Abstract
This study was conducted to evaluate the concentration and health risk of trace elements in milk powder and baby food samples marketed in Iran using inductive couple plasma/optical emission spectroscopy (ICP-OES) method. The limit of detection (LOD) and limit of quantification (LOQ) were ranged from 1.80 × 10–5 to 2.17 × 10–3 and 6.00 × 10–5 to 7.22 × 10–3 mg/kg, respectively, with recoveries ranged from 92 to 105%. Zinc (Zn) was found in a high mean concentration (8.49 × 10–1 ± 3.93 × 10–2 mg/kg) in milk powder, and iron (Fe) was found in the highest mean concentration (2.04 ± 3.61 × 10–2 mg/kg) in baby food. The Monte Carlo simulation results for the infants revealed that the rank order of the hazard quotient (HQ) index was mercury (Hg) > nickel (Ni) > arsenic (As) > cadmium (Cd) > aluminum (Al). Further, the result of non-carcinogenic and probability of carcinogenic risk was lower than the limits of safe risk (HQ > 1 and cancer risk (CR) > 1 × 10–4). In conclusion, the toxic elements content in the tested products was sufficiently low, and all of the milk powder and baby food sold in Iran could be considered safe for infants and children.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The growth of the child, or in other words the growth of the gastrointestinal tract, genitourinary, nervous, endocrine, muscular, bone and respiratory, etc., mainly depends on the food eaten. Animals and humans produce milk to feed their babies after birth. Breast milk is the first food that humans encounter which serves as a source of essential nutrients required for the biological functions and growth during early life stages. Likewise, milk contains valuable nutrients including protein, fat, vitamins, and minerals, including calcium [1, 2]. Sometimes, the mother is unable to breastfeed due to work outside the home or due to certain illnesses, and the baby also needs more food after six months, and breast milk alone does not provide enough nutrition. Raw buffalo or cow’s milk can be a good substitute for breast milk in feeding the baby. Milk powder is one of the most valuable milk products that is the main substitute for breast milk in baby nutrition. Milk powders stable and contain denatured proteins so that the curd is finer; they are available in a wide range to suit different ages [3, 4]. Also, recently, baby food, based on wheat, is becoming an important factor of daily intake in the diets of baby. Baby foods and formulae deserve high priority in plans aimed at sound child health, irrespective of cultural or religious considerations [5, 6].
Many trace elements can enter natural foods such as milk, formula, and baby food naturally through human activities such as processing, storage, agricultural and industrial activities, or fortifications. Essential trace elements (Fe, Zn, copper (Cu), chromium (Cr), cobalt (Co), manganese (Mn)) are essential for organisms, but can cause poisoning if consumed in excess. However, toxic elements (As, lead (Pb), Ni, Cd, Al, and Hg) can cause poisoning even in very small amounts [3, 7, 8]. Although the World Health Organization/United International Children’s Emergency Fund (WHO/UNICEF) reported in 1998 that Cu, Fe, sodium (Na), Zn, magnesium (Mg), and calcium (Ca) are essential for the human, the excess level of these elements can be toxic for human [9]. According to the other researches, trace elements have side effects such as neurological complications (Al, Pb, and As), renal toxicity (As, Al, Cr, Cu, and Pb), pulmonary effects (Fe and Al), hepatotoxicity (Cu), carcinogenicity (Pb and As), and gastrointestinal effects (Fe and Al) for various organs of the human body [5, 10]. Among the toxic elements, Al, Pb, and Cd are the most important toxic elements [4]. Toxic elements are pollutants that are persistent in the environment and have adverse effects on human health [11,12,13,14]. Infants and children are more sensitive to toxins (toxic metals) than other vulnerable groups [5, 15,16,17,18,19]. Toxic elements are found in abundance in the environment, including water, air, and soil, due to natural (volcanic) and man-made (mining) activities [12, 14, 20].
With these explanations, contamination of milk and other related product such as milk powder and baby food to toxic elements may occur when lactating animals consume contaminated water and feed or are exposed to environmental contamination, which ultimately leads to the production of contaminated milk [21, 22]. In addition, the use of contaminated milk and water in the production of baby food causes these elements to enter the body of the consumers and induce their toxic effects which become more dangerous at the infant’s stage that is characterized by fast immunological, biological, cognitive growth and development [23, 24].
Several devices were used to determine the quantity of trace elements in the food samples [4, 25, 26] such as the ICP-OES, flame atomic absorption spectrometry (FAAS), and graphite furnace atomic absorption spectrometry (GF-AAS). Among the methods, ICP-OES was reported as the most sensitive method, time saving, and specificity [7, 12, 13, 27]. It should be noted that preparation of samples is very important for accurate detection of contaminants. The traditional methods for sample preparation are time-consuming and need several reagents that are hazardous and expensive, and sample contamination by the analyst is possible. Improvement of the sample preparation has been led to the development of acid digestion of microwave-assisted, slurry, and extraction preparation, by ultrasound, and direct analysis of solid sampling [12, 28,29,30].
Regarding food safety regulatory system, risk assessment is a standard procedure used to evaluate and characterize hazards, better identify exposure sources, and assess the risks associated with contaminants transport and distribution in food [10, 31, 32].
Since no comprehensive study has been done on the amount of trace elements in baby food and milk powder in Iran, furthermore, due to the high consumption of these substances in children’s diets, the present study seems necessary. Therefore, the aim of the present study is firstly, to measure trace elements (essential and toxic elements) using ICP-OES and, secondly, to assess the potential health risk of these elements (including As, Al, Co, Cd, Pb, Cu, Cr, Hg, Fe, Mn, Ni, Zn, and Se) by HI method in milk powder and baby food in the Iranian market.
Materials and Methods
Sample Collection
In this study, a total of 120 samples (60 powdered milk and 60 baby food) from five different brands were collected from Iranian pharmacies during 2019. All samples were kept in the glass container with the condition of sterility in refrigerator (4 °C) until analyses at the same day. Milk powder ingredients include lactose, vegetable oil mix, demineralized whey protein, mineral premix, fish oil, emulsifier, and a number of other small ingredients. Ingredients of baby food (with based of wheat) including a mixture of flour, powdered milk, sugar, liquid oil, a mixture of vitamins, and minerals were determined.
