Abstract
The neurotoxic effects of aluminum (Al) are associated with the impairment of synaptic plasticity, the biological basis of learning and memory, the major form of which is long-term potentiation (LTP). The canonical glycogen synthase kinase-3β (GSK-3β)/β-catenin signaling–mediated brain–derived neurotrophic factor (BDNF) pathway has been suggested to play important roles in memory. Thus, Al may affect LTP through this pathway. In this study, a Sprague-Dawley rat model of neurotoxicity was established through intracerebroventricular (i.c.v.) injection of aluminum maltol (Al(mal)3), which was achieved by preimplantation of a cannula into the lateral ventricle. The rats in the control and Al-treated groups received a daily injection of SB216763, an inhibitor of GSK-3β. Electrophysiology and western blot analysis were used to investigate the regulatory effect of the GSK-3β/β-catenin signaling-mediated BDNF pathway on LTP impairment induced by Al(mal)3. The results confirmed that i.c.v. injection of Al(mal)3 significantly suppressed the field excitatory postsynaptic potential (fEPSP) amplitude, as indicated by a decrease in BDNF protein expression, which was accompanied by dose-dependent decreases in β-catenin protein expression and the phosphorylation of GSK-3β at Ser9. Rats that received SB216763, a GSK-3β inhibitor, exhibited higher fEPSP amplitudes than control rats. Furthermore, SB216763 treatment upregulated the hippocampal protein expression of BDNF and β-catenin while increasing the ratio of p-GSK-3β/GSK-3β. From the perspective of the identified β-catenin–BDNF axis, Al impairs hippocampal LTP, possibly through the GSK-3β/β-catenin signaling–mediated BDNF pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aluminum (Al), a neurotoxic metal that is found at abundant levels on Earth and has high melting and boiling points and a low density and exhibits resistance to corrosion, is commonly and widely used in various industrial fields and in consumer products such as vaccines, antacids, foods, skin care products, cosmetics, and cookware [1]. It has been shown that Al can be transported across the blood-brain barrier (BBB [2]. Moreover, a study of isotopically labeled 26Al suggested Al has a long half-life in the brain and indicated that it was present in the fetal rat brain [3]. Notably, a large number of epidemiological investigations have shown that long-term exposure to Al mainly harms the nervous system [1], is closely related to diseases associated with cognitive dysfunction [4, 5], and can cause neurological symptoms in the population, such as impaired learning and memory abilities and mild cognitive dysfunction [6, 7]. Moreover, studies have shown that exposure to Al can cause memory impairment in animal models and modify hippocampus-related signal pathways [8, 9] that are crucial to synaptic plasticity.
Synaptic plasticity, the basis of learning and memory [10], is a physiological phenomenon that is involved in neural activity and the consequential changes in synaptic efficacy and neural excitability [11], and the hippocampus plays a dominant and important role in synaptic plasticity [12]. In the brains of mammals, long-term potentiation (LTP) and long-term depression (LTD) are two major manifestations of synaptic plasticity [13]. LTP, which is long-term continuous enhancement of synaptic transmission efficiency caused by high-frequency stimulation (HFS) of presynaptic afferent fibers [14] in the cornu ammonis (CA) 1 area of hippocampus can be used to reliably study synaptic learning and memory functions [13]. Our previous studies confirmed that Al impairs LTP in vivo [15]. The ability of Al to inhibit LTP suggests that Al may impair synaptic transmission.
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family, regulates synaptic transmission and plays a crucial role in cognitive functions [16, 17], which depend on the establishment, maintenance, strengthening, or regulated elimination of synapses between neurons. In addition, inhibition of endogenous BDNF production using gene knockout or antisense RNA impairs spatial learning and memory, suggesting that BDNF plays an important role in learning and memory in the hippocampus [18, 19]. BDNF is one of the most potent mediators of synaptic plasticity, as it is secreted during LTP induction. BDNF can act on receptors in the hippocampus and enhance synaptic transmission efficiency by strengthening the activity of neurotransmitters and receptors [19,20,21]. In BDNF knockout mice, hippocampal LTP is significantly reduced. Moreover, LTP is increased by reversing exogenous BDNF expression or increasing BDNF expression. Other studies have confirmed that BDNF is functionally essential for the signaling cascades that lead to LTP [22, 23]. Furthermore, in our previous rat model of chronic Al-induced learning and memory impairment, Al-induced LTP impairment was accompanied by a decrease in the expression level of BDNF in the hippocampus [24]. However, the detailed mechanisms by which Al exerts its effect on LTP in the hippocampal CA1 region are only partially understood.
In recent years, glycogen synthase kinase-3β (GSK-3β)/β-catenin signaling has been proven to play important roles in hippocampal memory [25, 26]. GSK-3β, a ubiquitous serine/threonine protein kinase that is specifically expressed in the central nervous system [27], is constitutively active under basal conditions. The phosphorylation of GSK-3β at Ser9 inhibits the activity of GSK-3β in response to upstream signals [28]. GSK-3β plays a very important role in axonal growth and polarity formation and is related to nervous system dysfunction [29, 30]. Overexpression of GSK-3β inhibits LTP, and activation of GSK-3β promotes induction of LTD [31,32,33]. A study reported that downregulation of the expression of β-catenin, which is a transcription factor in the hippocampus, impairs memory consolidation [34, 35]. The level of β catenin is regulated by GSK-3β. GSK-3β activation reduces the stability of β-catenin and leads to its degradation through ubiquitination, whereas GSK-3β inhibition stabilizes β-catenin and increases its ability to regulate synaptic plasticity [36]. Another study reported that the β-catenin signaling pathway and BDNF regulate neural stem cell development in mice and that the BDNF pathway may engage in crosstalk with the β-catenin pathway [37]. Furthermore, researchers have confirmed that BDNF is a direct target of the β-catenin transduction pathway [38] and that BDNF expression is regulated by β-catenin [39]. Hence, whether the GSK-3β/β-catenin signaling–mediated BDNF pathway is involved in the negative effects of Al on LTP is still not known, and few reports on synaptic plasticity in this context have been published to date.
