Abstract
Infant exposure to neurotoxic elements is a public health issue that needs monitoring with regard to breast milk composition. We studied six neurotoxic elements in breast milk samples at different stages of lactation in mothers from Porto Velho, Brazil. We used a flow-injection mercury system (FIMS) to determine total Hg concentrations and an inductively coupled plasma optical emission spectrometer (ICP-OES) to determine the concentrations of Al, As, Cd, Pb, and Mn in 106 donors of a human milk bank. Association rules analyses were applied to determine the pattern of binary and ternary mixtures of the measured exposants. The metal concentration was mostly below the limit of detection (LOD) for Cd (99%), Pb (84%), and Hg (72%), and it was above the LOD for As (53%), Mn (60%), and Al (82%), respectively. Median concentrations (dry weight) of Al, As, Hg, Mn, and Pb were 1.81 μg/g, 13.8 ng/g, 7.1 ng/g, 51.1 ng/g, and 0.43 μg/g, respectively. Al is singly the most frequent element to which infants are exposed. Occurring binary combination (> LOD) was 56% for Al-Mn, 41% for Al-As, 22% for Al-Hg, and 13% for Al-Pb. In 100% of neonates, exposure to Al-ethylmercury (EtHg) occurred through immunization with thimerosal-containing vaccines (TCV). Association rules analysis revealed that Al was present in all of the multilevel combinations and hierarchical levels and that it showed a strong link with other neurotoxic elements (especially with Mn, As, and Hg). (a) Nursing infants are exposed to combinations of neurotoxicants by different routes, dosages, and at different stages of development; (b) In breastfed infants, the binary exposures to Al and total Hg can occur through breast milk and additionally through TCV (EtHg and Al); (c) The measured neurotoxic elements were found at low frequencies in breast milk and at concentrations that pose no public health concerns for milk banking.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human milk is a source of nourishment for newborns and infants, providing essential nutrition and immunoprotective features. The World Health Organization (WHO), policy makers, pediatricians, and health workers recommend breastfeeding as a public health priority to protect, promote, and support healthy infant development. The WHO recommends breastfeeding exclusively for the first 6 months of life [1]. And, in many parts of the world including Brazil, human milk is prescribed for premature and critically ill infants in neonatal units when the mother’s own milk is insufficient or not available [2]. The majority of newborns in the world are nursed by mothers living in less-developed countries.
The least fortunate in developing countries live in less than ideal urban environments, frequently in contaminated areas. Therefore, maternal environmental exposure to metals can occur mainly through lifestyle, air, and food [3,4,5]. In food, it will depend not only on the type and its origin but also on the cookware in which foods are prepared. Weidenhamer et al. [6] showed that artisanal aluminum cookware is a significant source of exposure to toxic metals (Al, Pb, As, Cd), and it can leach substantial amounts of these metals per serving. Toxic elements can cross both the placental and the mammary gland barriers [7,8,9]. As a result, simultaneous exposures to multiple neurotoxic substances are common in breastfed children living in contaminated environments [10, 11]. Furthermore, the toxicokinetics of neurotoxic elements seem exacerbated by high intestinal absorption in milk diet [12]. Therefore, the presence of toxic elements in human milk is an indicator of maternal exposure occurring during pregnancy and lactation.
Thus, human milk is a biomonitoring tool that indicates the level of accumulation and/or contamination of environmental toxic chemicals; it is useful to assess maternal exposure and respective hazardous risks for the developing infant [13]. However, exposures to more than one neurotoxic element do occur. Therefore, estimations of exposure to toxic metals by nursing infants are subject to unavoidable shortcomings; data on human milk intake and metal determination are rarely available from the same studies [11]. Nevertheless, risk analysis for metals in human milk has been addressed with available published results [14].
The issue of exposure and potential harm related to a combination of neurotoxic elements during early life has become a persistent problem; solutions are hindered by the complexity of such studies. Child neurodevelopment inequalities start during pregnancy and extend through lactation; indeed, exposure to toxic metals can accumulate when the child is still breastfeeding [15]. To understand the cumulative and/or synergistic effects of multiple exposures requires full assessment of the co-occurring substances. Additionally, it is also important to estimate binary and ternary interactions to interpret higher order interactions [16].
