Abstract
Selenium nanoparticles (Se-NPs) were added at 0, 0.5, 1, and 2 mg per kg diet to assess its effects on the performance, Se bioaccumulation, blood health, and antioxidant status of red sea bream. After 45 days, Se-NPs positively impacted the growth and feed efficiency of red sea bream especially by 1 mg per kg diet. No significant (P > 0.05) changes in survival and somatic indices were noticed among groups. Dietary Se-NPs significantly (P < 0.05) increased the protein, lipid, and Se contents in the whole body, muscle, and liver tissues, whereas decreasing the whole-body moisture content of treated groups compared with the Se-NP-free group. Using of Se-NPs at 2 mg per kg diet resulted in the highest Se content in the complete body, muscle, and liver. Significantly enhanced intestine protease activity and hematocrit levels accompanied with low cholesterol and triglyceride were observed in fish fed Se-NP-enriched diets. Fish fed on Se-NPs at 0.5, 1, and 2 mg Se-NPs per kg diet exhibited significantly higher values of biological antioxidant potential than the control group (P < 0.05). Therefore, the obtained results recommends adding 1 mg Se-NPs per kg diet to improve the growth, feed efficiency, blood health, and antioxidant defense system of red sea bream.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an indispensable microelement and vital for better performance and well-being of aquatic animals (Gobi et al. 2018; Pacitti et al. 2016). The importance of Se supplementation in fish feed has been well accepted since Se from ambient water and feed itself cannot supply the optimal level required by the cultured aquatic species. Even though Se is typically required in much lower quantity in fish diet, its supplementation at optimum level is a critical issue in fish feed formulation (Rider et al. 2009; Rotruck et al. 1973). Scientific reports gathered over last two decades indicate that Se is quite sensitive and proper care should be taken to ensure their inclusion at optimum level in fish feed formulation (Aliko et al. 2018; Le and Fotedar 2013; Sarkar et al. 2015; Watanabe et al. 1997; Zhu et al. 2012). Se bioaccumulation is affected by the interaction between environmental factors like hardness, temperature, salinity, and pH, as well as species-specific factors such as feeding habits, metabolism, digestion, and excretion efficiency, in addition to Se bioavailability in the water (McCarty and Mackay 1993). In a low concentration of Se in aquatic environments, aquatic animals can accumulate in their tissues at acceptable levels without side effects.
Se deficiency may depress growth and appetite, as well as cause peroxidative damage to cell membranes and result in high mortality, while excessive Se may reduce feed efficiency and result in reproductive failure, tissue destruction, and teratogenic deformities of organs (e.g., spine, head, mouth, and fins) (Watanabe et al. 1997). Hence, an optimal dietary Se level is important for different cultured fish species. Se can protect the animal cell from oxidation which happens by several stressors including, high density, transportation, poor water quality, and infectious diseases by increasing the activity of antioxidant-related enzymes and thyroid hormone metabolism and reproduction (Hefnawy and Tórtora-Pérez 2010; Pacitti et al. 2016). Se existed in fish meal as a major ingredient in fish diets, but further inclusion of Se is highly recommended to afford the requirements of some fish species (Le and Fotedar 2013; Le and Fotedar 2013; Watanabe et al. 1997; Zhu et al. 2012). The aquatic animals require more balanced diets which contain the macro and micro ingredients to meet the high metabolic rates associated with the increased growth performance (Aliko et al. 2018; Sarkar et al. 2015). The addition of Se in fish diets can be affected by Se forms, diet formulation, and fish body sizes and species (Zhu et al. 2016). Normally, Se which occurs in both organic and inorganic forms has been included in fish diets (Saffari et al. 2017). Recently, Se nanoparticle (Se-NP) form has been applied due to its high bioavailability and low harmfulness when fed to fish (Saffari et al. 2017, 2018; Wang et al. 2007; Zhou et al. 2009). Additionally, nano-Se improved the growth, feed utilization, and the antioxidant defense capacity of numerous cultured fish (Ashouri et al. 2015; Naderi et al. 2017a, b, c; Saffari et al. 2017).
Red sea bream (Pagrus major) is an important farmed fish in Japan, Korea, and China because of its suitability for aquaculture, marketability, and stable market prices (Dawood et al. 2017; El Basuini et al. 2017). The present experiment clearly demonstrated that red sea bream has a specific requirement level for dietary Se-NPs which cannot be met by regular feed and ambient water. Inclusion of dietary Se-NPs at optimum level is imperative to promote the maximum growth, tissue saturation, and antioxidative responses. The immunoprotective effects of Se-NPs on red sea bream were observed by Dawood et al. (2019a). However, there is no data about the efficacy of Se-NPs on the growth and antioxidative responses of red sea bream. Thus, this design was to find out the ameliorative properties of Se-NPs on the growth, blood health, oxidative status, and tissue bioaccumulation of red sea bream.
Materials and methods
Experimental diets and procedure
The Se-NPs (Nacalai Tesque, Inc., Kyoto, Japan; particle size is 38.7 nm) were added at the rates of 0, 0.5, 1, and 2 mg per kg semi-purified basal diets (Table 1). The diet components and Se-NPs were mixed to prepare the test diets by following the Dawood et al. (2019a). Characterization of Se-NPs was done according to Salahuddin et al. (2017) and Dawood et al. (2019a) (Supplementary file).