The characteristics of Al, i.e., its small size, near maximal charge, and high affinity for the metal binding sites of enzymes [40], may contribute to its effects on LTP and lead to the involvement of the GSK-3β/β-catenin signaling–mediated BDNF pathway. Here, Sprague-Dawley rats were treated with aluminum maltol (Al(mal)3), and changes in LTP in the hippocampal CA1 region induced by Al were analyzed. The ability of the GSK-3β inhibitor SB216763, a potent irreversible and cell-permeable pharmacological inhibitor of GSK-3 that is structurally distinct from other inhibitors and is highly selective for GSK-3β [41, 42], to regulate the phosphorylation of GSK-3β and the expression of β-catenin and BDNF during Al-induced changes in LTP is discussed.
Materials and Methods
Animals and Surgery
Two- to 3-month-old male Sprague-Dawley rats (weighing 250–300 g) supplied by the Laboratory Animal Center of Shanxi Medical University (certificate no. SCXK (Jin) 2009-0001, Taiyuan, China) were housed on a 12-h light/dark cycle (8:00–20:00 h), and the room temperature maintained at approximately 22 °C ± 2 °C. The rats were provided with food and drinking water ad libitum. Our studies were performed in accordance with the regulations of the Institutional Animal Care and Use Committee of Shanxi Medical University.
The surgical procedure was performed as described previously [43]. Animals were anesthetized with chloral hydrate (350 mg/kg) and placed in a stereotaxic apparatus, and a midline sagittal incision was made in the scalp. A small hole was drilled using the parameters reported by Paxinos et al. [44] for intracerebroventricular (i.c.v.) injection (0.8-mm posterior to bregma, 1.5-mm lateral to the midline, and 4.0 mm below the dura mater) for implantation of a guide cannula. A stainless steel guide cannula (RWD Life Science, Shenzhen, China) into which a stainless steel stylet was inserted was placed in the hole in the skull and secured to the skull with dental acrylic.
Chemicals and Treatment
According to the methods described in our previous study [45], AlCl36H2O (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in distilled water at concentrations of 4 and 20 mM. Maltolate (Sigma-Aldrich, USA) was dissolved in phosphate-buffered saline at concentrations of 12 and 60 mM. Al(mal)3 was freshly prepared for each experiment by mixing these two solutions in equal volumes, adjusting the pH to 7.4 with NaOH and filtering the mixture through a 0.22 μmol/L syringe filter. SB216763 (Abcam, UK), a selective GSK-3β inhibitor, was dissolved in DMSO and further diluted in phosphate-buffered saline (PBS) [46] to a final concentration of 2 μM, as described in a previous study [47]. Ten to 14 days after surgery, each rat received an i.c.v. injection of 5 μl Al(mal)3 or SB216763.
Before the formal experiment, we established two groups, namely, the no cannula implantation group and the cannula implantation group, to observe whether lateral ventricular cannula implantation had an impact on the rats. Then, the experiment was divided into two parts. First, to study the effects of Al exposure on LTP and related molecules, the rats were randomly divided into four groups (6 rats/group): the control group (0.9% normal saline) and three Al(mal)3 exposure groups (0 mM, 2 mM, and 10 mM). Second, to determine if the GSK-3β inhibitor SB216763 antagonizes the Al-induced suppression of hippocampal LTP and downstream signaling molecules, the rats were divided into four groups (6 rats/group): the control group, the GSK-3β inhibitor group (2 μM SB216763), the Al(mal)3 group (10 mM), and the GSK-3β inhibitor + Al(mal)3 group (2 μM SB216763 and 10 mM Al(mal)3). Each rat was administered an i.c.v. injection of 5 μl saline, Al(mal)3 and/or SB216763 at a rate of 1 μl/min through the stainless steel guide cannula with a microinjector (Gaoge, Shanghai, China). Drug administration was performed once every 2 days for 10 days.
In Vivo Electrophysiological Studies of the Hippocampus
Electrophysiology was performed using a methods previously reported by our group [15]. Rats from each treatment group were anesthetized with intraperitoneally (i.p.) administered 1.5 g/kg urethane (Sigma-Aldrich, St. Louis, MO, USA) and fixed on a stereotaxic device (Narishige Group). Two small holes were drilled on the right side of the skull at appropriate locations for electrode insertion. A bipolar stimulating electrode (FHC, Bowdoin, ME, USA) was inserted into the Schaffer collateral commissural pathway (4.2-mm posterior to bregma and 3.8-mm lateral to the midline), and a monopolar recording electrode (FHC) was inserted in the stratum radiatum of the CA1 region (3.8-mm posterior to bregma and 2.9-mm lateral to the midline). Stimulating electrodes and recording electrodes were inserted into preset locations in the hippocampus with a micropropulsion unit (Narishige Group). The electrodes were slowly lowered into the CA1 region of the hippocampus until field excitatory postsynaptic potentials (fEPSPs) were observed. Baseline fEPSPs were elicited by test stimuli with an intensity that elicited 50% of the maximal response at an interval of 30 s and were monitored for 30 min. LTP was induced by a high-frequency train.
