Abstract
Relative stability of mineral elements in tissues is necessary for health. High static magnetic fields (HiSMFs) have been widely used in biomedical research and industry. However, the bioeffect of HiSMFs on animals is still unclear. In this study, we investigated the effects of HiSMF exposure on the levels of Mg, Fe, Zn, Ca, and Cu in the main organs of mice. The 8-week male C57BL/6 mice were treated by 2–4 T, 6–8 T, 10–12 T HiSMFs for 28 days. The mass fractions of Mg, Fe, Zn, Ca, and Cu in the liver, brain, kidney, and heart in mice were respectively measured by atomic absorption spectroscopy, and used to evaluate mineral element content in tissues. The 2–4 T HiSMF exposure has increased the Mg, Fe, and Ca content in the kidney, as well as the Zn content in the brain. The 6–8 T HiSMF exposure has increased the Zn level in the liver; Mg, Fe, and Ca levels in the kidney; and Fe level in the heart, while the Zn in the kidney, and Zn and Ca in the heart was decreased by 6–8 T HiSMF exposure. For the 10–12 T HiSMF exposure, the Mg in the kidney, the Fe in the liver and kidney, and Cu in the brain have been increased significantly. However, the Zn in the kidney and the Ca in the brain and the heart were reduced by 10–12 T HiSMF exposure. The HiSMF exposure for 28 days can alter the Mg, Fe, Zn, Ca, and Cu content in mice, and change with the different magnetic flux density of HiSMFs (2–4 T, 6–8 T, 10–12 T), elements, and organ types.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the development of technology, artificial magnetic sources are gradually diversifying, and the magnetic environment that humans are exposed to is becoming more and more complex. Therefore, the biological effects of static magnetic fields (SMFs) have been valued gradually. Recently, the SMFs have been widely explored and utilized in biomedical field, such as bone repair and cancer research [1, 2]. According to the difference in magnetic flux density, the SMFs can be divided into the following categories: hypomagnetic field (HyMF, < 5 μT), weak (< 1 mT), moderate (1 mT–1 T), and high SMFs (HiSMFs) (> 1 T) [1, 3]. The magnetic resonance imaging (MRI) is the most famous application of HiSMFs in the biomedical field [4]. At present, the magnetic flux density of the HiSMFs in MRI systems as high as 7 T, and to pursue higher resolution and signal-to-noise ratio, 10.5 T MRI equipment for humans has also entered the experimental stage [5, 6]. There were various studies that focused on the influence of HiSMFs on animals, while most of them are based on temporary HiSMF exposure [5, 7]. Meanwhile, studies showed that the biological effects of SMFs are exposure time dependently [8, 9]. Nevertheless, the studies about the effect of HiSMF long-term exposure on biological systems are very limited.
Mineral elements are crucial for body health, and the changes of element content and distribution in cells and tissues may affect the normal physiological functions of organisms [10, 11]. As mineral elements, the imbalance of Mg, Fe, Zn, Ca, and Cu is associated with the occurrence of many diseases. Mg deficiency can cause arrhythmia; Fe accumulation in the brain is related to Alzheimer’s disease; Zn is essential for the development of the brain and reproductive system; Ca in the heart is related to heart contraction; and the disorder of Cu efflux could cause Wilson’s disease [12,13,14,15,16].
A previous study showed that the SMF exposure could affect the elements’ levels in cells and organs [17,18,19]. The 128 mT moderate SMF exposure could affect the Zn and Cu content in the liver and brain of mice, and the serum Zn and Fe level in rat [20, 21]. Our previous study found that 16 T HiSMF could increase Fe content in osteoblast MC3T3-E1 cells [22]. Furthermore, earlier studies indicated that the SMFs could change the membrane permeability of cells [23]. Homogeneous SMFs can change the cell membrane potential through the Lorentz force in ion diffusion, and the magnetic force under a gradient SMFs can create an ion flux across the membrane to change the membrane potential [24]. The transmembrane transport of many metal elements is regulated by the membrane potential, such as changing the structure of voltage-dependent/gated channels of Ca2+, etc. [25]. Therefore, determining the change of mineral elements in different organs is a worthy method to evaluate the biological effects of HiSMFs.
In this study, we investigated the content of Mg, Fe, Zn, Ca, and Cu in the liver, brain, kidney, and heart tissues to assess the effect of continuous exposure to 2–12 T HiSMFs for 28 days on the distribution of metal elements in mice. It can deepen the understanding of the mechanism of HiSMF biological effects, and explore the application potential of HiSMFs in the therapy of element imbalance–related diseases.