Red sea bream were transferred from Tawaki farm, Kumamoto, Japan, to the indoor wet Lab. at the Kamoike Marine Production Laboratory, Faculty of Fisheries, Kagoshima University, Japan, and kept in 500-L fiberglass tank for 2 weeks for acclimatization in accordance with “the guidelines on the care and use of animals for scientific purpose set by Kagoshima University.” Great attention was paid to minimize the suffering of fish. Similar-sized 20 fish (4.04 ± 0.02 g) per tank were randomly assigned in 12 tanks (100 L) (3 tanks per treatment, triplicates) provided with “a flow-through sea water system” with a continuous aeration. Water quality indices in each tank were as follows: “temperature, 26.5 ± 1.5 °C; dissolved oxygen, 6.1 ± 0.5 mg L−1; pH, 7.1; and light–dark photoperiod cycle was maintained at 12 h light and 12 h dark” (Dawood et al. 2015a, b; Zaineldin et al. 2018). For 45 days, the fish were fed by hand, twice daily, until apparent visual satiation level.
Final sampling
At the end of the trial, all fish in the different experimental groups were weighed individually to calculate the growth and survival parameters using the following formulae:
Weight gain (WG, %) = (W1 − W2) × 100/W1, where W1 and W2 are initial and final body weight (g) of fish.
where N2 and N1 are initial and final number of fish.
Randomly, 5 fish per tank were carefully chosen for carcass proximate analysis and the amount of Se bioaccumulation in whole body, muscle, and liver tissues, then stored at − 20 °C.
Another 3 fish were used to collect blood using 1.0 mL heparinized syringes (1600 UI mL−1, Nacalai Tesque, Kyoto, Japan) and divided for two portions. The hematocrit (HCT) was analyzed by following Dawood et al. (2015c). Then, the residual blood portion was centrifuged at 3000×g for 15 min, and resulting plasma samples were kept at − 80 °C for further analysis. Following blood sampling, the digestive tracts of fish were removed, cut into small pieces, rinsed with pure water, and finally kept at − 80 °C until protease activity assay. Then, the viscera and the liver were dissected out from the fish above and individually weighed and length was measured to calculate the condition factor (CF), viscerasomatic index (VSI), and hepatosomatic index (HSI) using the following formulae:
Biochemical analysis
The diets and carcass composition were analyzed for moisture, crude protein, crude lipid, and ash using standard methods (AOAC 1998). Se contents of test diets, whole body, muscle, and liver were assessed by the digestion of samples in nitric acid (AOAC 1998). The concentration of Se in the diluted digested solution was determined using Atomic Absorption Spectrophotometer-Graphite furnace (GBC Avanta E, Victoria, Australia).
Plasma chemical parameters including “total bilirubin, blood urea nitrogen, glutamyl oxaloacetic transaminase (GOT), glutamic-pyruvate transaminase (GPT), triglycerides (TG), and total cholesterol (T-CHO) were measured spectrophotometrically with an automated analyzer (SPOTCHEM™ EZ model SP-4430, Arkray, Inc., Kyoto, Japan)” according to Tatsumi et al. (2000). Samples of digestive tract were used to analyze the protease activity according to Sigma’s non-specific protease activity assay (casein as a substrate).
Antioxidant status
The antioxidative response was measured by analyzing the biological antioxidant potential (BAP) and reactive oxygen metabolites (d-ROMs). BAP and d-ROMs were measured spectrophotometrically from blood plasma with an automated analyzer (FRAS4, Diacron International s.r.l., Grosseto, Italy) by following Dawood et al. (2015c) and Morganti et al. (2002).
Statistical analysis
The normality and homogeneity of the data was checked with the Shapiro–Wilk test (SPSS software). The data (means ± standard error of means, SEM) were statistically analyzed by one-way analysis of variance (ANOVA) followed by Duncan to compare the means between levels of supplementation with SPSS (Version 22) at P < 0.05 level.
Results
Growth and protease activity
Table 2 represents the growth, feed efficiency, and survival results. The survival rates were 95% and 98.3% without significant (P > 0.05) alterations. Dose-related enhancements for the FBW, WG, SGR, FER, and PER, with the highest values in fish fed 1 mg. Fish fed Se-NPs displayed significantly (P < 0.05) higher FBW, WG, and SGR than in the control group. The highest values of FER and PER were significantly (P < 0.05) detected in fish fed 1 mg.
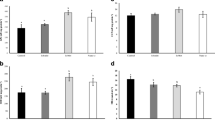
Dose-related increases were detected regarding the intestinal protease enzyme activity of fish fed Se-NPs (Fig. 1), with higher significant values in fish fed 0.5 and 1 mg (P < 0.05). However, insignificant (P > 0.05) changes were detected in protease activity between 0.5 and 1 mg.
Carcass composition and somatic indices
The carcass compositions of fish are displayed in Table 3. Compared with the control group, dietary Se-NPs had insignificant (P > 0.05) influences on the whole-body ash content and somatic indices including CF, HSI, and VSI. Dietary Se-NPs significantly (P < 0.05) decreased the values of whole-body moisture content compared with control group. Significantly higher carcass protein and lipid contents were observed in fish fed Se-NPs than the control group (P < 0.05). Dose-related increases were detected concerning the body protein and lipid contents, with the highest values significantly detected in fish fed 1 mg diet compared with the values found in fish fed the Se-NP-free diet (P < 0.05). However, insignificant variations were noticed in the carcass protein content of fish fed 1 and 2 mg (P > 0.05).