Western Blotting
Tissues from the hippocampal CA1 region were homogenized and sonicated on ice using ristocetin-induced platelet agglutination lysis buffer (Wuhan Boster Biological Technology, Wuhan, China) to extract protein. The samples were loaded and separated on 10% SDS-polyacrylamide electrophoresis gels to detect GSK-3β, pGSK-3β, β-catenin, BDNF, and GAPDH. The proteins were transferred to PVDF membranes (EMD Millipore, Bedford, MA, USA), which were incubated with rabbit antipGSK3β (Ser9) (1:1000, Cell Signaling Technology, USA), rabbit antiGSK3β, rabbit antiβ-catenin (1:1000, Cell Signaling Technology, USA), rabbit antiBDNF (1:1000, Cell Signaling Technology, USA), or mouse antiGAPDH (1:3000, CoWin Biotech) overnight at 4 °C. The next day, the membranes were rinsed with Tris-buffered saline containing Tween (TBST; 20 mM Tris-HCl, pH 7.4; 225 mM NaCl; and 0.05% Tween 20). After three washes in TBST, the blots were then incubated with one of the following HRP-conjugated antibodies: antirabbit IgG (Wuhan Boster Biological Technology) or antimouse IgG (CoWin Biotech). Then, the immunolabeled protein bands were detected using an ECL western blot detection kit (CoWin Biotech). Images were obtained with a chemiluminescence imaging system (Bio-Rad). Densitometric quantification of the immunoblots was performed using Quantity One software (Bio-Rad).
Data Analysis
All data are expressed as the mean ± S.D. and were analyzed with SPSS 17.0. Differences were analyzed by one-way analysis of variance (ANOVA), and post hoc comparisons were performed using the least significant difference (LSD) test (equal variances assumed) or Dunnett’s C test (equal variances not assumed). P < 0.05 was considered statistically significant.
Results
Al Treatment Significantly Suppressed LTP in the CA1 Area
As shown in Fig. 1 a and b, after a stable baseline was established for 30 min, HFS resulted in successful induction of LTP after cannula implantation. The average standardized fEPSP amplitudes were 178% ± 16.0%, 159% ± 11.4%, and 151% ± 8.7% at 10 min, 30 min, and 60 min after HFS, respectively, in the cannula implantation group. There was no difference in the amplitude of fEPSPs between the cannula implantation group and the no cannula implantation group (P > 0.05) (Fig. 1c).
Effects of cannula implantation on typical LTP in the hippocampal CA1 region in vivo in rats in response to high-frequency stimulation (HFS, 200 Hz). HFS induced stable LTP after cannula implantation. a The effect of cannula implantation on LTP in the CA1 region. Each point represents the mean ± SD of the excitatory postsynaptic potential (fEPSP) amplitude. fEPSP (% baseline) was calculated as (fEPSP/fEPSPbaseline) * 100. b Representative original data traces, with the dotted line representing the trace before HFS and the solid line representing the trace 60 min after HFS. c Average fEPSP amplitudes at different time points before and following HFS. Each column shows the mean ± SD of the fEPSP amplitude, n = 6. * P < 0.05 compared with the no cannula implantation group
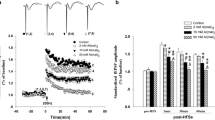
No significant change in the mortality rate was observed in any of the control or treatment groups. To determine whether Al affects synaptic plasticity, the effects of Al on LTP in the CA1 region in vivo in rats were further investigated. As shown in Fig. 2a, there was no difference in the amplitude of fEPSPs between the 0 mM Al(mal)3 group and the saline control group after HFS. However, administration of 2 mM or 10 mM Al(mal)3 significantly impaired LTP in a concentration-dependent manner. As shown in Fig. 2 b and c, the amplitude of fEPSPs in rats from the 2 mM group (156% ± 14.7%) and 10 mM group (146 ± 10.4) at 10 min after HFS was significantly lower than that in the control group (177% ± 16.2%) in the first minute after HFS. After LTP induction for 60 min, the amplitude of fEPSPs in rats from the control group remained above 153% ± 9.6%, while repression of LTP was obviously induced in the 10 mM group, and the amplitude of fEPSPs was significantly decreased in the 10 mM group (120% ± 10.9%) compared with the control group (Fig. 2c) (F(3, 20) = 19.517, P < 0.05).