Studies have shown substantial risk for both forms of organic-Hg exposure – methyl-Hg (MeHg) from maternal fish consumption and ethylmercury (EtHg) from thimerosal-containing vaccines (TCV) still used in developing countries [17, 18]. Occurrence of exposure to the binary mixture of Al-EtHg (adjuvant and thimerosal in hepatitis B vaccine) is by far higher than the combination of Al and total Hg in colostrum given to term and pre-term newborns [19]. The potential additive or synergistic effects on children of combined (binary or ternary) exposure will depend on the type of the multiple co-occurring exposures [10, 19]. Von Stackelberg et al. [20] reviewed neurotoxic outcomes of combined exposures to As-Pb-Cd-Mn; it appears that there is a synergistic effect of the binary combination of Pb-As and Pb-Cd. Therefore, research exploring intrinsic (maternal diet) and extrinsic (TCV) metal exposure is seldom undertaken in postnatal infants.
Due to the importance of exposure to low-doses of neurotoxic substances in early life, the study of breast milk composition is critical to assess levels of contaminants relevant to human lactation and public health. This study characterizes all sources of exposures to neurotoxic elements (Al, As, Cd, Hg, Pb, and Mn) during breastfeeding. We assessed multiple exposures of co-occurring elements in binary and ternary combinations from intrinsic (breast milk) and extrinsic (routine immunization with TCVs) sources.
Materials and Methods
Sampling
The study was approved by the institutional Ethics Committee (Ethics Committee for Studies in Humans of the Federal University of Rondônia, #002/2010) according to Brazilian regulations (#196, Conselho Nacional de Saúde). Mothers were recruited during the year of 2015 among donors to the Human-Milk Bank of Santa Ágata (Hospital Dr. Ary Pinheiro) in the city of Porto Velho-RO (population, 510,000). This Milk Bank provides specialized services supporting and counseling mothers with difficulties in lactating. Furthermore, the services centralize the social diversity of mothers and recipients in society; there is thus, a good societal representation for both donors and recipient infants. During visits to the milk bank, after face-to-face interviews to explain the purpose of the study participating mothers provided informed consent. Volunteer mothers that agreed to participate in the study were in good health (without signs of mastitis and chronic diseases) at the time of milk collection and considered eligible by the milk bank to be donors. In the present study, they had term babies from singleton pregnancy, and breastfeeding between 0 and 240 days. Because of organizational structure (located within the Hospital facility), some mothers were still in the maternity ward. The inclusion/exclusion criteria are an “a priori” decision by the milk bank procedures, thus reducing sampling bias. During the interview, the child’s vaccination card was used to obtain information on TCV received at the time of the milk sampling.
Samples of human milk (10 to 20 mL) were collected directly from both breasts by hand expression, before donation to the Milk Bank. Samples were collected in sterile plastic containers that had been especially prepared (trace-metal free), taken under refrigeration to the laboratory, properly aliquoted and stored at – 80 °C until analysis. In order to preserve the anonymity of the volunteers, all personal information was appropriately disguised.
Sample Preparation and Analysis
Samples of breast milk were defrosted and homogenized by gentle shaking and were properly weighed on analytical scales before and after lyophilization (Terroni-Mod. LH 0500, São Carlos, São Paulo, Brazil). Before analysis, an aliquot of the freeze-dried material was chemically digested with nitric acid. A sample (circa 300 mg dry weight) was weighed into a beaker of 100 mL and mixed with 5.0 mL of HNO3 (65%, Merck). A few drops of H2O2 (30%, Merck) were used with controlled temperature at 80 °C on a hot plate (Nova Instruments, Model NI 1512, São Paulo, Brazil) for circa 60 min until total solubilization of the samples. Subsequently, 0.1 Mol/L of HCl (v/v, 37% Merck) was added with final volume titrated into a 10-mL falcon tube [21,22,23].
The concentrations of the chosen elements were measured using appropriate techniques.
For the determination of Al, As, Cd, Mn, and Pb, we used an inductively coupled plasma optical emission spectrometer (ICP-OES-Perkin-Elmer, mod. Optima 8300, Waltham, MA, USA); this analytical technique allows the detection of nanogram quantities of multiple elements in breast milk, but is not suitable for Hg; more details on instrumental condition and method are described elsewhere [21]. Hg determination is best done by cold-vapor atomic absorption spectroscopy. All chemicals used in sample processing were of high purity grade.