Materials and Methods
High Static Magnetic Fields and Mice Feeding System
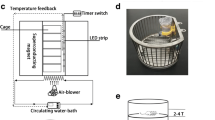
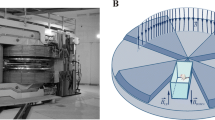
HiSMF was generated by a non-refrigerant superconducting magnet (CRYOF12/150, OXFORD) (Fig. 1a). The maximum magnetic flux density of the superconducting magnet is 12 T, and the magnetic flux density decreases from the middle to the sides. The magnetic flux density distribution is measured and acquired by a gauss meter (421, LakeShore). Relying on the magnet device, we built a special mice feeding system to ensure stable and controllable environmental conditions as previously described (Fig. 1b) [26].
Experimental HiSMF environment device. (a) Non-refrigerant superconducting magnets (CRYOF12/150, OXFORD); (b) schematic diagram of the mouse feeding system in the HiSMF; (c) the arrangement of the mice cages (three mice per cage) and magnetic field distribution. The magnetic field gradients in 2–4 T, 6–8 T, and 10–12 T groups were 24–35 T/m, 55–60 T/m, and 55–70 T/m
Animals and Treatments
Forty-eight 8-week-old male C57BL/6 mice were used for this study. After adapting for a week, the mice were randomly assigned to one of the following four groups (n = 12): (1) the sham control group; (2) the 2–4 T HiSMF group, (3) the 6–8 T HiSMF group, and (4) the 10–12 T HiSMF group. The three HiSMFs groups were fed in a special animal feeding system based on the superconducting magnet and were continuously exposed to the HiSMFs for 28 days. The sham control group (control group) was fed in the same system with the geomagnetic field (GMF), and with the magnetic flux, the density was between 40 and 50 μT. The 2–4 T, 6–8 T, and 10–12 T HiSMF groups were distinguished and named by the magnetic flux density and the active area of free movement for the mice (5 cm above the bottom of the cage), with the magnetic field gradients which were 24–35 T/m, 55–60 T/m, and 55–70 T/m (Fig. 1c). The mice were maintained free access to standard rodent food and water. The environment was maintained at 25 ± 2 °C, and kept on a 12-h light/dark cycle. The experiments were performed twice and data were pooled. All animal protocols used in this study were approved by the Lab Animal Ethics and Welfare Committee of Northwestern Polytechnical University (No. 2017027).
Determination of Mg, Fe, Zn, Ca, and Cu in Tissues
The Mg, Fe, Zn, Ca, and Cu in the liver, brain, kidney, and heart were determined using an atomic absorption spectrometer (AAS; Analytik Jena, Jena, Germany). The tissues were collected from the sacrificed mice after the cardiac perfusion by normal saline solution. We dried the tissues under 120 °C for 6 h and measured its dry weight. After that, we heated the tissues at 600 °C for 6 h to ash the tissue, then were dissolved in 1 ml 65% HNO3. We diluted the samples with ddH2O to a suitable concentration; then the Mg, Fe, Zn, Ca, and Cu concentrations were measured by flame AAS; the parameter settings of atomic absorption spectrometry are shown in Table 1. The element’s content in tissues was calculated as the mass fraction between the mass of the element and tissue dry weight, respectively.
Statistics
All statistical analyses were performed using the GraphPad Prism statistical software for Windows (version 5, GraphPad Software, Inc.). The differences of element contents between the control group and the three HiSMFs groups were revealed by using an ordinary one-way ANOVA followed by Duncan’s test. The normal distribution was tested by Kolmogorov-Smirnov test with p > 0.05, and equal variances were tested by using Bartlett’s test with p > 0.05 for the requirements of the ANOVA test. The results are expressed as the mean ± standard deviation. For all statistical tests, p < 0.05 was considered to indicate statistical differences.
Result
Liver, Brain, Kidney, and Heart Mg Level
The Mg in the liver, brain, kidney, and heart samples is shown in Fig. 2. The 2–4 T, 6–8 T and 10–12 T HiSMF exposure for 28 days cannot change the Mg levels in the liver, brain, and heart tissues. The average value of Mg content in the kidney showed that there is a significant increase in 2–4 T, 6–8 T, and 10–12 T groups compared to the control group.