Chemicals
All chemicals were of analytical grade and were purchased from Merck Co. (Darmstadt, Germany). Deionized water and nitric acid (HNO3) with a purity of 65% were used for the preparation of solutions.
Preparation of Samples
The concentration of thirteen trace elements (Al, As, Cd, Cr, Co, Fe, Hg, Cu, Mn, Pb, Ni, Zn, and Se) in milk powder and baby food samples were evaluated in duplicated. A closed vessel, acid decomposition in a microwave digestion oven (MLS GmbH, D-88299 Leutkirch, Germany) was applied to minimize the organic matrix effects and also to prevent the possibility of sample contamination and losses of analysts. For analyses of samples, 2 g of milk powder or 2 g of baby food were poured into the vessels of the microwave system. Then, 10 mL of HNO3-hydrogen peroxide (H2O2) mixture (9:1, v/v) was poured into each vessel, shaken, and kept at room temperature for 10 min in order to homogenize the samples. After one-step digestion of the program, the samples were heated for 15 min at 80% of total power (1800 W). After cooling, the subsequent solutions were evaporated to semi-dried mass to eliminate the extra acid and afterward diluted up to 10 mL in volumetric flasks with deionized water and retained as a stock sample solution.
ICP-OES Conditions
The total analyses of milk powder and baby food samples were conducted by ICP-OES techniques. Spectro Arcos ICP-OES (Germany) have, Quartz Torch 2.5 mm injector, Flared End for Spectro. The optimal operation parameters were: RF generator (1400 W), argon gas was used for plasma and nebulizer auxiliary gas. Gas flow for plasma was 14.50 L/min, flow of auxiliary gas was 0.90 L/min, and flow of nebulizer gas was 0.85 L/min, respectively. Afterward, sample uptake time, rinse time, and initial stabilization time were 240 total, 45, and preflush 45 s, respectively; delay time and time of between replicate analyses were ziro. The analysis was a three times, and the frequency (resonance frequency) of the generator of RF was 27.12 MHz. Type of spray chamber and detector Solid state was Cyclonic, modified Lichte and CCD, respectively Type of sample delivery pump was four-channel, software controlled; peristaltic pump enables exact sample flows. Also the speed of prewash pump (rpm) was 60 (for 15 s) and 30 (for 30 s); at the end, sample injection pump speed was 30 rpm, and time of prewash was 45 s. The wavelengths used for the determination of element concentration based on baseline signals and their interferences at selected lines observed experimentally during the measurements are presented in (Table 1).
Validation of the Analytical Method
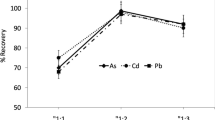
The validation of the analytical procedure for quantitative analysis of elements in milk powder and baby food samples was performed by evaluating selectivity, working and linear ranges, LOD, LOQ, repeatability, and reproducibility (precision). Matrix effects were studied by adding 200 μL of mixed standard solutions to the original samples (Mix standard CRM: 92,091 Supelco LOT BCCB9855, TraceCERT®, 33 elements, 10 mg/L in nitric acid, Hg standard CRM:28,941 Supelco, LOT BCCB8927, 1000 mg/L, Hg in nitric acid). The graphs of calibration for the 13 trace elements were prepared from the standard solutions at seven points, ranged from 1.00 × 10–6 to 3.5 mg/kg. All the trace elements showed linear relationships of the instrumental response and the solutions containing the elements with insignificant intercepts and correlation coefficients of 0.988 or higher. The LOD was ranged from 1.80 × 10–5 to 2.17 × 10–3, and the LOQ was ranged from 6.00 × 10–5 to 7.22 × 10–3 mg/kg. The recoveries were ranged from 92 to 105% that is a suitable range of all essential and nonessential elements (Table 1).
Assessment of Human Health Risk
Estimated Daily Intake (EDI) and Non-carcinogenic Risk Evaluation
In order to determine the oral exposure dose of the harmful compounds, estimated daily intake was used for characterizing the concentration of the sample values that EDI in mg/kg of sample in the day according to Eq. (1) proposed by [10, 33]:
where EDI is the estimated daily intake (mg/kg/day); ED is the exposure duration; IR is the daily ingestion rate of milk powder or baby food by infants (0–6 months) that equal to 120 g/day, infants (6–12 months) 160 g/day, toddlers (1–2 years) 200 g/day, and toddlers (6 months to 2 years) 100 g/day according to [34]; C is the metal concentration in milk powder or baby food samples (mg/kg dry weight); BW is the reference body weight and it was (0–6a) 5.9 kg for infants (0–6 months), 9.3 kg for infants (from 6 to 12 months), 12.2 kg for toddlers (with 1 to 2 years old), and 11 kg for toddlers (6 months to 2 years) [34]; and AT is the mean time [2, 32].
The human non-carcinogenic risk assessment was calculated by reference dose, which established by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) [35] and the United States Environmental Protection Agency (USEPA) [36]. The HQ, which characterizes the health risk of non-carcinogenic indices for the toxic elements in milk powder and baby food, is calculated using Eq. (2) detailed by [10, 37, 38]:
RfD is the oral reference dose which are 1 × 10–3, 1, 1 × 10–4, 2 × 10–2, and 3 × 10−4 mg/kg/day, for Cd, Al, MeHg (Methylmercury), Ni, and As,respectively [39]. Also, the total hazard index (THQ) is determined to calculate the potential risk of adverse health effects for toxic property in the samples which was the sum of the hazard quotient (HQ) through Eq. (3) proposed by [8, 18]:
So the chronic risks are passable if the hazard index was less than 1, while non-carcinogenic risks most probably happen in the target people by hazard index higher than one [40]. For the determination of the carcinogenic risk for each trace element, the cancer risk (CR) is calculated based on Eq. (4) proposed by [41, 42]:
In this equation, ADAF is the age dependent adjustment factor that is 3 over a lifetime. Also, SF is the slope factor of cancer, which is 1.5 mg/kg/day for As; however, SF for Pb, Sn, Cd, Hg, and Ni was not available [43].
