Abstract
It has been shown that static magnetic field (SMF) of moderate intensity produces considerable impact on biological systems. SMF can be homogeneous or inhomogeneous. In many studies, inhomogeneous SMF was employed. Aware that inhomogeneous SMF could result in experimental variability, we investigated the influence of a vertical homogeneous SMF of different orientation. Male Swiss-Webster 9- to 10-week-old mice were subacutely exposed to upward- and downward-oriented SMF of 128 mT generated by a cyclotron for 1 h/day during a 5-day period. We found that SMF affected various organs and that these effects were, to some degree, dependent on SMF orientation. Both upward- and downward-oriented SMF caused a reduction in the amount of total white blood cells (WBC) and lymphocytes in serum, a decrease of granulocytes in the spleen, kidney inflammation, and an increase in the amount of high-density lipoprotein (HDL). In addition, upward-oriented SMF caused brain edema and increased spleen cellularity. In contrast, downward-oriented SMF induced liver inflammation and a decrease in the amount of serum granulocytes. These effects might represent a specific redistribution of pro-inflammatory cells in blood and among various organs. It appears that homogeneous SMF of 128 mT affected specific organs in the body, rather than simultaneously and equally influencing the entire body system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Two types of magnetic fields are widely present in the human environment—an alternating and a static magnetic field (SMF). Biological effects of alternating magnetic field have been extensively investigated since alternating electromagnetic fields are widely used in electrical power systems as well as in information and telecommunications technologies. In contrast, there is scarce data on SMF activity impact on human health (Heinrich et al. 2011). The main sources of SMF that is stronger than a geomagnetic field are found in various medical devices. SMF, which is difficult to shield and easily penetrates biological tissues, may be classified as weak (<1 mT), moderate (1 mT to 1 T), strong (1 to 5 T), and ultrastrong (>5 T). Unlike SMF of weak and, to some degree, SMF of strong intensity, SMF of moderate intensity has considerable effects on biological systems (Rosen 2003, Dini and Abbro 2005). These effects are mediated by interaction with moving charges (ions, proteins, etc.) and magnetic materials found in tissues as a consequence of several physical mechanisms (Schenck 2005, Saunders 2005). Homogeneous as well as inhomogeneous SMF was employed in many studies that report its significant effects (Abdelmelek et al. 2006, Elferchichi et al. 2007, Hashish et al. 2008, Amara et al. 2009, Elferchichi et al. 2010, Lahbib et al. 2010, László et al. 2007, Vergallo et al. 2013, Csillag et al. 2014, Kiss et al. 2015). Although it can be argued that spatial dependence introduced by field inhomogeneity is more likely to mimic realistic exposures to environmental fields, homogeneous magnetic fields offer an advantage for in vivo experiments with moving animals in that they ensure identical exposure conditions at every point of the experimental volume. Exposure dosage is equal at every point of animal body, regardless of animal position during the course of experiment. We therefore decided to employ a homogeneous SMF.
A significant influence of inhomogeneous SMF of moderate intensities with checkerboard configuration, i.e., of spatially alternating SMF with a period of 2 cm in both directions was reported (László et al. 2007, Vergallo et al. 2013, Csillag et al. 2014, Kiss et al. 2015). Thorough description, analysis, and optimization of the field (László et al. 2007) that was later used in a number of biomedical experiments (for example, in Vergallo et al. 2013, Csillag et al. 2014, Kiss et al. 2015) established justifiable cause-and-effect relationship between the applied field and the obtained results. Averaging of a spatially alternating field over the entire surfaces parallel to the magnetic checkerboard would result in zero magnetic flux density vector and mean field gradient. Therefore, the mean intensity of the applied SMF and strong local field gradients were the cause of the observed effects and the overall beneficial influence of SMF. Homogeneity of the cyclotron SMF that is used in our study is much better than that of the exposure systems commonly used in biomedical experiments. Consequently, any possible biological effect of field gradients is excluded and all observed results are due to the applied field intensity in the chosen direction. We exploited the possibilities of the cyclotron magnet to check if the field orientation as well as the field intensity applied throughout the experimental volume is of importance, the motivation for the former being the accounts of significance of field orientation in the inhomogeneous (Djordjevich et al. 2012) as well as in the homogeneous (Todorović et al. 2015) SMF. With the intention to single out the influence of the field orientation, we used downward as well as upward-oriented vertical, highly homogeneous SMF of moderate intensity. The intensity of the SMF was adjusted to 128 mT motivated by the existence of research data on the effects of the horizontal homogeneous SMF of the same intensity (Chater et al. 2006, Elferchichi et al. 2007, Amara et al. 2009, Lahbib et al. 2010, Elferchichi et al. 2010, Ghodbane et al. 2014), allowing for the comparison of the results.