Liver, Brain, Kidney and Heart Fe Level
The Fe in the liver, brain, kidney, and heart samples are shown in Fig. 3. The Fe content in the liver was increasing by the 10–12 T HiSMF exposure; the 2–4 T and 6–8 T HiSMF treatment for 28 days did not infect the Fe content in the liver. The Fe level in the brain had no significant change after 2–4 T, 6–8 T, and 10–12 T HiSMF exposure for 28 days. The Fe content in the kidney of 2–4 T, 6–8 T, and 10–12 T HiSMF group was significantly higher than the control. Interestingly, the Fe content of heart in the 6–8 T group has been significantly increased compared to the control group, but changes in the 2–4 T and 10–12 T groups are not significant.
Liver, Brain, Kidney and Heart Zn Level
The Zn in the liver, brain, kidney, and heart samples are shown in Fig. 4. The Zn level in the liver and heart has been significantly decreased by 6–8 T HiSMF exposure, but the 2–4 T and 10–12 T groups have no significant change compared to the control group. In the kidney, the Zn content was decreased by 6–8 T and 10–12 T HiSMF exposure, and not changed by the 2–4 T HiSMF exposure for 28 days. Interestingly, the significant elevation was found between 2–4 T HiSMF group and control group in terms of the Zn content in the brain, but the 6–8 T and 10–12 T exposure have no effect on the Zn level in the brain.
Liver, Brain, Kidney and Heart Ca Level
The Ca in the liver, brain, kidney, and heart samples is shown in Fig. 5. Compared to the control group, the Ca content in the liver was increased by 6–8 T HiSMF exposure for 28 days. However, the liver’s Ca level had no significant difference in 2–4 T, 6–8 T and 10–12 T groups compared to the control group. The Ca in the brain has no significant change by the 2–4 T and 6–8 T HiSMF exposure and increased by the 10–12 T HiSMF exposure for 28 days. The kidney Ca level was increased by 2–4 T and 6–8 T HiSMF exposure compared to the control, and no significant change in the 10–12 T group. The heart Ca content in 2–4 T group was the same as the control group, but the heart Ca contents in the 6–8 T and 10–12 T groups were significantly lower than the control group.
Liver, Brain, Kidney and Heart Cu Level
The Cu in the liver, brain, kidney, and heart samples is shown in Fig. 6. The data showed that there were no significant differences between control and three HiSMFs treatment groups in the liver, kidney, and heart Cu content. The average Cu content of the brain showed that there was a significant increase in the 10–12 T group compared to the control group, but no significant difference in the 2–4 T and 4–8 T groups compared to the control group.
Discussion
Under physiological conditions, the content of elements in various tissues of the organism is in a relatively stable state, and the imbalance of element content in tissues caused by changes in the external environment may cause many pathological reactions, such as oxidative stress toxicity and functional impairment [27]. Due to the difference in experimental conditions and subjects, the tissue element content showed different changes under SMF exposure [21, 28, 29]. There are few studies on the effect of HiSMFs on intracellular element content, and studies based on animals are unprecedented. In this study, we found that the 2–4 T, 6–8 T, and 10–12 T HiSMF continuous exposure for 28 days could affect the content and distribution of Mg, Fe, Zn, Ca, and Cu in the liver, brain, kidney, and heart tissues of mice. Besides, these changes vary from the difference in elements, tissues, and magnetic flux density.
The kidney is an important place for Mg excretion and reabsorption [30]. In this study, the Mg content in the kidney has been increased by HiSMF exposure. It suggests that the HiSMF exposure could enhance the reabsorption of Mg in the kidney.
Fe is involved in a variety of physiologic processes in organisms, and is an important component of many enzymes, such as a series of enzymes involved in DNA synthesis and mitochondria respiration. Changes in the Fe content may lead to an imbalance of related enzymatic reactions and affect the normal physiologic functions of the tissues. It is well-known that Fe plays an important role in the intracellular redox balance [31]. Firstly, Fe is a component of oxidoreductases such as peroxidase and catalase. Moreover, Fe in cells can generate ROS through the Fenton reaction, and directly affects the oxidative stress level of cells. Fe overload in the brain has been proved as the triggers of neurodegenerative diseases, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) [12]. In this study, the brain’s Fe level has no significant change under HiSMF exposure. The liver is an important organ for Fe storage, and the myoglobin in myocardial cells contains a large amount of Fe. We found that the HiSMFs can increase the Fe content in the liver and heart, which may be related to the active iron metabolism of these cells. The previous research in our group indicated that SMFs can increase the Fe content in osteoblasts, while some studies have found that a 10-mT SMF can decrease the Fe content in MCF7 and HFF cells [22, 32]. This is probably due to the SMFs having diverse effects on different cells, or it may be caused by differences in magnetic flux density. In this study, it was also found that different HiSMFs have different effects on the Fe content of different tissues.