Effects of Al(mal)3 exposure on LTP in the hippocampal CA1 region in rats. a Representative original data traces, with the dotted line representing the trace before HFS and the solid line representing the trace at 60 min after HFS. b The suppressive effect of Al(mal)3 on LTP. Each point represents the mean ± SD of the fEPSP amplitude. c Summary histogram comparing the effects of different doses of Al(mal)3 on fEPSPs at 10 min, 30 min, and 60 min after HFS. The data are shown as the mean ± SE. n = 6. *P < 0.05 compared with the control group
Al-Induced Alterations in Hippocampal BDNF, GSK-3β and β-Catenin Levels
Because of the important role of the GSK-3β/β-catenin signaling pathway in axonal growth and memory consolidation [34, 35], we assumed that Al can inhibit LTP, which can be reliably used to study learning and memory, by inhibiting GSK-3β/β-catenin signaling. To verify our hypothesis, we first investigated the phosphorylation status of GSK-3β in the hippocampus after LTP induction. As shown in Fig. 3a–f, 10 mM Al reduced the inhibitory phosphorylation of GSK-3β at Ser9, and decreased the ratio of p-GSK-3β/GSK-3β to 37.7 ± 10.1% (n = 6 for each group, P < 0.05 vs the control). No obvious change was seen in the total level of GSK-3β in any of the treatment groups. To further explore the role of the GSK-3β pathway in the modulatory effect of Al on LTP, we performed western blot analysis to quantify β-catenin and BDNF expression in the hippocampus. As shown in Fig. 3a–c, treatment with Al at various concentrations resulted in dose-dependent decreases in the expression of β-catenin (F[3, 20] = 31.857, P < 0.05) and BDNF (F[3, 20] = 23.3764, P < 0.05). Compared with that in the control group, the expression of β-catenin and BDNF in the 10 mM Al group was obviously decreased (n = 6 for each group, P < 0.05 vs the control).
Al(mal)3 induced alterations in BDNF, GSK-3β and β-catenin levels in the hippocampus. a Protein levels of BDNF, β-catenin and p-GSK3β after exposure to Al(mal)3 were measured using western blotting. b, c, d, and e The ratio of the expression level of the corresponding protein to the expression level of GAPDH. GAPDH was used as a gel loading control. f The level of p-GSK-3β, which is expressed as a ratio of p-GSK-3 β expression to total GSK-3β expression. The data are shown as the mean ± SE. n = 6 and were analyzed by one-way ANOVA followed by the LSD test. *P < 0.05 compared with the control group
GSK-3β Inhibitors Prevented the Effects of Al on Hippocampal LTP In Vivo
To address the question of whether GSK-3β is involved in the effect of Al on LTP, we first evaluated the effects of i.c.v. injection of SB216763 (2 μM), an inhibitor of GSK-3β, combined with 10 mM Al on LTP. As shown in Fig. 4, the average standardized fEPSP amplitudes in the SB216763-treated group were 170 ± 16.7%, 155 ± 11.3%, and 146 ± 10.0% at 10, 30, and 60-min postHFS, respectively. We did not find any significant change in HFS-induced LTP in rats following SB216763 injection (n = 6 for each group, P > 0.05 vs the control group). However, pretreatment with inhibitors of GSK-3β antagonized the effects of 10 mM Al on the induction of LTP in vivo. SB216763 reversed the 10 mM Al-induced impairment of LTP, restoring fEPSP amplitudes to 149.2 ± 15.7%, 143.5 ± 10.4%, and 134 ± 9.4% of baseline levels at 10-, 30-, and 60-min postHFS, respectively (n = 6 for each group, P < 0.05 vs 10 mM Al alone).
Effects of SB216763 on LTP in the hippocampal CA1 region in rats. a Representative original data traces, with the dotted line representing the traces before HFS and the solid line representing the traces at 60 min after HFS. b The inhibitory effect of Al on LTP magnitude was reversed by SB216763. c Histograms showing the normalized fEPSP amplitude at 10 min, 30 min, and 60 min after HFS relative to that of the control, Al-, SB216763-, and SB216763 plus Al-treated rats. The data are shown as the mean ± SE (n = 6) and were analyzed by one-way ANOVA followed by the LSD test. *P < 0.05 compared with the control group, #P < 0.05 compared with the 10 mM Al(mal)3 group
SB216763 Reversed the Alterations in the Levels of GSK-3β-Related Signaling Molecules in the Hippocampi of Rats Treated with Al
SB216763 is a selective inhibitor that can inactivate GSK-3β by dephosphorylating it at the inhibitory Ser9 site [41]. We observed that SB216763 reversed the effects of Al on LTP in the electrophysiological experiment, and we then further investigated the phosphorylation status of GSK-3β in the hippocampus after HFS. As shown in Fig. 5 a and b, Al reduced the inhibitory phosphorylation of GSK-3β at Ser9 and decreased the ratio p-GSK-3β/GSK-3β to 27.5 ± 10.8% (n = 6 for each group, P < 0.05 vs the control). SB216763 could slightly prevent the decrease in the ratio of p- GSK-3β to GSK-3β (51.0 ± 9.1%) (n = 6 for each group, p < 0.05 vs the 10 mM Al group), suggesting that SB216763 may inhibit GSK-3β partially through modulating its phosphorylation. We tested whether i.c.v. administration of SB216763 has effects on GSK-3β-related signaling activity in the brain. As shown in Fig. 5a–d, the hippocampal protein levels of β-catenin and BDNF were lower in the Al-exposed rats than in the control rats. However, these differences were ameliorated by SB216763 (F[3, 20] = 8.677, P = 0.001; F[3, 20] = 12.085, P < 0.05;). These results indicate that SB216763 effectively reversed the Al-induced decrease in β-Catenin and BDNF expression.