Data Analysis
The mean concentration ± SD of results were explained. The data were analyzed by SPSS22 with one-way ANOVA test for comparing toxic metal factors (indices) means based on brand type (http://www.spss.com). Significant differences were considered at p < 0.05. Half of LOQ was used in order to calculate the trace elements mean concentrations in the samples when trace element level was not found.
Results and Discussion
The Concentration of Trace Elements
The mean values of the trace element factors based on milk powder and baby food are presented in Table 2. The highest mean of metal level was related to Zn (8.49 × 10–1 ± 3.93 × 10–2 mg/kg) in milk powder and Fe (2.04 ± 3.61 × 10–2 mg/kg) in baby food; however, the lowest was detection limit was related to Al, As, Cd, Co, and Se in milk powder and Al and Se in baby food.
The mean level of Ni in baby food was 8.60 × 10–1 ± 4.81 × 10–2 mg/kg and in milk powder was 1.30 × 10–2 ± 1.65 × 10–3 mg/kg. The mean concentrations of Al, Se, Cd, As, Pb, and Hg (6 toxic elements) in milk powder were < LOD, < LOD, < LOD, < LOD, 3.48 × 10–3 ± 4.50 × 10–4, and 5.00 × 10–4 ± 9.00 × 10–5 mg/kg, respectively. Also, the mean concentration of As, Al, Se, Cd, Pb, and Hg in baby food were 1.48 × 10–3 ± 7.80 × 10–4, < LOD, < LOD, 7.33 × 10–3 ± 3.78 × 10–3, 8.50 × 10–4 ± 2.00 × 10–4, and 4.43 × 10–4 ± 6.00 × 10–5 mg/kg, respectively. As shown in Table 2, our results were lower than the permissible standards levels of Iran, Egypt, and Codex.
Consumption of baby foods with toxic elements can be very harmful for the baby’s development.
However, baby food should have the necessary and appropriate elements in order to create better conditions for development, nutritional support, and safety of growing infants. The results show that the level of carcinogenic elements in baby food is relatively low and is within the acceptable level recommended by the European Commission. The current results are more or less agreed with the previous reports in different countries [2, 4, 20, 25, 44].
Dasbası et al. showed that concentration of toxic elements Cu(II),Zn(II), Fe(III), and Cr(III) were 2.7 × 10–1 ± 3 × 10–2, 4.89 × 10–1 ± 2 × 10–1, and 7.72 × 10–1 ± 2 × 10–1 mg/kg and not detected (n.d.), respectively, in milk powder in Turkey; however, Pb(II), Co(II), and Cd(II) were not detected which was more than our study [9].
Ghorbani-Kalhor et al. reported that the concentrations of Cd, Pb, and Zn were 7.70 –1.53 × 10+1, 1.94 × 10+1–1.21 × 10+2, and 1.67 × 10+1–2.43 × 10+1 mg/kg, respectively, in infant formula samples [45].
Sager et al. showed that the concentration of Cd in milk powder was at the range of less than 1.00 × 10–3–7.00 × 10–3 mg/kg of sample dry weight, but in our study was not detected [3].
Salah et al. reported that in all samples of milk powder, the concentrations of Cd, Pb, Mn, and Fe were higher than the EU standard and 58–96% of the samples had Se and Al levels higher than the standard limit. These authors found that the mean concentration of Cd, Pb, Fe, Al, Se, and Mn were 3.22 × 10–1 mg/kg, 7.91 × 10–1 mg/kg, 2.041 × 101 mg/kg, 1.57 mg/kg, 1.4 × 10–2 mg/kg, and 4.97 × 10–1 mg/kg, respectively [4].
Sadeghi et al. reported that total mean levels of Zn were 1.20 × 10–1 ± 1.5 × 10–2 mg/kg samples, Pb was 4.5 × 10–3 ± 0.00 mg/kg, Cu was 5.1 ± 8.30× 10–1 mg/kg, and Cd was 5 × 10–3 ± 0.00 mg/kg in 240 baby food samples. Also, total mean levels Pb, Cd, Cu, and Zn were 1 × 10–3 ± 0.00 mg/kg, 6 × 10–3 ± 4 × 10–3 mg/kg, 4.04 ± 1.34 × 10–2 mg/kg, and 3.62× 10–1 ± 5.3 × 10–2 mg/kg samples in 240 milk powder samples, respectively, which is consistent with the present study [46].
In a study on the 437 infant formula samples in Canada by Dabeka et al., the levels of Pb and Cd were 4.00 × 10–2 and 1.00 × 10–2 mg/kg, respectively [44], which in this study results were lower than our study.
Khan et al. in South Korea reported that the levels of Mo, Se and Cr in infant formula ranged from 7.80 × 10–2 ± 9.00 × 10–3 to 2.83 × 10–1 ± 2.50 × 10–2, 7.90 × 10–2 ± 3.00 × 10–3 to 2.21 × 10–1 ± 1.30 × 10–2 and < LOD to 1.19 × 10–1 ± 1.10 × 10–2 mg/kg, respectively but in our study Se was not detected [47].
Sorbo et al. in Italy reported that As concentration was lower than LOQ in infant formula, while Cd and Pb were 1.20 × 10–3 to 5.00 × 10–3 and 4.50 × 10–3 to 1.16 × 10–2 mg/kg, respectively which Pb concentration in our study was less than Sorbo study [48].
Al-Zahrani showed that the mean levels of Zn, Fe, Cu, Pb, and Mn were 2.91 mg/kg, 3.033 mg/kg, 1.82 × 10–1 mg/kg, 3.4 × 10–2 mg/kg, and 3.1 × 10–2 mg/kg, respectively, in milk powder samples and that all of them were higher than our study [30].