Total Hg determination was done by cold-vapor atomic absorption spectroscopy with a flow-injection mercury system (FIMS) (FIMS-400 – Perkin Elmer, Ueberlingen, Germany) equipped with an automatic sampler (AS90) and software Winlab-PerkinElmer according to our routine method [24]. Chemical treatment of freeze-dried samples was done with H2SO4:HNO3 (98 and 65%, respectively, Merck) solution and KMnO4 (5% w/v, Merck) for oxidation. For about 500 mg of sample (dry weight), 5.0 mL of acid mixture (H2SO4:HNO3—1:1) was added and digested in a digestion block at 60 °C for 1 h (Tecnal-Mod.007A, Piracicaba, São Paulo, Brazil). After digestion, 4.0 mL of KMnO4 solution (5% w/v) was added to the sample, leaving it for 30 min more in the digestion block. After cooling to room temperature, drops of hydroxylamine hydrochloride (12% w/v, Merck) were added, and the volume was made up to 10 mL with ultra-pure water (Milli-Q Plus, Millipore, Bedford, MA, USA). All glassware was washed clean in 10% (w/v) HNO3 and rinsed with ultra-pure H2O.
Quality control was done according to routine laboratory analyses for all samples and using certified reference materials; analyses were run in duplicate, following methodology published elsewhere [25, 26]. The limit of detection (LOD) for each chemical element, as well as the percentages of recovery of reference materials are shown in Table 1. For quality control, we used reference material (NIST-1549). The quality control procedure is routinely employed within the laboratory. All analytical work was done by the same investigator. The chosen elements are backed by literature indicating existing neurological issues of concern related to environmental exposure.
Statistical Analysis
Graphics were created to visually represent cumulative distribution of lactation stages of the collected samples as well as concentrations of the measured elements. We also created histograms to analyze the binary occurrence in the same sample of combined exposure of elements occurring above LOD. To further analyze the data, we used a statistical method to calculate the association between the analyzed elements that could reveal the frequency of patterns of binary and ternary mixtures.
We applied association rules (AR) to discover associations and to verify the data adjustment degree for a set of multiple exposures of measured elements in human milk. The AR technique uses the Apriori algorithm involving two steps: (i) generation of subsets of I with a support greater than a minimum support, and (ii) generation of association rules from the subsets of I whose confidence is greater than a fixed minimum confidence [27]. The AR takes into account a hierarchy for grouping the items and examining the existing rules at different levels. The method creates association rules for which the causes or consequences belong either to the same level or to two different levels of hierarchy. The quality measurements are probabilistic values which limit the combinatorial explosion during the two phases of the algorithm and allow the sorting of the results [27]; to eliminate redundant and unnecessary rules, the reading of the results was simplified by two indexes, alpha and beta, between 0 and 1 [28]. Additionally, multidimensional scaling (MDS) was applied to integrate the multiple associations in the milk composition data; analyses were performed with software XLSTAT 2017.1202 (XLStat data analysis and statistics add-in for MS Excel; ADDINSOFT, Paris, France). The outcomes showed existing patterns of interactions between chosen elements in a simultaneously occurring set (binary or ternary mixture). In the present case, AR revealed which elements of the same transaction (> LOD) occurred with higher frequency.
Results
Table 1 summarizes data on concentrations of neurotoxic elements (Al, Hg, Pb, Cd, As, and Mn) in milk and additional uptake of Al and EtHg in the hepatitis B vaccine (TCV) that is taken by all newborns. Stage of lactation of donor milk varied from 0 to 210 days (Fig. 1). Most samples (87%) were from mothers within 60 days postpartum; colostrum samples (0 to 4 days) comprised 43% (Fig. 1). Vaccination records traceable by immunization cards were only possible for 92 infants. In these infants, the hepatitis B vaccine (TCV) was given on the day of birth to 61 infants (66.3%) and within the first 24 h to 88.0% (81/92); only 6.5% (6/92) received this vaccine on postnatal days 3 to 12.
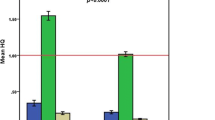
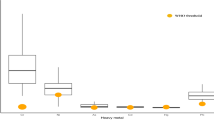
The cumulative frequency distribution of the measured element concentrations is illustrated in Fig. 1; the values are represented as mass/volume for easier comparison with literature. Elements measured above LOD ranged from a negligible 1% (Cd) to 82% (Al); of the six elements measured, only Al, Mn, and As had a detection frequency > 50%. Among the measured toxic elements, Al was the most commonly occurring element, and it was also found at the highest concentrations (Fig. 1); it occurred in all infants through hepatitis B immunization. Therefore, the high prevalence of Al in samples determined the binary exposures (Figs. 1 and 2).