SB216763 upregulated the hippocampal protein expression of BDNF and β-catenin while increasing the ratio of p-GSK-3β/GSK-3β. a Protein levels of BDNF, β-catenin, and p-GSK3β after application of SB216763 or Al(mal)3g. b The level of p-GSK-3β, which is expressed as a ratio of p-GSK-3 β expression to total GSK-3β expression. c, d The expression level of the corresponding protein relative to the expression level of GAPDH. The data are shown as the mean ± SE (n = 6) and were analyzed by one-way ANOVA followed by the LSD test. *P < 0.05 compared with the control group, #P < 0.05 compared with the 10 mM Al(mal)3 group
Discussion
The effect of Al on cognitive impairment has been studied for decades. Occupational epidemiologists have reported that workers exposed to Al show worse performance on various neurobehavioral tests than nonexposed individuals [48, 49]. Our group previously reported that Al exposure causes spatial memory deficits in rats [50], and the same conclusion was reached in a previous epidemiological study [7]. In the present study, an animal model of Al-induced neurotoxicity was established by i.c.v. injection of Al(mal)3, and changes in LTP, which is well recognized to reflect synaptic plasticity, a crucial neurobiological basis for learning and memory, were detected in vivo. As previously reported by our group [15], we implanted a hollow cannula into the lateral ventricle and fixed it to the skull of the rat for i.c.v. injection. After 2 weeks of adaptation, the agent was injected through the cannula with a microinjector. As shown in the present study, after a stable baseline was established for 30 min, HFS resulted in successful induction of LTP after cannula implantation, and there was no difference in the amplitude of fEPSPs between the cannula implantation group and the no cannula implantation group. The method of i.c.v. injection applied in our study has been widely used in neuropharmaceutical, neurobiological, and neurotoxicological studies, as it is a convenient method for facilitating the precise entry of the injected substance into brain tissue and avoids the influences of gastrointestinal absorption and metabolism [51,52,53]. The current study indicated that i.c.v. injection of Al(mal)3 significantly suppressed the amplitude of fEPSPs in a dose-dependent manner and that LTP was significantly inhibited in rats in the 10 mM group, indicating that LTP is a sensitive target of Al. Electrophysiological evidence confirmed that Al caused impairments in cognition, learning, and memory [54, 55].
This study shows that the impairment of LTP may be related to the GSK-3β/β-catenin signaling pathway, which provides a molecular basis of Al-induced neurotoxicity. GSK-3β, a kinase that is inactivated by phosphorylation at Ser9 and is specifically expressed in the central nervous system [56], plays a very important role in axonal growth, polarity formation, and memory processes and is related to nervous system dysfunction [29, 30, 57]. One of the main downstream targets of GSK3β is β-catenin. Inhibition of GSK3β allows β-catenin to be stabilized, accumulate in the cytoplasm, and translocate to the nucleus, activating downstream target genes responsible for synaptogenesis [25]. It has also been proven that GSK3β/β-catenin signaling plays an essential role in learning, memory and psychological status, the dysfunction of which is involved in several neuropsychiatric disorders, such as Alzheimer’s disease and depression [58]. The main functions of the brain are information storage, learning, and memory, which involve changes in synaptic transmission efficiency; thus, abnormalities in GSK-3β also affect synaptic plasticity, such as LTP and LTD. Several studies have noted that inhibition of GSK-3β activity by phosphorylation promotes LTP induction, overexpression of GSK-3β inhibits LTP, and activation of GSK-3β activity promotes induction of LTD [31,32,33]. GSK-3β mutant mice show LTP inhibition because GSK-3β activity cannot be inhibited through phosphorylation at Ser9 [59]. This phenomenon provides some context for the findings of the present study, in which we confirmed that increased exposure to Al decreased the protein level of β-catenin and the phosphorylation of GSK-3β at Ser9, causing increased accumulation of active GSK-3β. Given that LTP deficits can be attenuated/rescued by chronic treatment with a GSK-3β inhibitor [42], we applied SB216763, a potent irreversible and cell-permeable pharmacological inhibitor of GSK-3 that is structurally distinct from other inhibitors, is highly selective for GSK-3β and has no significant influence on the activity of other kinases [41], in the present study to determine whether GSK-3β is involved in the Al-induced impairment of LTP. In the present study, Al-induced suppression of LTP was partly but significantly reversed by SB216763. Furthermore, SB216763 significantly reversed the decrease in β-catenin protein levels in the hippocampus. These results suggest that the GSK-3β/β-catenin signaling pathway participates in the Al-induced inhibition of LTP. However, the downstream mechanisms that this pathway regulates require further investigation.
BDNF, which is mainly synthesized in brain tissue and is found at abundant levels in the cerebral cortex and hippocampus, is a key factor in the maintenance of synaptic plasticity [60]. BDNF can enhance synaptic transmission by acting on receptors in the hippocampus and other brain regions and by enhancing the activity of neurotransmitters and receptors involved in learning and memory [19,20,21]. In the present study, BDNF expression was also decreased by Al treatment, especially 10 mM Al. These findings are in line with previous studies showing decreased BDNF expression [24] and pathological lesions of synaptic structure [53]. This study provides new evidence that BDNF expression is decreased, indicating that the fEPSP amplitudes of functional synapses involved in neurotransmission are reduced.