Selenium bioaccumulation in fish tissues
Se in the carcass, liver, and muscle tissues were increased by Se-NP supplementation in fish diets (Table 4). Fish fed 2 mg exhibited significantly higher Se levels in the whole body over the other diets (P < 0.05). Regarding liver and muscle Se contents, dose-related increments were observed, being significantly higher in fish fed 1 and 2 mg (P < 0.05) compared with the values of fish fed the control. Se tended to be more bioaccumulated in the liver than in muscle tissue.
Blood parameters
Se-NP supplementation had no influence on the blood biochemistry of fish except for HCT, TG, and T-CHO (Table 5). Se-NPs showed significantly (P < 0.05) improved HCT especially for 0.5 or 1 mg. On contrary, TG was significantly (P < 0.05) lower in fish fed 2 mg, while fish fed 1 and 2 showed insignificant differences (P > 0.05). T-CHO was significantly increased in fish fed the Se-NP-free diet regarding the fish fed 1 mg (P < 0.05).
Antioxidative response
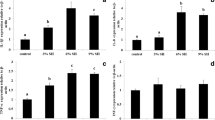
The antioxidative response of fish is shown in Table 6 and Fig. 2. The BAP was significantly (P < 0.05) upregulated in fish fed the Se-NPs, while d-ROMs showed insignificant (P > 0.05) differences (Table 6). The combined d-ROMs and BAP responses showed that 0.5 and 2 mg groups are located in zone A reflecting a lower intensity of oxidative stress and a higher tolerance ability against oxidative stress (Fig. 2). Moreover, the fish group fed Se-NPs at 1 mg is located in zone B showing higher BAP and d-ROMs while the control group is located in zone C that refers to lower BAP and low d-ROMs (Fig. 2).
Oxidative stress parameters in red sea bream fed test diets for 45 days. Values are expressed as mean ± SEM. Central axis based on mean values of reactive oxygen metabolites (d-ROMs) and biological antioxidant potential (BAP) from each treatment. Zone (A): high BAP and low d-ROMs (good condition); zone (B): high BAP and high d-ROMs (acceptable condition); zone (C): low BAP and low d-ROMs (acceptable condition); zone (D): low BAP and high d-ROMs (stressed condition)
Discussion
Micronutrients are highly recommended at adequate levels in the formulated diets to afford cultured fish requirements in order to keep the standard growth and health condition (Dawood et al. 2018; Dawood and Koshio 2018; El Basuini et al. 2016, 2017; Pacitti et al. 2016; Yan et al. 2017). The obtained results revealed that FBW, WG, and SGR parameters of fish were upregulated at 1 mg Se-NPs per kg, but presented no changes between 0.5 and 2 mg Se-NPs per kg, indicating that Se could be supplemented for red sea bream at 1 to 2 mg Se-NPs per kg.
In general, our observations agree with previous trials conducted to assess the role of Se in different fish species including grouper (Lin and Shiau 2005), cobia (Liu et al. 2010), carp (Ashouri et al. 2015), Nile tilapia (Lee et al. 2016), and rainbow trout (Wang et al. 2018). Specifically, a significant enhancement in growth performance has been previously demonstrated in carp (Ashouri et al. 2015; Saffari et al. 2017; Zhou et al. 2009) and tilapia (Lee et al. 2016) fed Se-NPs. Interestingly, Ashouri et al. (2015) reported an improved growth performance at a similar inclusion level (1 mg Se-NPs per kg) for common carp using nanoform of Se. However, beneficial effects were achieved by higher levels (5.56 mg) in yellowtail kingfish (Le and Fotedar 2013) and by lower levels in grouper (0.77 mg) (Lin and Shiau 2005). The minimum Se level giving maximum growth performance may vary according to Se forms, period of administration, and experimental procedures as well as fish species and sizes. Nonetheless, remarks of this study more confirm the hypothesis that identical growth performance in aquatic animal species can be achieved by lower levels of microminerals supplemented in nanoforms rather than inorganic and organic ones (Apines et al. 2003; Lee et al. 2008). It is generally accepted that Se-NPs are more readily available to fish (Ashouri et al. 2015; Saffari et al. 2017) and probably display wider and greater biological efficacy (Wang et al. 2007) compared with other Se sources.
Oversupplementation of Se can negatively affect the fish growth performance (Kim and Kang 2014, 2015; Pham and Fotedar 2017). Excess Se supplementation may force P. major to use energy for detoxification of Se rather than for toxicity for growth and development, and accordingly growth is retarded. Regardless, our results indicate that Se could be supplemented to red sea bream at 1 to 2 mg Se-NPs per kg diets without antagonistic effects on fish growth and feed efficiency. However, the declined performance at 2 mg, compared with 1 mg Se-NPs per kg, suggests that a dietary Se-NPs up to 1 mg Se-NPs per kg diet may be beneficial for growth of red sea bream under the circumstances of this study. Also, these results confirmed the crucial demand for dietary Se for red sea bream. Yet, specific Se requirements under different farming conditions remain to be determined for red sea bream.