Gasmalla et al. reported that the mean concentration of Pb in fresh milk of cows was 6.88 × 10–1 ± 1.5 × 10–1 mg/kg, in milk powder was 3.32 ± 1.66 mg/kg, and in infant milk was 3.64 ± 1.79 mg/kg. In the same study, Cd concentration was 1.97 ± 4 × 10–1 mg/kg in fresh milk, 4.06 ± 1.9 mg/kg in powder of milk, and 2.11 ± 5.6 × 10–1 mg/kg in infant formula which both of them were higher than results of this study [49].
Abdulkhaliq et al. found that the mean levels of Pb, Cu, and Cd were ND-9.3×10–1 mg/kg, 6.2×10–1-8.5×10–1 mg/kg, and 2.2 × 10–2- 5.7 × 10–2 mg/kg in the milk and dairy samples, which were somewhat more than our study [29].
Choi et al. reported that the mean concentration of Cd (for modified milk and follow-up formula), Pb, and As (for modified milk and infant formula) were 2 × 10–3 and 7 × 10–3 mg/kg, 4 × 10–4 and 1 × 10–2 mg/kg, and 4 × 10–3 and 4 × 10–2 mg/kg, respectively, which were somewhat more than our study [50].
Akhtar et al. reported that the concentrations of Cd and Pb were lower than LOD in the infant formula milk sample; the recorded concentrations of Fe, Zn, and Ni ranged 4.54 × 10–2–9.7 × 10–2, 2.97 × 10–2–1.14 × 10–1, and < 1.00 × 10–6–5.09 × 10–2 mg/kg, respectively, which all of them were less than our study [51].
Mania et al. showed that the mean levels of Pb were 2.8 × 10–2 mg/kg in the 90th percentile that was not higher than 1.3 × 10–2 mg/kg, and in the infant formulas, the Pb concentration was below the 1 × 10–2 mg/kg, but the mean level of Pb was 5 × 10–3 mg/kg. The highest mean level of Cd was 1 × 10–2 mg/kg in infant formulas (undiluted) based on milk in vegetable meal, Pb was 9 × 10–3 in infant formulas (undiluted) based on milk soya-based, and Pb was less than 3 × 10–3 in infant formulas (undiluted) based on milk in vegetable meal. The highest mean level of Hg in fish-based infant foods was 1.3 × 10–2 mg/kg and As in the foods that based on fish was 1.8 × 10–1 mg/kg, and the level of Hg was less than 1.4 × 10–1 mg/kg in rice products used for the infant formulas, which all of them were higher than our study [27].
Mehrnia et al. reported that the mean concentration of Cd, Ni, Mn, and Pb in various infant formulas ranged from 4.03 × 10–2 to 5.80 × 10–2, 4.48 to 6.42, 2.30 to 4.88, and 3.18 × 10–2 to 3.19 × 10–2 mg/kg, respectively, which all of them were higher than our study [26].
Ataro et al. reported that the concentrations of Cr, V, Sr, and Mn ranged from 1.86 × 10–1 to 3.71 × 10–1, 2.34 × 10–2 to 4.20 × 10–2, 1.88 to 3.15, and 1.09 × 10–1 to 2.99 × 10–1 mg/kg, respectively, in the whole raw milk samples; however, Cd was not detected in all samples, and the concentration of Pb was in the range of less than LOD to 1.97 × 10–2 mg/kg, which Cr, Mn, and Pb were higher and Cd was less than our study [20].
Cruz et al. determined the concentration of Pb, Al, and Hg in three samples of infant formula milk and reported that Al and Pb were not detected, but 2 out of the 3 infant formulas tested were positive for mercury with a level of 8.33 × 10–1 and 6.33 × 10–1 mg/kg [43].
Saracoglu et al. showed that the metal concentrations in baby food samples ranged from 5.20 × 10–1 to 4.38 mg/kg for Cu, 2.20 × 10–1 to 7.20 mg/kg for Mn, 1.02 to 6.75 × 10+1 mg/kg for Fe, 9.20 × 10–1 to 3.72 × 10+2 mg/kg for Zn, 1.20 × 10–1 to 3.20 × 10–1 mg/kg for Se, 2.02 × 10–3 to 6.88 × 10–2 mg/kg for Cr, 1.07 × 10–2 to 6.68 × 10–2 mg/kg for Al, 5.00 × 10–2 to 1.03 × 10–2 mg/kg Ni, and 2.67 × 10–3 to 2.54 × 10–2 mg/kg for Co, in which Cu, Mn, Fe, Zn, and Co were higher and Cr, Al and Ni were less than our study [14].
Kazi et al. analyzed Al, Cd, and Pb in infant soy-based formulae and infant milk-based in Pakistan and found that the concentration of Cd, Al, and Pb were 1.05 × 101 to 3.44 × 101, 1.07 × 103 to 2.17 × 103, and 2.87 × 101 to 1.19 × 102 mg/kg, respectively [15].
Comparison of the Trace Elements Detected with the National and International Standards
In the current study, the levels of trace elements in milk powder and baby food were compared to the permissible limit of the Codex and standard levels of Iran and Egypt [4] (Table 2). The levels of Al, Cd, Cr, Co, Fe, Hg, Cu, Mn, Pb, Ni, Zn, and Se in milk powder and baby food samples were lower than the mentioned standard level (of course for the elements that exist). The higher content of heavy elements in baby food and milk powder may be resulted from the contamination during handling, exposure to processing equipment during processing, and storage. During the processing of steel and Al equipment, milk powder producer, such contaminants, may be the consequences [5, 50, 52]. The difference in the concentration of heavy elements in food can be due to several factors such as species differences, specifications of the production methods, and contamination probably by the equipment’s during the production process [4]. The equipment and container oxidation were affected by several items like the quality of the raw materials used. Additionally, pH and the oxidation improvement may increase the level of heavy metal in the samples. The presence of heavy elements in raw milk may affect the heavy elements concentration in powdered milk. Increased concentration of Pb in milk powder may be due to the contamination of raw milk used in the production of milk powder [43, 49]. One of the causes of milk contamination and consequently the dry milk contamination is the location of farms near highways, which may lead to the contamination of water and animal feed [13, 30]. Dairy products such as milk and milk powder usually contain very low concentration of Cd except when animals consumed contaminated feeds and water [9, 44, 50]. In addition, the contamination with Cd and other elements may occur during washing machines, storage, and transportation of milk. Pb was mainly associated with caseins in cow’s milk and freezing or heating did not cause significant changes in distribution of Pb in cow’s milk and milk products [15, 47]. Also, the mean of Al concentration was higher than standard limit which may be due to the migration of Al from packaging materials to the milk [2, 27, 53]. The milk powder and baby food is widely consumed; in infant, therefore, fortification of baby food and milk powder can provide children with most of the nutrient deficiency. Also, according to several studies, the high concentration of Fe and Zn in milk powder may be due to the fortification of the sample by Fe and Zn [4, 54,55,56]. Moreover, the level of Se in milk mainly depends on feeding of animals and geographically distribution [7, 29, 30].