A recent study further confirmed this direct relationship; similar to BDNF expression, β-catenin signaling is inhibited in neurodegenerative diseases [61]. Similar to β-catenin signaling inhibition, BDNF dysregulation has been implicated in neurodegenerative disorders [62, 63]. BDNF mRNA and protein levels are decreased in various brain regions in patients with Alzheimer’s disease, and administration of exogenous BDNF or stimulation of its receptor can attenuate pathology in animal models [60]. Recent evidence has indicated that BDNF is a direct target of the β-catenin transduction pathway. Seven binding motifs for Wnt-dependent TCF/LEF transcription factors that associate with β-catenin interact with specific DNA sequence motifs were identified in the BDNF gene promoter [39]. Thus, downregulation of β-catenin expression via deletion of one copy of the gene in neurons abolishes capsaicin-induced BDNF expression [38]. Thus, the regulation of β-catenin/BDNF is important for Al-induced impairment of LTP. The results of the current study indicated that i.c.v. injection of 10 mM Al decreased BDNF protein expression, which is consistent with a previous study in a rat model of chronic aluminum exposure [24], and that this decrease was accompanied by reductions in β-catenin protein expression and the phosphorylation of GSK-3β at Ser9. We applied SB216763 to the same animal model, and the data suggested that the change in BDNF protein expression was significantly reversed. According to the findings of other researchers showing that BDNF or Wnt signaling inhibition reduces BDNF levels [64] and therefore reduces dendritic arbor size in cultured cortical neurons [65], BDNF levels may correlate with GSK-3β/β-catenin signaling.
Conclusion
The present study was designed to investigate the effects of Al on the induction of LTP in the CA1 region of the rat hippocampus and to further elucidate whether GSK-3β, a cognition-related serine/threonine kinase, is involved in these effects. However, in this study, an inhibitor of GSK-3β partially reversed the impairment of LTP induced by Al because synaptic plasticity is regulated by many signaling pathways, and these temporal profiles may not be directly comparable. Nonetheless, our results provide new insight into the effect of Al on synaptic plasticity and its neurotoxicity. From the perspective of the identified β-catenin–BDNF axis, we propose that the GSK-3β/β-catenin signaling–mediated BDNF pathway may be a critical pathogenic pathway in Al-induced impairment of synaptic plasticity.
Data Availability
Not applicable.
References
Niu Q (2018) Overview of the relationship between aluminum exposure and health of human being. Adv Exp Med Biol 1091:1–31
Yokel RA (2002) Brain uptake, retention, and efflux of aluminum and manganese. Environ Health Perspect 110(Suppl 5(Suppl 5)):699–704
Yumoto S, Nagai H, Matsuzaki H, Matsumura H, Tada W, Nagatsuma E, Kobayashi K (2001) Aluminium incorporation into the brain of rat fetuses and sucklings. Brain Res Bull 55(2):229–234
Kawahara M, Kato-Negishi M (2011) Link between aluminum and the pathogenesis of Alzheimer’s disease: the integration of the aluminum and amyloid cascade hypotheses. Int J Alzheimers Dis 2011:276393
Flaten TP (2001) Aluminium as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain Res Bull 55(2):187–196
Colomina MT, Peris-Sampedro F (2017) Aluminum and Alzheimer’s disease. Adv Neurobiol 18:183–197
Lu X, Liang R, Jia Z, Wang H, Pan B, Zhang Q, Niu Q (2014) Cognitive disorders and tau-protein expression among retired aluminum smelting workers. J Occup Environ Med 56(2):155–160
Walton JR (2009) Functional impairment in aged rats chronically exposed to human range dietary aluminum equivalents. Neurotoxicology 30(2):182–193
Yu L, Jiang R, Su Q, Yu H, Yang J (2014) Hippocampal neuronal metal ion imbalance related oxidative stress in a rat model of chronic aluminum exposure and neuroprotection of meloxicam. Behav Brain Funct 10:6
Dudek SM, Bear MF (1993) Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci 13(7):2910–2918
Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ (2008) Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med 14(8):837–842
Bannerman DM, Sprengel R, Sanderson DJ, McHugh SB, Rawlins JNP, Monyer H, Seeburg PH (2014) Hippocampal synaptic plasticity, spatial memory and anxiety. Nat Rev Neurosci 15(3):181–192
Malenka RC, Bear MF (2004) LTP and LTD: an embarrassment of riches. Neuron 44(1):5–21
Whitlock JR, Heynen AJ, Shuler MG, Bear MF (2006) Learning induces long-term potentiation in the hippocampus. Science 313(5790):1093–1097
Zhang H, Yang X, Qin X, Niu Q (2016) Caspase-3 is involved in aluminum-induced impairment of long-term potentiation in rats through the Akt/GSK-3β pathway. Neurotox Res 29(4):484–494
Bambah-Mukku D, Travaglia A, Chen DY, Pollonini G, Alberini CM (2014) A positive autoregulatory BDNF feedback loop via C/EBPβ mediates hippocampal memory consolidation. J Neurosci 34(37):12547–12559