The Se-induced improvement on the growth and feed effectiveness was due to the role of Se in the stimulation of growth hormone production, synthesis of selenoproteins, activation of intestinal protease enzyme, or enhancing in the intracellular protein of intestinal cells. Se can increase the production of growth hormone and enhance growth performance in fish (Khan et al. 2017). In this study, the best feed efficiency values (FER and PER) were in fish fed 1 mg Se-NPs per kg. Wang et al. (2013) reported an increase in the intracellular protein of intestinal epithelial cells of the crucian carp which may cause better utilization of the fed diets, resulting in higher feed efficiency and growth performance (Saffari et al. 2017). In addition, the expression of selenoprotein genes was upregulated due to the role of Se in selenoproteins synthesis, which resulted in the improved feed utilization and growth performance in rainbow trout (Wang et al. 2018). Furthermore, increased PER was verified in African catfish fed Se (Abdel-Tawwab et al. 2007). Micronutrients including Se act as co-enzymes in production/activation of digestive enzymes (Shenkin 2006). Previous observations reported that dietary Se enhances protein digestibility and utilization through increasing the amount and activity of intestinal microbes and digestive protease (Chaudhary et al. 2010; Shi et al. 2011). Thus, better feed efficiency values of sea bream fed Se-NPs may be explained by the high intestinal cell protein content which resulted in well metabolism of absorbed nutrients.
The high carcass protein and lipid contents observed in fish fed Se-NPs indicate that the protein and lipid contents of diets were utilized efficiently by fish. This could be attributed in part to the role of Se in enhancing growth hormone production (Ewan 1976) and protein synthesis in the intestinal epithelial cells (Wang et al. 2013) as discussed above. Wang et al. (2018) reported that selenoproteins accumulated more efficiently in rainbow trout tissues by Se supplementation and improved protein synthesis which might present an interpretation for the increased whole-body protein content. The increased carcass lipid content together with increased body weight in groups fed Se-NPs might be due to the high levels of lipids existed in the abdominal area in fish body. CF, HSI, and VSI are somatic indices to measure the energy kept nutritional state of the fish (Dawood et al. 2017). In this study, CF, HSI, and VSI reflect the enhancement in the body weight along with the length by Se-NPs. The absence of changes in somatic indices may be attributed to the short duration of Se feeding and the small size of our fish. Previous studies indicated inconsistent responses of hepatosomatic index to dietary Se supplementation (Khan et al. 2017).
The current findings revealed increased rate of Se accumulation in the carcass, liver, and the muscle of fish fed Se-NPs. Se was accumulated more in liver tissue than the muscle, suggesting that the Se level in liver mirrors a more distinct dose–response to dietary Se. Similarly, Se concentrations were increased in liver, muscle, and gill in Nile tilapia fed diets supplemented with Se (Lee et al. 2016). In contrast, the muscle Se concentrations were increased in the case of white sturgeon fed Se, whereas the liver Se was low (Tashjian et al. 2006). The liver is known as “the main site of Se accumulation in fish, and degeneration of liver tissue has been reported in fish exposed to an excess amount of Se” (Apines et al. 2003; Hodson et al. 1980; Lee et al. 2008). In our study, Se content in liver was similar in fish fed 1 and 2 mg which imply an enhanced detoxification mechanism in liver at higher levels of Se intake (Hilton et al. 1980) causing a shortage of energy available for growth, and this phenomenon may contribute to the interpretation of the little improvement in growth with Se intake at 2 mg compared with 1 mg Se-NPs per kg. However, the higher level of Se used in our study did not reach a detrimental level because of absence of changes in liver function enzymes.
The values of blood parameters founded in this study are similar to obtained results in red sea bream (Dawood et al. 2016a, b; Dossou et al. 2018a, b). Fish fed the control diet had meaningfully higher T-CHO and TG but lower HCT values than those fed Se-NPs. Previously, Se-NP supplementation had increased the HCT levels in mahseer fish (Khan et al. 2017) and reduced the T-CHO levels in carp (Ashouri et al. 2015). Physiologically, plasma T-CHO and TG are reliable indicators to present fish health status, immunity, and antioxidant ability (Yang et al. 2014) whereas the HCT count can be used as an indicator of the general health condition of fish (Song et al. 2014; Dossou et al. 2019). Thus, dietary Se-NPs can positively affect the welfare of cultured red sea bream.
During injury or damage of the liver cells, the GOT and GPT are released into the blood to evaluate the liver condition and functionality (Lemaire et al. 1991; Hossain et al. 2016). Although did not differ significantly among diets, the lower values of GOT and GPT in fish fed Se-NP-supplemented diets than those fed the Se-NP-free diet may indicate that Se-NPs are not detrimental to liver health. Available information on Se-induced changes in liver enzymes are controversial; Se-NP administration was reported to minimize liver enzymes in loach (Hao et al. 2014) and carp (Safari et al. 2017); however, Ashouri et al. (2015) observed an increased activity of these enzymes in carp receiving 1 and 2 mg.
The most vital role of Se is the antioxidant effect as it forms “selenocysteine”, which is a part of the active center of the glutathione peroxidase activity (GPx) (Köhrle et al. 2000). Se-NPs are an effective microelement for the prevention of fish against oxidative stress (Ashouri et al. 2015; Saffari et al. 2017). The role of antioxidant enzymes is to “remove damaging reactive oxygen species (ROS) from the cellular environment by catalyzing the dismutation of two superoxide radicals to hydrogen peroxide and oxygen (Fattman et al. 2003; Lin et al. 2010). Consequently, levels of antioxidant enzymes have been extensively used as an early warning indicator of toxicity and/or pollution (Burgos-Aceves et al. 2018; Dawood et al. 2019b; Faggio et al. 2016; Hodkovicova et al. 2019; Lin et al. 2001). Recently, oxidative stress could be determined by measuring d-ROM and BAP in plasma samples. Oxidative stress occurs when oxidant activity exceeds the neutralizing capacity of antioxidants (Celi et al. 2010). Using these parameters, our study showed that fish fed diets supplemented with Se-NPs were more tolerant of oxidative stress indicating a higher health status”.