In general, contamination with toxic metals in baby food may occur through contaminated raw materials and/or through contaminated equipment during the manufacturing process. As mentioned, industrial and agricultural processes can lead to increased concentrations of heavy elements in air, water, and soil, which in turn can accumulate in plant and animal tissues and lead to contamination of milk, milk powder, and other foods.
Estimated Daily Intake for Essential and Toxic Elements
The comparison of EDI for all of the essential and toxic elements in milk powder and baby food samples with their provisional tolerable daily intakes (PTDI) or their bench mark dose levels (BMDL) and recommended daily allowance (RDA) is presented in (Table 3). The mean EDI (accordance mg/day) of Cu, Fe, Mn, Se, and Zn in milk powder for infants (0–6 months) were 8.85 × 10–4, 1.12 × 46 × 10–3, 1.19 × 10–4, 9.98 × 10–4, and 1.46 × 10–2; for toddlers (12–24 months) were 7.13 × 10–4, 9.02 × 10–3, 1.14 × 10–4, 9.51 × 10–4, and 1.39 × 10–2 mg/day; and for baby toddlers (6 − 24 months) were 9.45 × 10–4, 1.85 × 10–2, 5.05 × 10–4, 5.27 × 10–4, and 1.65 × 10–2, respectively. The highest intake was for the Zn (at the range of 1.39 × 10–2 to 1.72 × 10–2 mg/day) in the milk powder. The Reference Daily Intake (RDI) contribution of the elements in milk powder and baby food was lower 10–2, 1.41 × 10–4, 1.18 × 10–4 and 1.72 × 10–2; for infants (6–12 months) were 7.48 × 10–4, 9. than 100 percent. These findings give an indication that consuming such milk powder and baby food were lower than the PTDI and BMDL guidelines [10].
Human Health Risk Assessment
The United States Environmental Protection Agency (EPA) applied the Monte Carlo simulation in the probabilistic risk assessments to minimize the uncertainties of the risk calculations with probability distribution for each parameter to avoid the overestimation or underestimation [57]. In this concern, several studies have been performed on the probabilistic health risk assessment by the Monte Carlo simulation for infants and toddlers in rice cereal in the USA [58], polycyclic aromatic hydrocarbons in infant formulae in Iran [34], and aflatoxins M1 and B1 in breast milk and ochratoxin A and deoxynivalenol in wheat noodles in Belgium [59]. The rank order of the HQ index according to 95% percentile for infants (0–6 months) was Hg (1.54 × 10–1) > Ni (3.66 × 10–2) > As (1.05 × 10–3) > Cd (6.16 × 10–4) > Al (6.32 × 10–7); for infants (6–12) was Hg (1.33 × 10–1) > Ni (1.74 × 10–2) > As (8.74 × 10–4) > Cd (5.34 × 10–4) > Al (5.35 × 10–7); for milk toddlers (12–24) was Hg (1.24 × 10–1) > Ni (1.64 × 10–2) > As (8.20 × 10–4) > Cd (5.06 × 10–4) > Al (5.03 × 10–7); and for baby toddlers (6 − 24) was Ni (5.90 × 10–1) > Cd (1.02 × 10–1) > As (6.90 × 10–2) > Hg (6.18 × 10–2) > Al (2.78 × 10–7) as shown in Table 4. Among all of the samples, TQ95 estimated below 1; therefore, milk powder and baby food are not hazardous to the consumer.
The literature also reports several studies on human health risk due to potentially toxic elements of baby food. Similar observations in Turkey, Demir et al. showed that the hazard indexes (HI) of elements (Fe, Cu, Al, Cd, As, Ni, Ba) in infant milk formulas samples were less than 1 [60]. Ghuniem et al. in the Egypt showed that the hazard quotient and the hazard index for toxic elements in rice-based and wheat-based baby cereals were safe (5.48 × 10–2- 1.86 × 10–1) for consumption by the baby [61].
The data in (Fig. 1) showed that Hg and Ni are the two main contributors in total hazard index (THQ), and whiles the other trace elements that measured have contribution of less than 3 percent. The mean of THQ value and contribution of toxic elements was used to compare hazard index between 4 subgroups: infants (with 0 to 6 months), infants (with 6 to 12 months), toddlers (with 1 to 2 year), and toddlers (with 6 to 24 months). Although the HQ associated with each heavy metal increases with increasing the ingestion rate, the contribution of Hg for infants and Ni for toddlers in milk powder and baby food becomes more dominant at higher ingestion rates. The potential human health risks from contaminants in food depends on different agents that these factors are such as exposure time, consumption of foodstuff per capita, body weight (BW) and toxicity. Also, harmful effects on health are high for people exposed to these foods when the THQ > 10. So, according to value of THQ, the milk powder and baby food that analyzed in this study are not harmful on healthy infants and toddlers. Thus, based on the results of this study, the examined milk powder and baby food consumption is safe for infants and toddlers.