Zinc deficiency is involved in various diseases, such as growth retardation and immune dysfunction [33]. The maintenance of the structure and function of the nerve synapse requires the participation of Zn. The Zn deficiency in the brain is related to the central nervous system malformations, while the rapid increase in zinc content can also cause neurotoxicity. Interestingly, studies have shown that both the deficiency and excess of zinc are related to the occurrence of Alzheimer’s disease [34, 35]. In this study, the 2–4 T HiSMF can increase the zinc content of brain, which can provide potential physical therapy for brain dysplasia caused by zinc deficiency, while we must also beware to its potential risks.
Lahbib Aida et al. found that 128 mT SMF exposure can increase the zinc content in the blood of rats and decrease the Fe content [21]. In this study, except for the 2–4 T HiSMF can increase the zinc content in the brains of mice; the other changes in the zinc content in the tissues caused by the HiSMFs are reduced. Unlike zinc, the changes in tissue Fe content caused by the HiSMFs are increased. In this study, the Fe and Zn content in the heart under the 6–8 T HiSMF exposure, and which in the kidney under 6–8 T and 10–12 T HiSMF exposure, showed the opposite trend. The difference and mechanism of the influence of HiSMFs on the content of Fe and Zn in the tissue are also worthy of further exploration. This phenomenon may be due to the disruption of redox balance leading to physiological disturbances as described by Aida et al., or it may be due to a certain difference in the response of the two elements to the magnetic field, such as the magnetism of ions.
Ca is playing a pivotally important role in many biological processes. It is a major constituent of the skeleton and could influence signal transduction pathways as a secondary messenger [36]. The influx of calcium in most tissues is mainly through voltage-gated calcium channels (VGCCs). In this study, the Ca content of various tissues under a HiSMF exposure has been significantly changed, which may be caused by the HiSMF introducing the change of the membrane voltage. Meanwhile, the plasma membrane Ca2+-ATPase (PMCA) is the key membrane protein that mediates Ca2+ efflux, and this process is ATP-dependent. Studies have shown that SMFs can affect the intracellular ATP level [37, 38]. It may also be the mechanism of tissue Ca level changes after HiSMF exposure.
Like the Fe, the Cu can participate in the generation of intracellular ROS through the Fenton-Like reaction, and causing membrane lipid peroxidation. Therefore, excessive intracellular Cu could cause cell oxidative stress damages. Besides, the increase in Cu content is associated with the occurrence of some degenerative diseases [35, 39]. In this study, the 12 T HiSMF exposure increased brain Cu content, which suggested that the 12 T HiSMF had potential risks to the central nervous system.
In this study, the results showed that the same magnetic field exposure has distinctive effects on the content of each element in the same tissue. The ion channels on cell membranes of different elements have different responses to SMFs which is a reasonable cause. Meanwhile, it is probably caused by different element ions that have different magnetic properties, and such that the Zn2+ is diamagnetic, while the Cu2+ and Fe2+/Fe3+ ions are paramagnetic. Furthermore, because the phospholipid molecule is diamagnetic, it could undergo conformational changes in the gradient SMFs [40]. This change in the cell membrane structure may also affect the ion transport of the cell and affect the intracellular element content. It could not be ignored that the functions of the intracellular different elements are similar or opposite, and the changes in one element content can affect the level of other elements. For example, the Mg, Fe, Zn, and Cu are widely involved in the regulation of intracellular redox metabolism.
The same element showed different changes in the same organ after the different HiSMF exposure, and it suggests that there is a threshold between the SMF’s characteristics and bioeffects. To determine the relationship between SMF’s bioeffects and characteristics, the influence of the intensity and gradient of the SMFs on its biological effects is worthy of further exploration, respectively. In addition, the biological effect of the SMFs is related to the cell type, which may be the reason for the different changes of the same element in different tissues [41, 42].
Preliminary studies have shown that, from the perspective of histomorphology, 2–12 T HiSMF treatment for 28 days will not cause significant pathological changes on tissues in mice [26]. In this study, the HiSMF exposure can affect the changes of the element content in the tissues of the mice. This may be because the effect of the HiSMFs on normal tissues is relatively mild.