Conclusion
Se-NPs are required for better growth, feed utilization, and physiological condition of red sea bream. For the optimal performances of red sea bream, Se-NPs could be added at 1 mg per kg. However, more detailed studies are needed to explain the molecular mechanisms of Se-NPs in aquatic fish species.
References
Abdel-Tawwab M, Mousa MA, Abbass FE (2007) Growth performance and physiological response of African catfish, Clarias gariepinus (B.) fed organic selenium prior to the exposure to environmental copper toxicity. Aquaculture 272(1-4):335–345. https://doi.org/10.1016/j.aquaculture.2007.09.004
Aliko V, Qirjo M, Sula E, Morina V, Faggio C (2018) Antioxidant defense system, immune response and erythron profile modulation in gold fish, Carassius auratus, after acute manganese treatment. Fish Shellfish Immunol 76:101–109. https://doi.org/10.1016/j.fsi.2018.02.042
AOAC (Association of Official Analytical Chemists) (1998) Official methods of analysis of official analytical chemists international, 16th edn. AOAC, Washington, DC
Apines MJS, Satoh S, Kiron V, Watanabe T, Aoki T (2003) Availability of supplemental amino acid-chelated trace elements in diets containing tricalcium phosphate and phytate to rainbow trout, Oncorhynchus mykiss. Aquaculture 225(1):431–444. https://doi.org/10.1016/S0044-8486(03)00307-7
Ashouri S, Keyvanshokooh S, Salati AP, Johari SA, Pasha-Zanoosi H (2015) Effects of different levels of dietary selenium nanoparticles on growth performance, muscle composition, blood biochemical profiles and antioxidant status of common carp (Cyprinus carpio). Aquaculture 446:25–29. https://doi.org/10.1016/j.aquaculture.2015.04.021
Burgos-Aceves MA, Cohen A, Smith Y, Faggio C (2018) MicroRNAs and their role on fish oxidative stress during xenobiotic environmental exposures. Ecotoxicol Environ Saf 148:995–1000. https://doi.org/10.1016/j.ecoenv.2017.12.001
Celi P, Sullivan M, Evans D (2010) The stability of the reactive oxygen metabolites (d-ROMs) and biological antioxidant potential (BAP) tests on stored horse blood. Vet J 183:217–218. https://doi.org/10.1016/j.tvjl.2008.09.018
Chaudhary M, Garg AK, Mittal GK, Mudgal V (2010) Effect of organic selenium supplementation on growth, Se uptake, and nutrient utilization in guinea pigs. Biol Trace Elem Res 133(2):217–226
Dawood MAO, Koshio S (2018) Vitamin C supplementation to optimize growth, health and stress resistance in aquatic animals. Rev Aquac 10(2):334–350
Dawood MAO, Koshio S, Ishikawa M, Yokoyama S (2015a) Interaction effects of dietary supplementation of heat-killed Lactobacillus plantarum and β-glucan on growth performance, digestibility and immune response of juvenile red sea bream, Pagrus major. Fish Shellfish Immunol 45(1):33–42
Dawood MAO, Koshio S, Ishikawa M, Yokoyama S (2015b) Effects of heat killed Lactobacillus plantarum (LP20) supplemental diets on growth performance, stress resistance and immune response of red sea bream, Pagrus major. Aquaculture 442:29–36
Dawood MA, Koshio S, Ishikawa M, Yokoyama S (2015c) Effects of partial substitution of fish meal by soybean meal with or without heat-killed Lactobacillus plantarum (LP20) on growth performance, digestibility, and immune response of amberjack, Seriola dumerili juveniles. BioMed Res Int 2015:514196. https://doi.org/10.1155/2015/514196
Dawood MAO, Koshio S, Ishikawa M, El-Sabagh M, Esteban MA, Zaineldin AI (2016a) Probiotics as an environment-friendly approach to enhance red sea bream, Pagrus major growth, immune response and oxidative status. Fish Shellfish Immunol 57:170–178. https://doi.org/10.1016/j.fsi.2016.08.038
Dawood, M.A., Koshio, S., Ishikawa, M. and Yokoyama, S. (2016b) Immune responses and stress resistance in red sea bream, Pagrus major, after oral administration of heat-killed Lactobacillus plantarum and vitamin C. Fish & shellfish immunology, 54:266–275
Dawood MAO, Koshio S, Ishikawa M, Yokoyama SE, Basuini MF, Hossain MS, Nhu TH, Moss AS, Dossou S, Wei H (2017) Dietary supplementation of β-glucan improves growth performance, the innate immune response and stress resistance of red sea bream, Pagrus major. Aquac Nutr 23(1):148–159. https://doi.org/10.1111/anu.12376
Dawood MAO, Koshio S, Esteban MÁ (2018) Beneficial roles of feed additives as immunostimulants in aquaculture: a review. Rev Aquac 10(4):950–974
Dawood MAO, Koshio S, Zaineldin AI, Van Doan H, Moustafa EM, Abdel-Daim MM, Esteban MA, Hassaan MS (2019a) Dietary supplementation of selenium nanoparticles modulated systemic and mucosal immune status and stress resistance of red sea bream (Pagrus major). Fish Physiol Biochem 45(1):219–230. https://doi.org/10.1016/j.fsi.2016.04.017