Carcinogenic Risk (CR)
Based on Monte Carlo simulation, the carcinogenic risk indexes of As (percentile 95%) in the milk powder and baby food for infants aged 0 to 6 months, infants aged 6 to 12 months, toddlers aged 1–2 years and toddlers with 6 months to 2 years, were 1.40 × 10–6, 1.17 × 10–6,1.16 × 10–6 and 6.29 × 10–6, respectively (Fig. 2). The simulation histogram or probabilistic distributions of carcinogenic risks via the oral pathway of As in the samples for infants and toddlers are shown in Fig. 2. The infants at 6–12 months has more exposure with As element, so could pose higher carcinogenic risk in comparison with other groups. This simulation histogram is very useful to characterize the potential adverse health effects of human exposures to As, because knowing the likelihood of achieving the needed level of CR index can help to make decisions for ensuring a minimum CR index.
The qualitatively describe of cancer risk specifications can be characterized in three modes; CR with limits less than 10–6 is the safe limit; CR with limits higher than 10–4 is the limit of threshold risk; CR higher than 10–3 is the limit of considerable risk. CR assessment comparison of inorganic As (milk powder and baby food) by USEPA regulatory standard was below 10–6. In similar studies in Kuwaiti, Jallad (2019) showed that the CR index for As due to the ingestion of baby food, growing-up milk, and infant cereal was < 10−4, indicating that the infants were at the safety limit [62]. Shibata et al. (2016) in the USA showed that the incremental lifetime cancer risk (ILCR) for As levels in rice cereal was safe for consumption by infants and toddlers. They reported the ILCR index ranged from 10−6 to 10−5 [58]. It is important to reduce public health risks by imposing strict regulations for all toxic elements, especially in baby food to prevent toxic elements contamination. Producers of baby food and other health-related goods should ensure the proper quality of their products by improving their processing method and selecting raw materials.
Conclusion
Trace elements concentration in milk powder and baby food collected from market of Iran as determined by ICP-OES revealed that the highest average concentration of Zn was found in milk powder and Fe was found in baby food. However, these amounts were less than the standard limit recommended by Codex. The lowest mean concentrations of elements in milk powder were Al, As, Cd, Co, and Se; meanwhile, Al and Se were the lowest in baby foods. Toxic elements concentration in the milk powder and baby food were lower than the recommended standard limits. This study, focusing on a sensitive contamination, will provide appropriate data on the dietary exposure of infant to trace elements and will be useful for predicting risk assessment analysis. The results of the health risk which assessed based on Monte Carlo simulation showed that HQ value was lower than 1. So consumption of the examined milk powder and baby food was not notable non-carcinogenic risk for consumer (CR and actual CR < 1 × 10–4 value).
Data Availability
All data related to this article are given in tables and figures and no other data is available.
References
Abdallah MIMI (2005) Evaluation of some heavy metal residues in whole milk powder used at confectionery plants regarding their public health significance. J Egypt Vet Med 65:1–5
Shariatifar N, Dadgar M, Fakhri Y, Shahsavari S, Moazzen M, Ahmadloo M, Kiani A, Aeenehvand S, Nazmara S, Khanegah AM (2020) Levels of polycyclic aromatic hydrocarbons in milk and milk powder samples and their likely risk assessment in Iranian population. J Food Compos Anal 85:103–331
Sager M, McCulloch CR, Schoder D (2018) Heavy metal content and element analysis of infant formula and milk powder samples purchased on the Tanzanian market: international branded versus black market products. Food Chem 255:365–371
Salah F, Esmat I, Mohamed A (2013) Heavy metals residues and trace elements in milk powder marketed in Dakahlia Governorate. Int Food Res J 20:4
Fernandes TA, Brito JA, Gonçalves LM (2015) Analysis of micronutrients and heavy metals in Portuguese infant milk powders by wavelength dispersive X-ray fluorescence spectrometry (WDXRF). Food Anal Methods 8(1):52–57
Abdelkhalek A, Elsherbini M, Gunbaej E (2015) Assessment of heavy metals residues in milk powder and infant milk formula sold in Mansoura City, Egypt. Alex J Vet Sci 47(1):71–77
Ikem A, Nwankwoala A, Odueyungbo S, Nyavor K, Egiebor N (2002) Levels of 26 elements in infant formula from USA, UK, and Nigeria by microwave digestion and ICP–OES. Food Chem 77(4):439–447
Abd-Elghany SM, Mohammed MA, Abdelkhalek A, Saad FSS, SALLAM K I, (2020) Health risk assessment of exposure to heavy metals from sheep meat and offal in Kuwait. J Food Prot 83(3):503–510
Daşbaşı T, Saçmacı Ş, Ülgen A, Kartal Ş (2016) Determination of some metal ions in various meat and baby food samples by atomic spectrometry. Food Chem 197:107–113
Abd-Elghany SM, Zaher HA, Elgazzar MM, Sallam KI (2020) Effect of boiling and grilling on some heavy metal residues in crabs and shrimps from the Mediterranean Coast at Damietta region with their probabilistic health risk assessment. J Food Compos Anal 93:103606
Tripathi R, Raghunath R, Sastry V, Krishnamoorthy T (1999) Daily intake of heavy metals by infants through milk and milk products. Sci Total Environ 227(2–3):229–235
Fathabad AE, Shariatifar N, Moazzen M, Nazmara S, Fakhri Y, Alimohammadi M, Azari A, Khaneghah AM (2018) Determination of heavy metal content of processed fruit products from Tehran’s market using ICP-OES: a risk assessment study. Food Chem Toxicol 115:436–446