Panja D, Kenney JW, D’Andrea L, Zalfa F, Vedeler A, Wibrand K, Fukunaga R, Bagni C, Proud CG, Bramham CR (2014) Two-stage translational control of dentate gyrus LTP consolidation is mediated by sustained BDNF-TrkB signaling to MNK. Cell Rep 9(4):1430–1445
Diógenes MJ, Costenla AR, Lopes LV, Jerónimo-Santos A, Sousa VC, Fontinha BM, Ribeiro JA, Sebastião AM (2011) Enhancement of LTP in aged rats is dependent on endogenous BDNF. Neuropsychopharmacology 36(9):1823–1836
Leal G, Bramham CR, Duarte CB (2017) BDNF and hippocampal synaptic plasticity. Vitam Horm 104:153–195
Gibon J, Barker PA (2017) Neurotrophins and proneurotrophins: focus on synaptic activity and plasticity in the brain. Neuroscientist 23(6):587–604
Lu B, Nagappan G, Lu Y (2014) BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol 220:223–250
Lu B, Nagappan G, Guan X, Nathan PJ, Wren P (2013) BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci 14(6):401–416
Park H, Poo MM (2013) Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14(1):7–23
Li H, Xue X, Li Z, Pan B, Hao Y, Niu Q (2020) Aluminium-induced synaptic plasticity injury via the PHF8-H3K9me2-BDNF signalling pathway. Chemosphere 244:125445
King MK, Pardo M, Cheng Y, Downey K, Jope RS, Beurel E (2014) Glycogen synthase kinase-3 inhibitors: rescuers of cognitive impairments. Pharmacol Ther 141(1):1–12
Jiang P, Li G, Zhou X, Wang C, Qiao Y, Liao D, Shi D (2019) Chronic fluoride exposure induces neuronal apoptosis and impairs neurogenesis and synaptic plasticity: role of GSK-3β/β-catenin pathway. Chemosphere 214:430–435
Goold RG, Gordon-Weeks PR (2004) Glycogen synthase kinase 3beta and the regulation of axon growth. Biochem Soc Trans 32(Pt 5):809–811
Li X, Jope RS (2010) Is glycogen synthase kinase-3 a central modulator in mood regulation? Neuropsychopharmacology 35(11):2143–2154
Eldar-Finkelman H (2002) Glycogen synthase kinase 3: an emerging therapeutic target. Trends Mol Med 8(3):126–132
Benedetti F, Poletti S, Radaelli D, Bernasconi A, Cavallaro R, Falini A, Lorenzi C, Pirovano A, Dallaspezia S, Locatelli C, Scotti G, Smeraldi E (2010) Temporal lobe grey matter volume in schizophrenia is associated with a genetic polymorphism influencing glycogen synthase kinase 3-β activity. Genes Brain Behav 9(4):365–371
Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, Matthews P, Isaac JTR, Bortolotto ZA, Wang YT, Collingridge GL (2007) LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron 53(5):703–717
Yu DF, Shen ZC, Wu PF, Guan XL, Chen T, Jin Y, Hu ZL, Ni L, Wang F, Chen JG, Long LH (2016) HFS-triggered AMPK activation phosphorylates GSK3β and induces E-LTP in rat hippocampus in vivo. CNS Neurosci Ther 22(6):525–531
Ma T, Tzavaras N, Tsokas P, Landau EM, Blitzer RD (2011) Synaptic stimulation of mTOR is mediated by Wnt signaling and regulation of glycogen synthetase kinase-3. J Neurosci 31(48):17537–17546
Fortress AM, Frick KM (2016) Hippocampal Wnt signaling: memory regulation and hormone interactions. Neuroscientist 22(3):278–294
Fortress AM, Schram SL, Tuscher JJ, Frick KM (2013) Canonical Wnt signaling is necessary for object recognition memory consolidation. J Neurosci 33(31):12619–12626
Garza JC, Guo M, Zhang W, Lu XY (2012) Leptin restores adult hippocampal neurogenesis in a chronic unpredictable stress model of depression and reverses glucocorticoid-induced inhibition of GSK-3β/β-catenin signaling. Mol Psychiatry 17(8):790–808
Chen BY, Wang X, Wang ZY, Wang YZ, Chen LW, Luo ZJ (2013) Brain-derived neurotrophic factor stimulates proliferation and differentiation of neural stem cells, possibly by triggering the Wnt/β-catenin signaling pathway. J Neurosci Res 91(1):30–41
Zhang W, Shi Y, Peng Y, Zhong L, Zhu S, Zhang W, Tang SJ (2018) Neuron activity-induced Wnt signaling up-regulates expression of brain-derived neurotrophic factor in the pain neural circuit. J Biol Chem 293(40):15641–15651
Yi H, Hu J, Qian J, Hackam AS (2012) Expression of brain-derived neurotrophic factor is regulated by the Wnt signaling pathway. Neuroreport 23(3):189–194
Martin RB (1986) The chemistry of aluminum as related to biology and medicine. Clin Chem 32(10):1797–1806
Coghlan MP, Culbert AA, Cross DAE, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, Mills D, Brown MJ, Haigh D, Ward RW, Smith DG, Murray KJ, Reith AD, Holder JC (2000) Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol 7(10):793–803
Hooper C, Markevich V, Plattner F, Killick R, Schofield E, Engel T, Hernandez F, Anderton B, Rosenblum K, Bliss T, Cooke SF, Avila J, Lucas JJ, Giese KP, Stephenson J, Lovestone S (2007) Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. Eur J Neurosci 25(1):81–86
Stepanichev MY, Kudryashova IV, Yakovlev AA, Onufriev MV, Khaspekov LG, Lyzhin AA, Lazareva NA, Gulyaeva NV (2005) Central administration of a caspase inhibitor impairs shuttle-box performance in rats. Neuroscience 136(2):579–591