Figure 2 illustrates the most common binary combination of the selected elements. Relevant binary combinations happened with Al and Hg by different media and routes (breastfeeding and TCV) as well as in different chemical forms: as Al-EtHg in hepatitis B (thimerosal preserved) vaccine and as total Hg (MeHg and inorganic Hg) in breast milk. Therefore, the Al and Hg combined exposure was the most prevalent in these infants. It is worth noting that this common occurrence should be taken into account when considering ternary combinations. Furthermore, when considering the TCV media, adjuvant Al can occur in distinct chemical forms (hydroxide and phosphate).
Therefore, ternary combinations (above LOD) in milk showed very low frequencies involving Hg and Pb; these were 0% for Mn-Hg-Pb, 1.8% for As-Hg-Pb, and 1.8% for Al-Hg-Pb. Coincidentally, all but two samples showed both Hg and Pb concentrations below LOD. However, low frequencies were observed for Mn-As-Hg (8.5%) and Al-Mn-As (21.7%). The overall outcome of the association rules applied to this sample is shown in Fig. 3 illustrating the hierarchy of all the possible combinations. The association rules obtained by the Apriori algorithm and the different values for each rule appear symmetrically organized. The size of the circle indicates the relative strength of the link between the items. The association rules (presence and/or absence) of neurotoxin metals (Al, Mn, Pb, As, and total Hg) in human milk were obtained by the Apriori algorithm as were differences for each rule. The values obtained for confidence, support, and lift were respectively 1.0, 0.079, and 1.74. Al is present at different hierarchical levels and in all multilevel combinations, thus indicating the strength of the link between Al and the other neurotoxic elements (especially Mn, Pb, and Hg).
A complementary analysis using the crosstab (antecedent and consequent) and the sorting criteria of rules (confidence, support, or lift) was performed. After applying the MDS method, the proximity of the five elements can be visualized in Fig. 4. The closest values meeting the criteria of minimum support and confidence were obtained for Al-Mn (0.785 or 78.5%), Al-As (0.602 or 60.2%), and Al-Hg (0.560 or 56%). These values are helpful in understanding the complexity of random mixtures and relative importance of the presence or absence of respective neurotoxic elements in human milk.
Illustration of the multidimensional scaling (MDS) outcome showing links among measured elements; results of the MDS analysis were based on the symmetric matrix averaging the confidences between neurotoxin metals involved in association rules and meeting the criteria of minimum support and confidence
Discussion
Samples of banked human milk are representative not only of lactating mothers (and their infants) but also of infants receiving donors’ milk. This study revealed that infants at different stages of development are exposed to combinations of neurotoxicants by different media and dosages. Nursing infants were exposed orally through breast milk and parenterally through TCV. Additionally to toxic elements (Al, Mn, As, Hg, and Pb) in breast-milk, neonates immunized against hepatitis B experience a very high burden of both Al and EtHg [29]. However, the results indicate that milk donated to the bank had a great variation in the frequency of measured toxic elements > LOD; only Al, Mn, and As showed concentrations > 50% of LOD. Because Al had a very high prevalence in intrinsic (82%) and extrinsic (100%) exposures, only the binary combination containing this element was of any relevance. Additionally, during the first day postpartum, neonates are likely to consume volumes near 15 mL [30]; therefore, exposure to most of the measured neurotoxicants is minimal.
The study revealed also that intrinsic Al and Hg (mother’s milk) varied greatly (Table 1, Fig. 1), but the TCV (hepatitis B vaccine) was the most consistent source of Al and EtHg exposures (Table 1, Fig. 2). While human milk showed a low frequency of Hg (28% > LOD) and an even lower binary combination of 22% (> LOD) in milk, the combination of Al-EtHg in the TCV was 100%. When newborns receive the hepatitis B vaccine (TCV), they experience a very high burden of both Al and EtHg [31]. Furthermore, substances intrinsically transferred from mothers to infants (during lactation) are gradual and proportional to the weight of the nursing infant [32]; in contrast, the combined mixture of Al:EtHg is taken in a bolus by neonates, and in this study, this was mostly within the first 24 h postpartum. In human milk, the median ratio of Al:THg (10) in samples with both elements measured above LOD is much lower (Fig. 2) than in TCV – circa 50 [10]. For comparison, in umbilical cord blood from Jamaican newborns, the median Al:THg ratio was circa 2.3 [33].
