accumulation, after which the cadmium is transferred to organs, such as liver, kidney, and intestines by way of the circulatory system or the enterohepatic circulation (Wu et al. 2007). Similar levels of cadmium accumulation was observed in gills when silver catfish were exposed to this metal for 7 and 14 days and elimination verified in this tissue after the recovery period. These results indicated that cadmium accumulation occurs in liver, kidney, and gills of silver catfish, and it appears that a long period of time is required for its elimination.
The induction of antioxidant enzymes, such as CAT, SOD, and GST, can be an important adaptation to pollutant-induced stress caused by cadmium. However, the activity of antioxidant enzymes can vary depending on the intensity and duration of the chemical stress applied to the organism in addition to the susceptibility of the exposed species (Ballesteros et al. 2009). One example of this is the observation that CAT and GST activity in liver and gills of silver catfish showed different responses to exposure to cadmium. In liver, an increase in the levels of CAT and GST was accompanied by a decrease in SOD activity in this tissue during exposure periods. During the same period, gill tissues showed a significant decrease in CAT and GST activity. The changes observed in liver and gill antioxidant responses as represented by the levels of CAT and GST suggested that cadmium toxicity is a tissue-specific response.
Different concentrations of metals, such as copper, cadmium, zinc, and chromium (0.1 to 1.5 mg l−1), also increased CAT activity in liver of Oreochromis niloticus in an in vivo experiment performed during a period of 96 h (Atli et al. 2006). In Leporinus obtusidens, exposure to zinc (2.3 and 4.6 mg l−1) and copper (0.02 and 0.04 mg l−1) for 30 and 45 days increased CAT activity (Gioda et al. 2007). Increased CAT activity may indicate a compensatory response to stress caused by cadmium exposure. In contrast, exposure of O. niloticus to copper (0.1 mg l−1) and chromium (0.1–1.5 mg l−1) for 96 h decreased gill CAT activity, whereas cadmium and zinc had no significant effects (Atli et al. 2006). Experiments exposing Carassius auratus to copper (0.01–0.25 mg l−1) for 40 days indicated that liver CAT and GPx activities were inhibited (Liu et al. 2006). Decreased antioxidant enzyme activity, such as CAT and GST in gills, observed in our study can be explained by the high production of ROS induced by the metal. Increased TBARS levels and protein carbonyl can lead to tissue damage and thus decrease the activity of the enzymes. In addition, some investigators have suggested that severe oxidative stress may suppress the activity of antioxidant defense enzyme due to oxidative damage and a loss of the compensatory mechanisms (Atli et al. 2006; Liu et al. 2006; Zhang et al. 2004).
As in liver, in kidney of silver catfish high levels of metal were also observed, but no changes in CAT activity were observed in kidney tissue during analysis of the experimental results. TBARS levels were found to increase in kidney after 14-day exposure, but they returned to control values after a recovery period in cadmium-free water. Conversely, increased TBARS levels occurred during both the exposure and recovery periods in both liver and gills. In this study, exposure to cadmium resulted in the suppression of the antioxidant enzymes in gills; however, liver tissue showed a different response, i.e., increased CAT and GST levels. Taken together, these results could be related to the enhancement of TBARS levels in these tissues, depending on the period of exposure. Different results concerning TBARS levels were observed in fish tissues after metal exposure by other investigators. Gioda et al. (2007) reported an increase of TBARS levels in brain, liver, and muscle of L. obtusidens exposed for 45 days to zinc (2.3 and 4.6 mg l−1), but copper (0.02 and 0.04 mg l−1) exposure for the same period resulted in decreased TBARS values in liver and brain. Fish exposed to pollutants can acclimate physiologic condition by changing their metabolic functions, such as varying the expression of the antioxidant enzymes or increasing pro-oxidants, such as TBARS and protein carbonyl levels. The formation of toxic hydroxyl radicals has direct consequences on cell integrity and cell function. In the present study, oxidative stress was verified in liver and gill tissues of silver catfish, and this stressful condition was followed by a tissue-specific antioxidant response. In the kidney a different response was observed because the increase of Cd in this organ did not induce any changes in the antioxidant enzymes. Another important parameter by which to evaluate oxidative stress damage is protein carbonyl formation. In the present study, protein carbonyl increase observed after 7-day exposure could be related to protein damage caused by exposure to cadmium. Many previous studies have reported protein carbonyl content as a biomarker of oxidative stress in humans, but this biomarker has also been used to determine oxidative damage in fish (Parvez and Raisuddin 2005). These investigators observed significant increases in protein carbonyl levels in tissues, such as liver, kidney, and gill of Channa punctata exposed to deltamethrin pesticide (0.75 μg l−1) for 28 days. In our study, we observed an increase in liver protein carbonyl in fish exposed to 414 μg l−1 cadmium, followed by a decrease after 7 days in cadmium-free water. Almroth et al. (2008) verified a similar increase in plasma protein carbonyl levels in fish captured in places where water and sediments were known to be contaminated with heavy metals. According to Almroth et al. (2005), decreased protein carbonyl levels may indicate that susceptibility to proteolytic degradation has been increased by mild oxidation of proteins, and an increase in this biomarker would indicate that normal protein metabolism had been altered by the accumulation of damaged molecules. The measurement of induction in protein carbonyl levels may serve as a useful indicator of exposure to oxidative stress-inducing chemical contaminants in fish (Parvez and Raisuddin 2005). In the present study, a stress condition indicated by protein carbonylation was observed only after 7 days of exposure. In addition, after these fish were transferred back to cadmium-free water, protein carbonyl levels returned to levels observed in untreated control fish. Thus, this parameter is not a reliable indicator of cadmium toxicity.