In conclusion, we reported that 2–12 T HiSMF exposure for 28 days could alter Mg, Fe, Zn, Ca, and Cu elements distribution in the mice. On one hand, the changes of the elements in the tissue caused by the HiSMF are a warning that we should attach importance to the potential physiological changes of organisms under HiSMF exposure. Meanwhile, it also provides potential non-invasive physical therapy methods of the diseases caused by element imbalances.
References
Zhang J, Ding C, Ren L, Zhou Y, Shang P (2014) The effects of static magnetic fields on bone. Prog Biophys Mol Biol 114(3):146–152
Yuan LQ, Wang C, Lu DF, Zhao XD, Tan LH, Chen X (2020) Induction of apoptosis and ferroptosis by a tumor suppressing magnetic field through ROS-mediated DNA damage. Aging-Us 12(4):3662–3681
Rosen AD (2003) Mechanism of action of moderate-intensity static magnetic fields on biological systems. Cell Biochem Biophys 39(2):163–173
Budinger TF, Lauterbur PC (1984) Nuclear magnetic-resonance technology for medical studies. Science 226(4672):288–298
Eryaman Y, Zhang P, Utecht L, Kose K, Lagore RL, DelaBarre L, Kulesa J, Eberly LE, Adriany G, Iles TL, Iaizzo PA, Vaughan JT, Ugurbil K (2018) Investigating the physiological effects of 10.5 Tesla static field exposure on anesthetized swine. Magn Reson Med 79(1):511–514
Nowogrodzki A (2018) The world’s strongest MRI machines are pushing human imaging to new limits. Nature 563(7729):24–26
Tian XF et al (2019) Effects of 3.5-23.0 T static magnetic fields on mice: a safety study. Neuroimage 199:273–280
Chionna A et al (2003) Cell shape and plasma membrane alterations after static magnetic fields exposure. Eur J Histochem 47(4):299–308
Tatarov I, Panda A, Petkov D, Kolappaswamy K, Thompson K, Kavirayani A, Lipsky MM, Elson E, Davis CC, Martin SS, DeTolla L (2011) Effect of magnetic fields on tumor growth and viability. Comp Med 61(4):339–345
World Health Organization, Food and Agriculture Organization of the United Nations, and International Atomic Energy Agency (1996) Trace elements in human nutrition and health. World Health Organization, Geneva xviii, 343 p
Brown CJ, Chenery SRN, Smith B, Mason C, Tomkins A, Roberts GJ, Sserunjogi L, Tiberindwa JV (2004) Environmental influences on the trace element content of teeth--implications for disease and nutritional status. Arch Oral Biol 49(9):705–717
Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L (2014) The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 13(10):1045–1060
Eisner D (2014) Calcium in the heart: from physiology to disease. Exp Physiol 99(10):1273–1282
Krishnan N, Felice C, Rivera K, Pappin DJ, Tonks NK (2018) DPM-1001 decreased copper levels and ameliorated deficits in a mouse model of Wilson’s disease. Genes Dev 32(13-14):944–952
Fairley JL et al (2019) Magnesium status and magnesium therapy in cardiac surgery: a systematic review and meta-analysis focusing on arrhythmia prevention. Intern Med J 49:5–5
Black RE et al (2013) Maternal and Child Nutrition Study Group. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382(9890):396–396
Rubio Ayala M, Syrovets T, Hafner S, Zablotskii V, Dejneka A, Simmet T (2018) Spatiotemporal magnetic fields enhance cytosolic Ca(2+) levels and induce actin polymerization via activation of voltage-gated sodium channels in skeletal muscle cells. Biomaterials 163:174–184
Erdem O, Akay C, Cevher SC, Canseven AG, Aydın A, Seyhan N (2018) Effects of intermittent and continuous magnetic fields on trace element levels in guinea pigs. Biol Trace Elem Res 181(2):265–271
Dogan MS, Yavaş MC, Yavuz Y, Erdoğan S, Yener İ, Şimşek İ, Akkuş Z, Eratilla V, Tanık A, Akdag MZ (2017) Effect of electromagnetic fields and antioxidants on the trace element content of rat teeth. Drug Des Devel Ther 11:1393–1398
De Luka SR et al (2016) Subchronic exposure to static magnetic field differently affects zinc and copper content in murine organs. Int J Radiat Biol 92(3):140–147
Aida L, Soumaya G, Myriam E, Mohsen S, Hafedh A (2014) Effects of static magnetic field exposure on plasma element levels in rat. Biol Trace Elem Res 160(1):67–72