Dawood MAO, Shukry M, Zayed MM, Omar AA, Zaineldin AI, El Basuini MF (2019b) Digestive enzymes, immunity and oxidative status of Nile tilapia (Oreochromis niloticus) reared in intensive conditions. Slov Vet Res 56(22-Suppl)
Dossou S, Koshio S, Ishikawa M, Yokoyama S, Dawood MA, El Basuini MF, Olivier A, Zaineldin AI (2018a) Growth performance, blood health, antioxidant status and immune response in red sea bream (Pagrus major) fed Aspergillus oryzae fermented rapeseed meal (RM-Koji). Fish Shellfish Immunol 75:253–262
Dossou S, Koshio S, Ishikawa M, Yokoyama S, Dawood MA, El Basuini MF, El-Hais AM, Olivier A (2018b) Effect of partial replacement of fish meal by fermented rapeseed meal on growth, immune response and oxidative condition of red sea bream juvenile, Pagrus major. Aquaculture 490:228–235
Dossou S, Koshio S, Ishikawa M, Yokoyama S, El Basuini MF, Zaineldin AI, Mzengereza K, Moss A, Dawood MAO (2019) Effects of replacing fishmeal with fermented and non-fermented rapeseed meal on the growth, immune and antioxidant responses of red sea bream (Pagrus major). Aquac Nutr 25:508–517
El Basuini MF, El-Hais AM, Dawood MAO, Abou-Zeid AES, EL-Damrawy SZ, Khalafalla MMES, Koshio S, Ishikawa M, Dossou S (2016) Effect of different levels of dietary copper nanoparticles and copper sulfate on growth performance, blood biochemical profiles, antioxidant status and immune response of red sea bream (Pagrus major). Aquaculture 455:32–40
El Basuini MF, El-Hais AM, Dawood MAO, Abou-Zeid AS, EL-Damrawy SZ, Khalafalla MS, Koshio S, Ishikawa M, Dossou S (2017) Effects of dietary copper nanoparticles and vitamin C supplementations on growth performance, immune response and stress resistance of red sea bream, Pagrus major. Aquac Nutr 23(6):1329–1340. https://doi.org/10.1111/anu.12508/abstract
Ewan RC (1976) Effect of selenium on rat growth, growth hormone and diet utilization. J Nutr 106:702–709
Faggio C, Pagano M, Alampi R, Vazzana I, Felice MR (2016) Cytotoxicity, haemolymphatic parameters, and oxidative stress following exposure to sub-lethal concentrations of quaternium-15 in Mytilus galloprovincialis. Aquat Toxicol 180:258–265. https://doi.org/10.1016/j.aquatox.2016.10.010
Fattman CL, Schaefer LM, Oury TD (2003) Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med 35(3):236–256. https://doi.org/10.1016/S0891-5849(03)00275-2
Gobi N, Vaseeharan B, Rekha R, Vijayakumar S, Faggio C (2018) Bioaccumulation, cytotoxicity and oxidative stress of the acute exposure selenium in Oreochromis mossambicus. Ecotoxicol Environ Saf 162:147–159. https://doi.org/10.1016/j.ecoenv.2018.06.070
Hao X, Ling Q, Hong F (2014) Effects of dietary selenium on the pathological changes and oxidative stress in loach (Paramisgurnus dabryanus). Fish Physiol Biochem 40(5):1313–1323. https://doi.org/10.1007/s10695-014-9926-7
Hefnawy AEG, Tórtora-Pérez JL (2010) The importance of selenium and the effects of its deficiency in animal health. Small Rumin Res 89(2):85–192. https://doi.org/10.1016/j.smallrumres.2009.12.042
Hilton JW, Hodson PV, Slinger SJ (1980) The requirement and toxicity of selenium in rainbow trout (Salmo gairdneri). J Nutr 110(12):2527–2535
Hodkovicova N, Chmelova L, Sehonova P, Blahova J, Doubkova V, Plhalova L, Fiorino E, Vojtek L, Vicenova V, Siroka Z, Enevova V, Berlinska J, Faldyna M, Svobodova Z, Faggio C (2019) The effects of a therapeutic formalin bath on selected immunological and oxidative stress parameters in common carp (Cyprinus carpio). Sci Total Environ 653:1120–1127. https://doi.org/10.1016/j.scitotenv.2018.11.035
Hodson PV, Spry DJ, Blunt BR (1980) Effects on rainbow trout (Salmo gairdneri) of a chronic exposure to waterborne selenium. Can J Fish Aquat Sci 37(2):233–240
Hossain MS, Koshio S, Ishikawa M, Yokoyama S, Sony NM, Dawood MAO, Kader MA, Bulbul M, Fujieda T (2016) Efficacy of nucleotide related products on growth, blood chemistry, oxidative stress and growth factor gene expression of juvenile red sea bream, Pagrus major. Aquaculture 464:8–16. https://doi.org/10.1016/j.aquaculture.2016.06.004
Khan KU, Zuberi A, Nazir S, Ullah I, Jamil Z, Sarwar H (2017) Synergistic effects of dietary nano selenium and vitamin C on growth, feeding, and physiological parameters of mahseer fish (Tor putitora). Aquac Rep 5:70–75. https://doi.org/10.1016/j.aqrep.2017.01.002
Kim JH, Kang JC (2014) The selenium accumulation and its effect on growth, and haematological parameters in red sea bream, Pagrus major, exposed to waterborne selenium. Ecotoxicol Environ Saf 104:96–102