Sources of contaminations differ for different elements; differences in barriers (placenta and mammary gland) and mineral metabolism also modulate fetuses’ and nursing infants’ exposures. In the case of Hg, maternal transfer is higher for the inorganic (maternal amalgam fillings) than for the organic form (MeHg in fish and seafood) from maternal diet [24]; nevertheless, during lactation, the retention of MeHg is relatively higher than that of inorganic Hg [34]. In the nursing infant, exposure to Hg is from human milk and additionally from TCV. Although both organic (MeHg) and inorganic Hg occur in breast milk, the proportion of MeHg in milk is always lower than inorganic Hg [24]; in the case of TCV, the Hg is totally in the organic form (EtHg). After a single shot of TCV, a neonate can receive an excessive load of Hg (12.5 μg EtHg). A neonate body burden by TCV can reach levels higher than those transferred by these urban mothers in colostrum; it has been estimated that the amount of Hg in hepatitis B vaccine is comparable to the total Hg received in breastfeeding during the first month of nursing [32]. In our study, total Hg > LOD occurred only in 28% of the samples.
Thus, partition of Pb, Hg, Cd, and Mn in the maternal/fetal unit (maternal blood and umbilical cord blood) indicated that Hg in the fetuses exceeds by more than threefold that of mothers; Mn also increased in fetal circulation compared to maternal levels; fetal Pb was nearly equivalent (only 10% lower) to maternal concentrations, while Cd levels were greatly diminished in fetal blood [35]. There are differences in barriers (placental and mammary) operating during early life; As seems to cross the placental barrier more easily than the mammary gland. Concha et al. [7] found similar levels of As in cord and maternal blood, but reported less As in breast milk [36]. The barrier function of the human mammary gland was evaluated by analyzing maternal sera and corresponding colostrum samples for Cd and Pb; the corresponding values were 200 and 325%, respectively [37]. Sharma et al. [38] reported blood:milk ratio which ranges for Mn (1.7 to 4.1), Pb (0.7 to 4.1), THg (0.5 to 2.4), As (0.5 to 2.8), and Cd (1.7 to 7).
Comparing studies of multiple elements in human milk across time and place is complex due to limitations related to differences in the populations examined (and pertaining to environmental issues), time of sampling, and analytical strategies (different methods). Furthermore, the effects of combined low-level exposures of neurotoxicants have been studied, but no consistent results have been possible. Multi-exposures seem to depend on the studied neurotoxicants and exposed population [17]. Experimental studies of exposure to binary mixtures of toxic metals can provide insights into the mechanism of actions of binary exposures. Gibson et al. [39] showed in vitro interactions of Cd Hg (at levels of dietary Hg per day and of Hg found as thimerosal per vaccine injection) with hemoglobin. Prato and Biandolino [40] showed that a binary mixture of Hg:Cd gave an antagonist response compared to single element exposure.
Because of analytical limitations, ICP is not suitable for determinations of Hg chemical forms in milk [24], but it is the method of choice for multi-element analysis of breast milk [14]. Therefore, human milk studies with simultaneous determination of elements that include Al and Hg are difficult to find, and our results are the first in recent literature. However, Al appears in the highest concentrations when in combination with other neurotoxic elements [41,42,43,44].
Exposure to binary mixtures of neurotoxicants are often considered additive. However, complex interactions with media and host organism may result in an antagonist and synergistic effect [16], depending on the elemental mixture [45]. Therefore, ternary combinations (above LOD) in milk occurred in considerable amounts only for Al-Mn-As (21.7%). Studies of nursing infants that address the effects of multiple exposures with neurotoxic elements and that take into consideration both organic Hg (MeHg and EtHg) exposures are rare [18]. However, in vitro studies point to the differences in the biochemical fate of Cd, Hg, (MeHg and thimerosal) that may lead to a cumulative “buildup” of Hg (from thimerosal) on Hb [46]. Therefore, the novelty of this paper is to show real scenarios of combined exposures occurring in early life that contribute to understanding toxic metal mixtures in infants.