Conclusion
In general, our results indicated that tissues, such as liver and gills, in silver catfish were highly affected by cadmium, likely due to the liver’s role as the main detoxification organ and to the fact that the gills interface directly with the external environment. These organs exhibited some differences in the oxidative stress markers analyzed. Thus, certain parameters, such as TBARS, CAT, GST, and SOD, can be considered effective markers of cadmium exposure in silver catfish.
References
Almroth BC, Sturve J, Berglund A, Förlin L (2005) Oxidative damage in eelpout (Zoarces viviparous), measured as protein carbonyl and TBARS, as biomarkers. Aquat Toxicol 73:171–180
Almroth BC, Sturve J, Stephensen E, Holt TF, Forlin L (2008) Protein carbonyls and antioxidant defenses in corkwing wrasse (Symphodus melops) from a heavy metal polluted and a PAH polluted site. Mar Environ Res 66:271–277
Asagba SO, Eriyamremu GE, Igberaese ME (2008) Bioaccumulation of cadmium and its biochemical effect on selected tissues of the catfish (Clarias gariepinus). Fish Physiol Biochem 34:61–69
Atli G, Alptekin Ö, Tükel S, Canli M (2006) Response of catalase activity to Ag+, Cd2+, Cr6+, Cu2+ and Zn2+ in five tissues of freshwater fish Oreochromis niloticus. Comp Biochem Physiol C 143:218–224
Ballesteros ML, Wunderlin DA, Bistoni MA (2009) Oxidative stress responses in different organs of Jenynsia multidentata exposed to endosulfan. Ecotoxicol Environ Saf 72:199–205
Barcellos LJG, Kreutz LC, Rodrigues LB, Fioreze I, Quevedo RM, Cericato L et al (2003) Haematological and biochemical characteristics of male jundiá (Rhamdia quelen Quoy & Gaimard Pimelodidae): changes after acute stress. Aquacult Res 34:1465–1469
Benaduce APS, Kochhann D, Flores EMM, Dressler VL, Baldisserotto B (2008) Toxicity of cadmium for silver catfish Rhamdia quelen (Heptapteridae) embryos and larvae at different alkalinities. Arch Environ Contam Toxicol 54:274–282
Bhakta JN, Munekage Y (2008) Role of ecosystem components in Cd removal process of aquatic ecosystem. Ecol Eng 32:274–280
Buege JA, Aust SD (1978) Microssomal lipid peroxidation. Methods Enzymol 52:302–309
Cattani O, Serra R, Isani G, Raggi G, Cortesi P, Carpene E (1996) Correlation between metallothionein and energy metabolism in sea bass, Dicentrarchus labrax, exposed to cadmium. Comp Biochem Physiol C 113:193–199
Cinier CC, Petit-Ramel M, Faure R, Garin D, Bouvet Y (1999) Kinetics of cadmium accumulation and elimination in carp Cyprinus carpio tissues. Comp Biochem Physiol C 122:345–352
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329:23–38
Gioda CR, Lissner LA, Pretto A, da Rocha JBT, Schetinger MRC, Neto JR et al (2007) Exposure to sublethal concentrations of Zn (II) and Cu (II) changes biochemical parameters in Leporinus obtusidens. Chemosphere 69:170–175
Gomes LC, Golombieski JI, Gomes ARC, Baldisserotto B (2000) Biologia do jundiá Rhamdia quelen (Teleostei, Pimelodidae). Cienc Rural 30:179–185
Habig WH, Pabst MJ, Jacoby WB (1974) Glutathione S-transferase, the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Liu H, Wang W, Zhang J, Wang X (2006) Effects of copper and its ethylenediaminetetraacetate complex on the antioxidant defenses of the goldfish, Carassius auratus. Ecotoxicol Environ Saf 65:350–354