Kim JH, Kang JC (2015) Oxidative stress, neurotoxicity, and non-specific immune responses in juvenile red sea bream, Pagrus major, exposed to different waterborne selenium concentrations. Chemosphere 135:46–52. https://doi.org/10.1016/j.chemosphere.2015.03.062
Köhrle J, Brigelius-Flohé R, Böck A, Gärtner R, Meyer O, Flohé L (2000) Selenium in biology: facts and medical perspectives. Biol Chem 381(9-10):849–864
Le KT, Fotedar R (2013) Dietary selenium requirement of yellowtail kingfish (Seriola lalandi). Agric Sci 4(6A):68–75
Lee S, Lee JH, Bai SC (2008) Effects of different levels of dietary selenium (Se) on growth, tissue Se accumulations and histopathological changes in black sea bream, Acanthopagrus schlegeli. Asian-Australian J Anim Sci 21:1794–1799
Lee S, Nambi RW, Won S, Katya K, Bai SC (2016) Dietary selenium requirement and toxicity levels in juvenile Nile tilapia, Oreochromis niloticus. Aquaculture 464:153–158. https://doi.org/10.1016/j.aquaculture.2016.06.027
Lemaire P, Drai P, Mathieu A, Lemaire S, Carriere S, Giudicelli J, Lafaurie M (1991) Changes with different diets in plasma enzymes (GOT, GPT, LDH, ALP) and plasma lipids (cholesterol, triglycerides) of sea-bass (Dicentrarchus labrax). Aquaculture 93(1):63–75. https://doi.org/10.1016/0044-8486(91)90205-L
Lin YH, Shiau SY (2005) Dietary selenium requirements of juvenile grouper, Epinephelus malabaricus. Aquaculture 250(1):356–363. https://doi.org/10.1016/j.aquaculture.2005.03.022
Lin CT, Lee TL, Duan KJ, Su JC (2001) Purification and characterization of Black porgy muscle Cu/Zn superoxide dismutase. ZOOLOGICAL STUDIES-TAIPEI 40(2):84–90
Lin YH, Shih CC, Kent M, Shiau SY (2010) Dietary copper requirement reevaluation for juvenile grouper, Epinephelus malabaricus, with an organic copper source. Aquaculture 310(1):173–177. https://doi.org/10.1016/j.aquaculture.2010.10.004
Liu K, Wang XJ, Ai Q, Mai K, Zhang W (2010) Dietary selenium requirement for juvenile cobia, Rachycentron canadum L. Aquac Res 41(10):e594–e601. https://doi.org/10.1111/j.1365-2109.2010.02562.x
McCarty LS, Mackay D (1993) Enhancing ecotoxicological modeling and assessment. Body residues and modes of toxic action. Environ Sci Technol 27(9):1718–1728
Morganti P, Bruno C, Guarneri F, Cardillo A, Del Ciotto P, Valenzano F (2002) Role of topical and nutritional supplement to modify the oxidative stress. Int J Cosmet Sci 24:331–339
Naderi M, Keyvanshokooh S, Salati AP, Ghaedi A (2017a) Combined or individual effects of dietary vitamin E and selenium nanoparticles on humoral immune status and serum parameters of rainbow trout (Oncorhynchus mykiss) under high stocking density. Aquaculture 474:40–47
Naderi M, Keyvanshokooh S, Salati AP, Ghaedi A (2017b) Effects of dietary vitamin E and selenium nanoparticles supplementation on acute stress responses in rainbow trout (Oncorhynchus mykiss) previously subjected to chronic stress. Aquaculture 473:215–222
Naderi M, Keyvanshokooh S, Salati AP, Ghaedi A (2017c) Proteomic analysis of liver tissue from rainbow trout (Oncorhynchus mykiss) under high rearing density after administration of dietary vitamin E and selenium nanoparticles. Comp Biochem Physiol Part D: Genomics Proteomics 22:10–19
Pacitti D, Lawan MM, Feldmann J, Sweetman J, Wang T, Martin SAM, Secombes CJ (2016) Impact of selenium supplementation on fish antiviral responses: a whole transcriptomic analysis in rainbow trout (Oncorhynchus mykiss) fed supranutritional levels of Sel-Plex®. BMC Genomics 17(1):116. https://doi.org/10.1186/s12864-016-2418-7
Pham HD, Fotedar R (2017) Do the dietary ingredients of low-protein formulated diet provide a sufficient selenium source in Australian snapper Pagrus auratus diet (Bloch & Schneider 1801)? Anim Feed Sci Technol 223:99–109. https://doi.org/10.1016/j.anifeedsci.2016.11.012
Rider SA, Davies SJ, Jha AN, Fisher AA, Knight J, Sweetman JW (2009) Supranutritional dietary intake of selenite and selenium yeast in normal and stressed rainbow trout (Oncorhynchus mykiss): implications on selenium status and health responses. Aquaculture 295:282–291
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra W (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179(4073):588–590. https://doi.org/10.1126/science.179.4073.588
Saffari S, Keyvanshokooh S, Zakeri M, Johari SA, Pasha-Zanoosi H (2017) Effects of different dietary selenium sources (sodium selenite, selenomethionine and nanoselenium) on growth performance, muscle composition, blood enzymes and antioxidant status of common carp (Cyprinus carpio). Aquac Nutr 23(3):611–617. https://doi.org/10.1111/anu.12428