Studies of human milk composition pose severe limitations when sampling from mothers in a relatively large city. The choice of a human milk bank is the most feasible approach to collecting a sufficient volume of milk that can be analyzed by different methods. However, the advantages of sampling in a human milk bank also carry limitations intrinsically associated with this convenient approach. As a cross-sectional study, it only allows us to infer the frequency of occurrences of the specific metal combinations. Another limitation is associated with different stages of lactation and its influence on milk protein and fat complexes with trace elements. Except for TCV, no other information or risk factors could be obtained during the specific interval of time. Therefore, it was not possible to study changes in composition due to intrinsic or constitutional features.
In environmental science, the study of one toxicant, especially at low levels, is not sufficient to establish interactions (additivity/synergism). Milk sample size poses a serious limitation; large volumes, especially at early stages of lactation, are often unfeasible, and pooled samples are not suitable for a relevant combination of neurotoxicants. Therefore, it is crucial to identify sources, exposure route (characterization), and a clear quantification of the elements’ contribution. These are the strengths of the used sampling strategy. Additionally, evidence of low toxic metal levels can promote breastfeeding and its use in human milk banks. In the present case, it was possible to (a) identify the routes and occurrence of neurotoxicants encountered by newborns; (b) quantify the commonly occurring low-dose mixtures during early life; (c) provide basic information on binary mixtures that can guide future research on low-dose mixtures; and (d) define mixtures of neurotoxicants based on observed occurrences.
Concluding Remarks
-
The exposure of neurotoxic elements (Al, As, Cd, Pb, Mn, and Hg) to the young is intrinsically through placenta and human milk. Additional exogenous exposure (Al-EtHg) can occur through thimerosal-containing vaccines.
-
We studied breast milk metal mixtures, specifying the individual elements with a focus on frequency of occurrence of concentrations > LOD. Multi-elements accessible to infants reveal the relevance of the combination of Hg and Al in early life.
-
Breast-milk analysis can provide a snapshot of the current exposure experienced by mother and infants; however, due to low concentrations, milk from banks poses no public health concerns. In these donors’ samples, neurotoxic elements are mostly low and probably of no public health concern, and therefore safe for milk bank use.
-
Association rules analysis demonstrated the complexity of early life exposure to single and multiple (binary and ternary) combinations of neurotoxic elements, and it was useful to guide public health policies to protect vulnerable groups—pregnant mothers and nursing infants.
-
Human milk is the only source of nutrition for exclusively breastfed infants, but in early life, it is not the only source of multi-element exposure. In nursing infants, this type of exposure can occur both through breast milk and through TCV (which are also Al-adjuvanted).
References
WHO (2003) World Health Organisation, Global strategy for infant and young child feeding. WHO, Geneva
Almeida SG, Dórea JG (2006) Quality control of banked milk in Brasilia, Brazil. J Hum Lact 2:335–339
Vahter M, Berglund M, Akesson A, Lidén C (2002) Metals and women’s health. Environ Res 88:145–155
Esteban M, Castano A (2009) Non-invasive matrices in human biomonitoring: a review. Environ Int 35:438–449
Callan AC, Winters M, Barton C, Boyce M, Hinwood AL (2012) Children's exposure to metals: a community-initiated study. Arch Environ Contam Toxicol 62:714–722
Weidenhamer JD, Fitzpatrick MP, Biro AM, Kobunski PA, Hudson MR, Corbin RW, Gottesfeld P (2017) Metal exposures from aluminum cookware: an unrecognized public health risk in developing countries. Sci Total Environ 579:805–813
Concha G, Vogler G, Lezcano D, Nermell B, Vahter M (1998a) Exposure to inorganic arsenic metabolites during early human development. Toxicol Sci 44:185–190