Al Khalifa A, Ahmad D (2010) Determination of key elements by ICP-OES in commercially available infant formulae and baby foods in Saudi Arabia. Afr J Food Sci 4(7):464–468
Saracoglu S, Saygi KO, Uluozlu OD, Tuzen M, Soylak M (2007) Determination of trace element contents of baby foods from Turkey. Food Chem 105(1):280–285
Kazi TG, Jalbani N, Baig JA, Afridi HI, Kandhro GA, Arain MB, Jamali MK, Shah AQ (2009) Determination of toxic elements in infant formulae by using electrothermal atomic absorption spectrometer. Food Chem Toxicol 47(7):1425–1429
Landrigan PJ, Goldman LR (2011) Children’s vulnerability to toxic chemicals: a challenge and opportunity to strengthen health and environmental policy. Health Aff 30(5):842–850
Shariatifar N, Seilani F, Jannat B, Nazmara S, Arabameri M (2020) The concentration and health risk assessment of trace elements in commercial soft drinks from Iran marketed. Int J Environ Anal Chem 5:1–15
Sallam KI, Abd-Elghany SM, Mohammed MA (2019) Heavy metal residues in some fishes from Manzala Lake, Egypt, and their health-risk assessment. J Food Sci 84(7):1957–1965
Yao BM, Chen P, Sun GX (2020) Distribution of elements and their correlation in bran, polished rice, and whole grain. Food Sci Nutr 8(2):982–992
Ataro A, McCrindle RI, Botha B, McCrindle CME, Ndibewu P (2008) Quantification of trace elements in raw cow’s milk by inductively coupled plasma mass spectrometry (ICP-MS). Food chem 111(1):243–248
Lima JF, Delerue-Matos C, Vaz MCV (1998) Automation of iron and copper determination in milks using FIA systems and colourimetric detection. Food Chem 62(1):117–121
Yebra M, Moreno-Cid A, Cespón R, Cancela S (2004) Preparation of a soluble solid sample by a continuous ultrasound assisted dissolution system for the flow-injection atomic absorption spectrometric determination of iron in milk powder and infant formula. Talanta 62(2):403–406
Zamir T, Hussain A (2001) Determination of lead and cadmium level in powdered milk in Quetta (Pakistan) by atomic absorption spectrometry. Online J Biol Sci 1(5):412–413
Licata P, Trombetta D, Cristani M, Giofre F, Martino D, Calo M, Naccari F (2004) Levels of “toxic” and “essential” metals in samples of bovine milk from various dairy farms in Calabria, Italy. Environ Int 30(1):1–6
Vinas P, Pardo-Martınez M, Hernandez-Cordoba M (2000) Rapid determination of selenium, lead and cadmium in baby food samples using electrothermal atomic absorption spectrometry and slurry atomization. Anal Chim Acta 412(1–2):121–130
Mehrnia MA, Bashti A (2014) Evaluation of toxic element contents in infant foods commercially available in Iran. BEPLS 3:249–253
Mania M, Wojciechowska-Mazurek M, Starska K, Rebeniak M, Szynal T, Strzelecka A, Postupolski J (2015) Toxic elements in commercial infant food, estimated dietary intake, and risk assessment in Poland. Pol J Environ Stud 24(6):2525–2536
Abd-El Aal SFA (2012) Assessment of toxic heavy metals in some dairy products and the effect of storage on its distribution. Am J Sci 8(8):655–670
Abdulkhaliq A, Swaileh K, Hussein RM, Matani M (2012) Levels of metals (Cd, Pb, Cu and Fe) in cow’s milk, dairy products and hen’s eggs from the West Bank, Palestine. Int Food Res J 19(3):1089–1094
Al-Zahrani J (2012) Natural radioactivity and heavy metals in milk consumed in Saudi Arabia and population dose rate estimates. J Life Sci 9(2):651–656
Arabameri M, Mohammadi Moghadam M, Monjazeb Marvdashti L, Mehdinia SM, Abdolshahi A, Dezianian A (2020) Pesticide residues in pistachio nut: a human risk assessment study. Int J Environ Anal Chem 5:1–14
Eghbaljoo-Gharehgheshlaghi H, Shariatifar N, Arab A, Alizadeh-Sani M, Sani IK, Asdagh A, Rostami M, Alikord M, Arabameri M (2020) The concentration and probabilistic health risk assessment of trace metals in three type of sesame seeds using ICP- OES in Iran. Int J Environ Anal Chem 4:1–15
Jahanbakhsh M, Afshar A, Momeni Feeli S, Pabast M, Ebrahimi T, Mirzaei M, Akbari-Adergani B, Farid M, Arabameri M (2019) Probabilistic health risk assessment (Monte Carlo simulation method) and prevalence of aflatoxin B1 in wheat flours of Iran. Int J Environ Anal Chem 6:1–12
Badibostan H, Feizy J, Daraei B, Shoeibi S, Rajabnejad SH, Asili J, Taghizadeh SF, Giesy JP, Karimi G (2019) Polycyclic aromatic hydrocarbons in infant formulae, follow-on formulae, and baby foods in Iran: An assessment of risk. Food Chem Toxicol 131:110640
Singla RK, Bhat VG (2011) Crocin: an overview. Indo Glob J Pharm 1(4):281–286
Mousavi SZ, Bathaie SZ (2011) Historical uses of saffron: identifying potential new avenues for modern research. Avicenna J Phytomed 1(2):57–66
Gorji MEh, Ahmadkhaniha R, Moazzen M, Yunesian M, Azari A, Rastkari N (2016) Polycyclic aromatic hydrocarbons in Iranian Kebabs. Food Control 60:57–63
Iranian National Standardization Organization. (2010) Candy sugar-Specifications and test methods. Record Number, 168. http://www.isiri.gov.ir/portal/files/std/739.pdf
United States Environmental Protection Agency (2015) quantitative risk assessment calculations 7–9. https://www.epa.gov/sites/production/files/2015-08/toxicity_weighting_spreadsheet_v2.3.3.xlsx
Cao S, Duan X, Zhao X, Wang B, Ma J, Fan D, Sun C, He B, Wei F, Jiang G (2015) Health risk assessment of various metal (loid) s via multiple exposure pathways on children living near a typical lead-acid battery plant, China. Environ Pollut 200:16–23
Sultana MS, Rana S, Yamazaki S, Aono T, Yoshida S (2017) Health risk assessment for carcinogenic and non-carcinogenic heavy metal exposures from vegetables and fruits of Bangladesh. Cogent Environ Sci 3(1):1291107
Antoine JM, Fung LAH, Grant CN (2017) Assessment of the potential health risks associated with the aluminium, arsenic, cadmium and lead content in selected fruits and vegetables grown in Jamaica. Toxicol Rep 4:181–187