Yang J, Zhang J, Ding C, Dong D, Shang P (2018) Regulation of osteoblast differentiation and iron content in MC3T3-E1 cells by static magnetic field with different intensities. Biol Trace Elem Res 184(1):214–225
Liu Y et al (2011) An investigation into the combined effect of static magnetic fields and different anticancer drugs on K562 cell membranes. Tumori 97(3):386–392
Zablotskii V, Polyakova T, Dejneka A (2018) Cells in the non-uniform magnetic world: how cells respond to high-gradient magnetic fields. Bioessays 40(8):e1800017
Zablotskii V et al (2016) How a high-gradient magnetic field could affect cell life. Sci Rep 6:37407
Wang S, Luo J, Lv H, Zhang Z, Yang J, Dong D, Fang Y, Hu L, Liu M, Liao Z, Li J, Fang Z, Wei Y, Han W, Shaikh AB, Yin D, Shang P (2019) Safety of exposure to high static magnetic fields (2 T-12 T): a study on mice. Eur Radiol 29(11):6029–6037
Brodziak-Dopierala B, Kwapulinski J, Kusz D, Gajda Z, Sobczyk K (2009) Interactions between concentrations of chemical elements in human femoral heads. Arch Environ Contam Toxicol 57(1):203–210
Salem A et al (2005) Zinc prevents hematological and biochemical alterations induced by static magnetic field in rats. Pharmacol Rep 57(5):616–622
Miryam E, Aida L, Samira M, Mohsen S, Hafedh A (2010) Effects of acute exposure to static magnetic field on ionic composition of rat spinal cord. Gen Physiol Biophys 29(3):288–294
Curry JN, Yu ASL (2018) Magnesium handling in the kidney. Adv Chronic Kidney Dis 25(3):236–243
Crielaard BJ, Lammers T, Rivella S (2017) Targeting iron metabolism in drug discovery and delivery. Nat Rev Drug Discov 16(6):400–423
Hajipour Verdom B, Abdolmaleki P, Behmanesh M (2018) The static magnetic field remotely boosts the efficiency of doxorubicin through modulating ROS behaviors. Sci Rep 8(1):990
Prasad AS (2009) Impact of the discovery of human zinc deficiency on health. J Am Coll Nutr 28(3):257–265
Fitzgerald DJ (1995) Zinc and Alzheimer’s disease. Science 268(5219):1920 author reply 1921-3
Sensi SL, Granzotto A, Siotto M, Squitti R (2018) Copper and zinc dysregulation in Alzheimer’s disease. Trends Pharmacol Sci 39(12):1049–1063
Carafoli E, Klee CB (1999) Calcium as a cellular regulator. Oxford University Press, New York xiii, 642 p
Zhao GP et al (2011) Cellular ATP content was decreased by a homogeneous 8.5 T static magnetic field exposure: role of reactive oxygen species. Bioelectromagnetics 32(2):94–101
Wang D, Wang Z, Zhang L, Li Z, Tian XF, Fang J, Lu Q, Zhang X (2018) Cellular ATP levels are affected by moderate and strong static magnetic fields. Bioelectromagnetics 39(5):352–360
Arnal N, Cristalli DO, de Alaniz MJT, Marra CA (2010) Clinical utility of copper, ceruloplasmin, and metallothionein plasma determinations in human neurodegenerative patients and their first-degree relatives. Brain Res 1319:118–130
Braganza LF, Blott BH, Coe TJ, Melville D (1984) The superdiamagnetic effect of magnetic fields on one and two component multilamellar liposomes. Biochim Biophys Acta 801(1):66–75
Short WO, Goodwill L, Taylor CW, Job C, Arthur ME, Cress AE (1992) Alteration of human tumor cell adhesion by high-strength static magnetic fields. Investig Radiol 27(10):836–840
Zhang L, Ji X, Yang X, Zhang X (2017) Cell type- and density-dependent effect of 1 T static magnetic field on cell proliferation. Oncotarget 8(8):13126–13141
Funding
This work was supported by the Natural Science Foundation of Shenzhen, the Technology and Innovation Commission of Shenzhen Municipality of China (JCYJ20190806145818081), the National Natural Science Foundation of China (52037007), and the Innovation Foundation for Doctor Dissertation of Northwestern Polytechnical University (CX201970).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, S., Huyan, T., Zhou, L. et al. Effect of High Static Magnetic Field (2 T–12 T) Exposure on the Mineral Element Content in Mice. Biol Trace Elem Res 199, 3416–3422 (2021). https://doi.org/10.1007/s12011-020-02469-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02469-1