Iyengar GV, Rapp A (2001) Human placenta as a ‘dual’ biomarker for monitoring fetal and maternal environment with special reference to potentially toxic trace elements. Part 3: toxic trace elements in placenta and placenta as a biomarker for these elements. Sci Total Environ 280:221–238
Rudge CV, Röllin HB, Nogueira CM, Thomassen Y, Rudge MC, Odland JØ (2009) The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of South African delivering women. J Environ Monit 11:1322–1330
Marques RC, Bernardi JV, Dórea JG, de Fatima R, Moreira M, Malm O (2014) Perinatal multiple exposure to neurotoxic (lead, methylmercury, ethylmercury, and aluminum) substances and neurodevelopment at six and 24 months of age. Environ Pollut 187:130–135
Bansa DK, Awua AK, Boatin R, Adom T, Brown-Appiah EC, Amewosina KK, Diaba A, Datoghe D, Okwabi W (2017) Cross-sectional assessment of infants’ exposure to toxic metals through breast milk in a prospective cohort study of mining communities in Ghana. BMC Public Health 17:505
Kostial K, Kello D, Jugo S, Rabar I, Maljković T (1978) Influence of age on metal metabolism and toxicity. Environ Health Perspect 25:81–86
Marchitti SA, LaKind JS, Naiman DQ, Berlin CM, Kenneke JF (2013) Improving infant exposure and health risk estimates: using serum data to predict polybrominated diphenyl ether concentrations in breast milk. Environ Sci Technol 47:4787–4795
Rebelo FM, Caldas ED (2016) Arsenic, lead, mercury and cadmium: toxicity, levels in breast milk and the risks for breastfed infants. Environ Res 151:671–688
Walker SP, Wachs TD, Grantham-McGregor S, Black M, Nelson CA, Huffman SL, Baker-Henningham H, Chang SM, Hamadani JD, Lozoff B, Gardner JM, Powell CA, Rahman A, Richter L (2011) Inequality in early childhood: risk and protective factors for early child development. Lancet 378:1325–1338
Claus-Henn B, Coull BA, Wright RO (2014) Chemical mixtures and children’s health. Curr Opin Pediatr 26:223–229
Dórea JG (2017) Low-dose thimerosal in pediatric vaccines: adverse effects in perspective. Environ Res 152:280–293
Dórea JG (2017) Abating mercury exposure in young children should include thimerosal-free vaccines. Neurochem Res. https://doi.org/10.1007/s11064-017-2277-x
Dórea JG (2015) Exposure to mercury and aluminum in early life: developmental vulnerability as a modifying factor in neurologic and immunologic effects. Int J Environ Res Public Health 12:1295–1313
von Stackelberg K, Guzy E, Chu T, Claus Henn B (2015) Exposure to mixtures of metals and neurodevelopmental outcomes: a multidisciplinary review using an adverse outcome pathway framework. Risk Anal 35:971–1016
Silva PR, Dorea JG, Boaventura GR (1997) Multielement determination in small samples of human milk by inductively coupled plasma atomic emission spectrometry. Biol Trace Elem Res 59:57–62
Kira CS, Maihara VA (2007) Determination of major and minor elements in dairy products through inductively coupled plasma optical emission spectrometry after wet partial digestion and neutron activation analysis. Food Chem 100:390–395
Birghila S, Dobrinas S, Stanciu G, Soceanu A (2008) Determination of major and minor elements in milk through ICP-AES. Environ Eng Manag J 7:805–808
Vieira SM, de Almeida R, Holanda IB, Mussy MH, Galvão RC, Crispim PT, Dórea JG, Bastos WR (2013) Total and methyl-mercury in hair and milk of mothers living in the city of Porto Velho and in villages along the Rio Madeira, Amazon, Brazil. Int J Hyg Environ Health 216:682–689
Malm O, Pfeiffer WC, Bastos WR, Souza CMM (1989) Utilização do Acessório de Geração de Vapor Frio para Análise de Mercúrio em Investigações Ambientais por Espectrofotometria de Absorção Atômica. Ciênc Cult 41:88–92
Bastos WR, Malm O, Pfeiffer WC, Cleary D (1998) Establishment and analytical quality control of laboratories for Hg determination in biological and geological samples in the Amazon, Brasil. Ciênc Cult 50:255–260
Agrawal R, Srikant R (1994) Fast algorithms for mining association rules. In Proceedings of the 20th int. conf. very large data bases, (VLDB). 1215:487-499