Lowry DH, Rosenbrough NJ, Far AL, Randal RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Mc Cord JM, Fridovich I (1969) Superoxide dismutase: an enzymatic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Monserrat JM, Martínez PE, Geracitano LA, Amado LL, Martins CMG, Pinho GLL et al (2007) Pollution biomarkers in estuarine animals: critical review and new perspectives. Comp Biochem Physiol C 146:221–234
Monteiro DA, Almeida JA, Rantin FT, Kalinin AL (2006) Oxidative stress biomarkers in the freshwater characid fish, Brycon cephalus, exposed to organophosphorus insecticide Folisuper 600 (methyl parathion). Comp Biochem Physiol C 143:141–149
Nelson DP, Kiesow LA (1972) Enthalpy of decomposition of hydrogen peroxide by catalase at 25°C (with molar extinction coefficients of H2O2 solution in the UV). Anal Biochem 49:474–478
Parvez S, Raisuddin S (2005) Protein carbonyl: novel biomarkers of exposure to oxidative stress-inducing pesticides in freshwater fish Channa punctata (Bloch). Environ Toxicol Pharmacol 20:112–117
Pretto A, Loro VL, Morsch VM, Moraes BS, Menezes C, Clasen B et al (2010) Acetylcholinesterase activity, lipid peroxidation, and bioaccumulation in silver catfish (Rhamdia quelen) exposed to cadmium. Arch Environ Contam Toxicol 58:1008–1014
Roméo M, Bennani N, Gnassia-Barelli M, Lafaurie M, Girard JP (2000) Cadmium and copper display different responses towards oxidative stress in the kidney of the sea bass Dicentrarchus labrax. Aquat Toxicol 48:185–194
Sampaio FG, Boijink CL, Tie Oba E, Santos LRB, Kalinin AL, Rantin FT (2008) Antioxidant defenses and biochemical changes in pacu (Piaractus mesopotamicus) in response to single and combined copper and hypoxia exposure. Comp Biochem Physiol C 147:43–51
Sanchez W, Palluel O, Meunier L, Coquery M, Porcher J, Aït-Aïssa S (2005) Copper-induced oxidative stress in three-spined stickleback: relationship with hepatic metal levels. Environ Toxicol Pharmacol 19:177–183
Sies H (1993) Strategies of antioxidant defence. Eur J Biochem 215:213–219
Soares SS, Martins H, Gutiérrez-Merino C, Aureliano M (2008) Vanadium and cadmium in vivo effects in teleost cardiac muscle: metal accumulation and oxidative stress markers. Comp Bochem Physiol C 147:168–178
Wu SM, Shih M, Ho Y (2007) Toxicological stress response and cadmium distribution in hybrid tilapia (Oreochromis sp.) upon cadmium exposure. Comp Biochem Physiol C 145:218–226
Yan LJ, Traber MG, Packer L (1995) Spectrophotometric method for determination of carbonyls in oxidatively modified apolipoprotein B of human low-density lipoproteins. Anal Biochem 228:349–351
Zhang J, Shen H, Wang X, Wu J, Xue Y (2004) Effects of chronic exposure of 2, 4-dichlorophenol on the antioxidant system in liver of freshwater fish Carassius auratus. Chemosphere 55:167–174
Zirong X, Shijun B (2007) Effects of waterborne Cd exposure on glutathione metabolism in Nile tilapia (Oreochromis niloticus) liver. Ecotoxicol Environ Saf 67:89–94
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pretto, A., Loro, V.L., Baldisserotto, B. et al. Effects of Water Cadmium Concentrations on Bioaccumulation and Various Oxidative Stress Parameters in Rhamdia quelen . Arch Environ Contam Toxicol 60, 309–318 (2011). https://doi.org/10.1007/s00244-010-9586-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-010-9586-2