Saffari S, Keyvanshokooh S, Zakeri M, Johari SA, Pasha-Zanoosi H, Mozanzadeh MT (2018) Effects of dietary organic, inorganic, and nanoparticulate selenium sources on growth, hemato-immunological, and serum biochemical parameters of common carp (Cyprinus carpio). Fish Physiol Biochem 44(4):1087–1097
Salahuddin NA, El-Kemary M, Ibrahim EM (2017) High-performance flexible epoxy/ZnO nanocomposites with enhanced mechanical and thermal properties. Polym Eng Sci 57(9):932–946
Sarkar B, Bhattacharjee S, Daware A, Tribedi P, Krishnani KK, Minhas PS (2015) Selenium nanoparticles for stress-resilient fish and livestock. Nanoscale Res Lett 10(1):371
Shenkin A (2006) Micronutrients in health and disease. Postgrad Med J 82(971):559–567. https://doi.org/10.1136/pgmj.2006.047670
Shi L, Xun W, Yue W, Zhang C, Ren Y, Liu Q, Wang Q, Shi L (2011) Effect of elemental nano-selenium on feed digestibility, rumen fermentation, and purine derivatives in sheep. Anim Feed Sci Technol 163(2-4):136–142
Song SK, Beck BR, Kim D, Park J, Kim J, Kim HD, Ringø E (2014) Prebiotics as immunostimulants in aquaculture: a review. Fish Shellfish Immunol 40(1):40–48. https://doi.org/10.1016/j.fsi.2014.06.016
Tashjian DH, Teh SJ, Sogomonyan A, Hung SS (2006) Bioaccumulation and chronic toxicity of dietary l-selenomethionine in juvenile white sturgeon (Acipenser transmontanus). Aquat Toxicol 79(4):401–409
Tatsumi N, Tsuji R, Yamada T, Kubo K, Matsuda T (2000) Spot chem. EZ SP- 4430 no kisotekikento. J Clin Lab Instrum Reagents 23(6):427–433
Wang Y, Han J, Li W, Xu Z (2007) Effect of different selenium source on growth performances, glutathione peroxidase activities, muscle composition and selenium concentration of allogynogenetic crucian carp (Carassius auratus gibelio). Anim Feed Sci Technol 134(3):243–251. https://doi.org/10.1016/j.anifeedsci.2006.12.007
Wang Y, Yan X, Fu L (2013) Effect of selenium nanoparticles with different sizes in primary cultured intestinal epithelial cells of crucian carp, Carassius auratus gibelio. Int J Nanomedicine 8:4007
Wang L, Zhang X, Wu L, Liu Q, Zhang D, Yin J (2018) Expression of selenoprotein genes in muscle is crucial for the growth of rainbow trout (Oncorhynchus mykiss) fed diets supplemented with selenium yeast. Aquaculture. 492:82–90. https://doi.org/10.1016/j.aquaculture.2018.03.054
Watanabe T, Kiron V, Satoh S (1997) Trace minerals in fish nutrition. Aquaculture 151(1):185–207. https://doi.org/10.1016/S0044-8486(96)01503-7
Yan J, Li Y, Liang X, Zhang Y, Dawood MAO, Matuli'c D, Gao J (2017) Effects of dietary protein and lipid levels on growth performance, fatty acid composition and antioxidant-related gene expressions in juvenile loach Misgurnus anguillicaudatus. Aquac Res 48(10):5385–5393. https://doi.org/10.1111/are.13352
Yang Q, Yang R, Li M, Zhou Q, Liang X, Elmada ZC (2014) Effects of dietary fucoidan on the blood constituents, anti-oxidation and innate immunity of juvenile yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol 41(2):264–270. https://doi.org/10.1016/j.fsi.2014.09.003
Zaineldin AI, Hegazi S, Koshio S, Ishikawa M, Bakr A, El-Keredy AM, Dawood MA, Dossou S, Wang W, Yukun Z (2018) Bacillus subtilis as probiotic candidate for red sea bream: Growth performance, oxidative status, and immune response traits. Fish Shellfish Immunol 79:303–312
Zhou X, Wang Y, Gu Q, Li W (2009) Effects of different dietary selenium sources (selenium nanoparticle and selenomethionine) on growth performance, muscle composition and glutathione peroxidase enzyme activity of crucian carp (Carassius auratus gibelio). Aquaculture 291:78–81
Zhu Y, Chen Y, Liu Y, Yang H, Liang G, Tian L (2012) Effect of dietary selenium level on growth performance, body composition and hepatic glutathione peroxidase activities of largemouth bass Micropterus salmoide. Aquac Res 43(11):1660–1668. https://doi.org/10.1111/j.1365-2109.2011.02972
Zhu L, Han D, Zhu X, Yang Y, Jin J, Liu H, Xie S (2016) Dietary selenium requirement for on-growing gibel carp (Carassius auratus gibelio var. CAS III). Aquac Res. https://doi.org/10.1111/are.13118
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 580 kb)
Rights and permissions
About this article
Cite this article
Dawood, M.A.O., Koshio, S., Zaineldin, A.I. et al. An evaluation of dietary selenium nanoparticles for red sea bream (Pagrus major) aquaculture: growth, tissue bioaccumulation, and antioxidative responses. Environ Sci Pollut Res 26, 30876–30884 (2019). https://doi.org/10.1007/s11356-019-06223-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06223-6