Han J, Fu Y (1999) Mining multiple-level association rules in large databases. IEEE Trans Knowl Data Eng 11:798–805
Dórea JG, Marques RC (2010) Infants’ exposure to aluminium from vaccines and breast milk during the first 6 months. J Expo Sci Environ Epidemiol 20:598–601
Santoro W, Martinez FE, Ricco RG, Jorge SM (2010) Colostrum ingested during the first day of life by exclusively breastfed healthy newborn infants. J Pediatr 156:29–32
Dórea JG, Marques RC, Brandão KG (2009) Brandão, Neonate exposure to thimerosal mercury from hepatitis B vaccines. Am J Perinatol 26:523–527
Marques RC, Dórea JG, Fonseca MF, Bastos WR, Malm O (2007) Hair mercury in breast-fed infants exposed to thimerosal-preserved vaccines. Eur J Pediatr 166:935–941
Rahbar MH, Samms-Vaughan M, Dickerson AS, Hessabi M, Bressler J, Desai CC, Shakespeare-Pellington S, Reece JA, Morgan R, Loveland KA, Grove ML, Boerwinkle E (2015) Concentration of lead, mercury, cadmium, aluminum, arsenic and manganese in umbilical cord blood of Jamaican newborns. Int J Environ Res Public Health 12:4481–4501
Björnberg KA, Vahter M, Berglund B, Niklasson B, Blennow M, Sandborgh-Englund G (2005) Transport of methylmercury and inorganic mercury to the fetus and breast-fed infant. Environ Health Perspect 113:1381–1385
Kopp RS, Kumbartski M, Harth V, Brüning T, Käfferlein HU (2012) Partition of metals in the maternal/fetal unit and lead-associated decreases of fetal iron and manganese: an observational biomonitoring approach. Arch Toxicol 86:1571–1581
Concha G, Vogler G, Nermell B, Vahter M (1998b) Low-level arsenic excretion in breast milk of native Andean woman exposed to high levels of arsenic in drinking water. Int Arch Environ Health 71:42–46
Rossipal E, Krachler M, Li F, Micetic-Turk D (2000) Investigation of the transport of trace elements across barriers in humans: studies of placental and mammary transfer. Acta Paediatr 89:1190–1195
Sharma R, Pervez S (2005) Toxic metals status in human blood and breast milk samples in an integrated steel plant environment in Central India. Environ Geochem Health 27:39–45
Gibson MA, Sarpong-Kumankomah S, Nehzati S, George GN, Gailer J (2017) Remarkable differences in the biochemical fate of Cd(2+), Hg(2+), CH(3)Hg(+) and thimerosal in red blood cell lysate. Metallomics. https://doi.org/10.1039/c7mt00069c
Prato E, Biandolino F (2007) Combined toxicity of mercury, copper and cadmium on embryogenesis and early larval stages of the Mytilus galloprovincialis. Environ Technol 28:915–920
Björklund KL, Vahter M, Palm B, Grandér M, Lignell S, Berglund M (2012) Metals and trace element concentrations in breast milk of first time healthy mothers: a biological monitoring study. Environ Health 11:92
Matos C, Moutinho C, Almeida C, Guerra A, Balcão V (2014) Trace element compositional changes in human milk during the first four months of lactation. Int J Food Sci Nutr 65:547–551
Chao HH, Guo CH, Huang CB, Chen PC, Li HC, Hsiung DY, Chou YK (2014) Arsenic, cadmium, lead, and aluminium concentrations in human milk at early stages of lactation. Pediatr Neonatol 55:127–134
Sun Z, Yue B, Yang Z, Li X, Wu Y, Yin S (2013) Determination of 24 minerals inhuman milk by inductively coupled plasma mass spectrometry with microwave digestion. Wei Sheng Yan Jiu 42:504–509
Bellés M, Albina ML, Sánchez DJ, Corbella J, Domingo JL (2002) Interactions in developmental toxicology: effects of concurrent exposure to lead, organic mercury, and arsenic in pregnant mice. Arch Environ Contam Toxicol 42:93–98
Gibson MA, Sarpong-Kumankomah S, Nehzati S, George GN, Gailer J (2017) Remarkable differences in the biochemical fate of Cd(2+), Hg(2+), CH(3)Hg(+) and thimerosal in red blood cell lysate. Metallomics 9:1060–1072
Acknowledgements
We thank CT-Universal and INCT-INPeTAm/CNPq/MCT for the support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bastos, W.R., Vieira, S.M., Manzatto, Â.G. et al. Heterogeneity of Multimedia Exposures to Neurotoxic Elements (Al, As, Cd, Pb, Mn, and Hg) in Breastfed Infants from Porto Velho, Brazil. Biol Trace Elem Res 184, 7–15 (2018). https://doi.org/10.1007/s12011-017-1165-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1165-1