Paxinos G, Watson C, Pennisi M, Topple A (1985) Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods 13(2):139–143
Liang RF et al (2012) Aluminium-maltolate-induced impairment of learning, memory and hippocampal long-term potentiation in rats. Ind Health 50(5):428–436
Singh S, Mishra A, Bharti S, Tiwari V, Singh J, Parul, Shukla S (2018) Glycogen synthase kinase-3β regulates equilibrium between neurogenesis and gliogenesis in rat model of Parkinson’s disease: a crosstalk with Wnt and notch signaling. Mol Neurobiol 55(8):6500–6517
Wang J, Lin F, Cai F, Yan W, Zhou Q, Xie L (2013) Microcystin-LR inhibited hippocampal long-term potential via regulation of the glycogen synthase kinase-3β pathway. Chemosphere 93(2):223–229
Buchta AM et al (2005) Neurotoxicity of exposures to aluminium welding fumes in the truck trailer construction industry. Environ Toxicol Pharmacol 19(3):677–685
Zawilla NH, Taha FM, Kishk NA, Farahat SA, Farghaly M, Hussein M (2014) Occupational exposure to aluminum and its amyloidogenic link with cognitive functions. J Inorg Biochem 139:57–64
Wang L, Hu J, Zhao Y, Lu X, Zhang Q, Niu Q (2014) Effects of aluminium on β-amyloid (1-42) and secretases (APP-cleaving enzymes) in rat brain. Neurochem Res 39(7):1338–1345
Birch AM, Kelly ÁM (2013) Chronic intracerebroventricular infusion of nerve growth factor improves recognition memory in the rat. Neuropharmacology 75:255–261
Khan MB, Ahmad M, Ahmad S, Ishrat T, Vaibhav K, Khuwaja G, Islam F (2015) Bacopa monniera ameliorates cognitive impairment and neurodegeneration induced by intracerebroventricular-streptozotocin in rat: behavioral, biochemical, immunohistochemical and histopathological evidences. Metab Brain Dis 30(1):115–127
Pan B, Li Y, Zhang J, Zhou Y, Li L, Xue X, Li H, Niu Q (2020) Role of mGluR 1 in synaptic plasticity impairment induced by maltol aluminium in rats. Environ Toxicol Pharmacol 78:103406
McLachlan DR et al (1991) Would decreased aluminum ingestion reduce the incidence of Alzheimer's disease? Cmaj 145(7):793–804
Shuchang H et al (2008) Protective effects of gastrodia elata on aluminium-chloride-induced learning impairments and alterations of amino acid neurotransmitter release in adult rats. Restor Neurol Neurosci 26(6):467–473
Luo J (2009) GSK3beta in ethanol neurotoxicity. Mol Neurobiol 40(2):108–121
Shim SS, Stutzmann GE (2016) Inhibition of glycogen synthase kinase-3: an emerging target in the treatment of traumatic brain injury. J Neurotrauma 33(23):2065–2076
Takashima A (2012) GSK-3β and memory formation. Front Mol Neurosci 5:47
Dewachter I, Ris L, Jaworski T, Seymour CM, Kremer A, Borghgraef P, de Vijver H, Godaux E, van Leuven F (2009) GSK3beta, a centre-staged kinase in neuropsychiatric disorders, modulates long term memory by inhibitory phosphorylation at serine-9. Neurobiol Dis 35(2):193–200
Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S (2008) New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev 59(1):201–220
Oliva CA, Vargas JY, Inestrosa NC (2013) Wnts in adult brain: from synaptic plasticity to cognitive deficiencies. Front Cell Neurosci 7:224
Nagahara AH, Tuszynski MH (2011) Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov 10(3):209–219
Andero R, Choi DC, Ressler KJ (2014) BDNF-TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. Prog Mol Biol Transl Sci 122:169–192
Yang JW, Ru J, Ma W, Gao Y, Liang Z, Liu J, Guo JH, Li LY (2015) BDNF promotes the growth of human neurons through crosstalk with the Wnt/β-catenin signaling pathway via GSK-3β. Neuropeptides 54:35–46
Hiester BG, Galati DF, Salinas PC, Jones KR (2013) Neurotrophin and Wnt signaling cooperatively regulate dendritic spine formation. Mol Cell Neurosci 56:115–127
Acknowledgments
We sincerely thank our colleagues for their help and work on the study.
Funding
This work was financially supported by the National Natural Science Foundation of China (nos. 81703202 and 81872599).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
All experiments complied with the rulings of the Institute of Laboratory Animal Research, Division on Earth and Life Sciences, National Institute of Health, USA (Guide for the Care and Use of Laboratory Animals). The research protocol was performed in accordance with the regulations of the Institutional Animal Care and Use Committee of Shanxi Medical University.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
First authors: Huifang Zhang and Yingchao Han
Rights and permissions
About this article
Cite this article
Zhang, H., Han, Y., Zhang, L. et al. The GSK-3β/β-Catenin Signaling–Mediated Brain–Derived Neurotrophic Factor Pathway Is Involved in Aluminum-Induced Impairment of Hippocampal LTP In Vivo. Biol Trace Elem Res 199, 4635–4645 (2021). https://doi.org/10.1007/s12011-021-02582-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02582-9