Cruz GC, Din Z, Feri CD, Balaoing AM, Gonzales EM, Navidad HM, Schlaaff MMF, Winter J (2009) Analysis of toxic heavy metals (arsenic, lead and mercury) in selected infant formula milk commercially available in the Philippines by AAS. Int J Sci Res 1(1):40–51
Dabeka R, Fouquet A, Belisle S, Turcotte S (2011) Lead, cadmium and aluminum in Canadian infant formulae, oral electrolytes and glucose solutions. Food Addit Contam 28(6):744–753
Ghorbani-Kalhor E, Hosseinzadeh-Khanmiri R, Babazadeh M, Abolhasani J, Hassanpour A (2015) Synthesis and application of a novel magnetic metal-organic framework nanocomposite for determination of Cd, Pb, and Zn in baby food samples. Can J Chem 93(5):518–525
Sadeghi N, Oveisi MR, Jannat B, Hajimahmoodi M, Behfar A, Behzad M, Norouzi N, Oveisi M, Jannat B (2014) Simultaneous measurement of zinc, copper, lead and cadmium in baby weaning food and powder milk by DPASV. Iran J Pharm Sci 13(1):345
Khan N, Jeong IS, Hwang IM, Kim JS, Choi SH, Nho EY, Choi JY, Kwak B-M, Ahn J-H, Yoon T (2013) Method validation for simultaneous determination of chromium, molybdenum and selenium in infant formulas by ICP-OES and ICP-MS. Food Chem 141(4):3566–3570
Sorbo A, Turco A, Di Gregorio M, Ciaralli L (2014) Development and validation of an analytical method for the determination of arsenic, cadmium and lead content in powdered infant formula by means of quadrupole Inductively Coupled Plasma Mass Spectrometry. Food Control 44:159–165
Gasmalla MA, Khadir KE, Musa A, Aboshora W, Zhao W (2013) Evaluation of some physicochemical parameters of three commercial milk products. PJFS 23(2):62–65
Choi H, Kim H-S, Park S-H (2013) Risk assessment of heavy metals through modified milk powder and formulas. Food Sci Anim Resour 33(5):617–625
Akhtar S, Shahzad MA, Yoo S-H, Ismail A, Hameed A, Ismail T, Riaz M (2017) Determination of aflatoxin M1 and heavy metals in infant formula milk brands available in Pakistani markets. Korean J Food Sci Anim Resour 37(1):79
Ji K, Kim J, Lee M, Park S, Kwon H-J, Cheong H-K, Jang J-Y, Kim D-S, Yu S, Kim Y-W (2013) Assessment of exposure to heavy metals and health risks among residents near abandoned metal mines in Goseong, Korea. Environ Pollut 178:322–328
Yaman M, Çokol N (2004) Determination of trace elements in human milk, cow’s milk, and baby foods by flame AAS using wet ashing and microwave oven sample digestion procedures. Norwalk CT 25:185–190
Meshref AM, Moselhy WA, Hassan NE-HY (2014) Heavy metals and trace elements levels in milk and milk products. J Food Meas Charact 8(4):381–388
Lutfullah G, Khan AA, Amjad AY, Perveen S (2014) Comparative study of heavy metals in dried and fluid milk in Peshawar by atomic absorption spectrophotometry. Sci World J 2014 (2014): 715845. https://doi.org/10.1155/2014/715845
Moraes ML, Feijó M, Melo FM, Campos RC, Hauser-Davis RA (2009) Iron, copper and zinc in substitute foods for maternal milk: comparison with infant nutritional requirements. J Braz Chem Soc 20(9):1724–1731
United States Environmental Protection Agency (2016) Use of Monte Carlo Simulation in Risk Assessments. https://www.epa.gov/risk/use-monte-carlo-simulation-risk-assessments
Shibata T, Meng C, Umoren J, West H (2016) Risk assessment of arsenic in rice cereal and other dietary sources for infants and toddlers in the US. Int J Environ Res Public Health 13(4):361
Ortiz J, Jacxsens L, Astudillo G, Ballesteros A, Donoso S, Huybregts L, De Meulenaer B (2018) Multiple mycotoxin exposure of infants and young children via breastfeeding and complementary/weaning foods consumption in Ecuadorian highlands. Food Chem Toxicol 118:541–548
Demir F, Yildirim M, Mermer N K, Derun E M (2017) Determination of some element concentrations of first infant milk formula and health risk assessment. CBU International Conference Proceedings 937–942
Ghuniem MM, Khorshed MA, El-safty SM, Souaya ER, Khalil MMH (2020) Assessment of human health risk due to potentially toxic elements intake via consumption of Egyptian rice-based and wheat-based baby cereals. Int J Environ Anal Chem 5:1–19
Jallad KN (2019) The hazards of a ubiquitary metalloid, arsenic, hiding in infant diets: detection, speciation, exposure, and risk assessment. Biol Trace Elem Res 190(1):11–23
Funding
This study was carried out by the researchers in the Fasa University of Medical Sciences and Health Services with grant number (NO. 97380). Funding obtained for this study has been fully spent.
Author information
Authors and Affiliations
Contributions
Nabi Shariatifar: Conceptualization; Supervision; Design of study; Writing, reviewing and editing. Amin Kiani.: Data curation; Writing, original draft preparation. Gholamreza Jahed Khaniki: Visualization, Investigation. Mojtaba Moazzen: Writing, reviewing and editing; Software. Saeed Aeenehvand: Writing, reviewing and editing; Methodology; Validation. Majid Arabameri: Software, Methodology, Validation. Saeed Shahsavari: Software, Methodology, Validation. Mossad Abdel-Wahhab: Writing, reviewing and editing; Design of study; Methodology; Validation.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This study does not involve any human or animal testing, or this study was approved by the Institutional Review Board of Food Safety of Fasa University of Medical Sciences.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kiani, A., Arabameri, M., Moazzen, M. et al. Probabilistic Health Risk Assessment of Trace Elements in Baby Food and Milk Powder Using ICP-OES Method. Biol Trace Elem Res 200, 2486–2497 (2022). https://doi.org/10.1007/s12011-021-02808-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02808-w