The thorough study reported by Colbert et al. (2009) revealed that magnetic fields are often poorly described, lacking critical data on magnet characteristics, measured field strength, and estimated distance of the magnet from the target tissue. As a result, inferences drawn from such reports cannot be precisely associated with the applied SMFs. To the contrary if, as suggested by Colbert et al. (2009), all ten SMF dosage and treatment parameters deemed necessary for the full description of the applied SMF were systematically reported, as for example, in our study and in László et al. (2007), replication of experiments by other investigators as well as comparison with the results of exposures to different magnetic fields is enabled.
Biological response to moderate SMF is wide-ranging, involving different types of cells in various tissues and organs. For example, it has been shown in in vitro system that exposure to SMF caused a significant initial decline in ROS production by human peripheral blood neutrophils that was reversible after longer incubation time (Poniedzialek et al. 2013). Moreover, it has been demonstrated that SMF exposure had beneficial effects in a murine model of allergic inflammation via mobilization of cellular ROS-eliminating mechanism (Csillag et al. 2014). It has been also shown that SMF of moderate intensity induces hematological changes in exposed mice that resemble hypoxia-like status (Elferchichi et al. 2010). In addition, the hypoxia-like status is associated with a sympathetic hyperactivity that could be attributed to stress reaction of exposed animals (Abdelmelek et al. 2006). Using an in vivo experimental model of mouse ear edema, it has been suggested that the place of SMF action may be in the lower spinal region (Kiss et al. 2015). In vivo experiments also demonstrated antinociceptive effects of SMF in invertebrate (László and Hernádi 2012), mice (László et al. 2007, László and Gyires 2009) and humans (László et al. 2012). In vitro analysis of the effects of SMF on human macrophages and lymphocytes provided a possible explanation for the effect observed in vivo (Vergallo et al. 2013). Namely, it was shown that SMF exposure (of 200 mT average intensity) suppresses inflammation by reducing release of pro-inflammatory cytokines IL-6, IL-8, and TNF-α from macrophages and by enhancing release of anti-inflammatory cytokine IL-10 from lymphocytes (Vergallo et al. 2013). SMF of 128 mT can also induce tissue changes in various organs such as the liver, kidney, and brain of exposed animals (Amara et al. 2007, Amara et al. 2009), as well as in cell culture (Sahebjamei et al. 2007). These changes were attributed to oxidative stress. Another possibility is that the tissue changes induced by SMF were caused by the redistribution of inflammatory cells (Djordjevich et al. 2012). We were specifically interested to assess whether changes in hematological parameters could be directly related to changes observed in various organs from the same animals. Since changes in the blood may be specific to the hematological system and may not cause or reflect changes in the organs, we investigated whether changes in the blood corresponded to changes in selected organs, which had previously been studied by others in SMF of 128 mT. In our case, the spleen and kidney were selected for the experiment since they are involved in regulation of blood content. On the other hand, the liver and brain were studied as target organs for possible redistribution of blood cells and consequent oxidative damage. In addition, we chose the liver as an example of a target body organ because of its central role in lipid metabolism, which we studied in-depth.
Phospholipids are a class of lipids in which a phosphate group replaces one of the fatty acids. They are a major structural component of all cell membranes where they are involved in forming lipid bilayers. Phospholipids are amphipathic molecules containing a hydrophilic phosphate group and a hydrophobic fatty acid tail. The four phospholipids that predominate in the plasma membrane of mammalian cells are phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and sphingomyelin. Besides a structural role, phospholipids also have a metabolic role. They act as sources of intracellular signals in response to extracellular signals which interact with receptors on the outer layer of the plasma membrane. Phospholipid fatty acids of the cell membrane are precursors of lipid mediators, with eicosanoids (prostaglandins, thromboxanes, leukotrienes) being one of the most important. They, also, play a role in signal transduction and gene transcription pathways (Kremmyda et al. 2011). The liver plays an important role in the synthesis and metabolism of phospholipids. The structure of hepatocyte membrane phospholipids is dependent on nutritional intake and affects liver metabolic functions (Oguzhan et al. 2006). The fatty acid profile of liver phospholipids and triglycerides is known to be influenced by many factors, including dietary intake, age, gender, and endogenous metabolism (Oguzhan et al. 2006). However, magnetic field influence on the total fatty acids in an organism has been scarcely reported (Lahbib et al. 2010, Elferchichi et al. 2010). In the present study, we monitored the liver as a key representative organ of possible SMF influence on phospholipids.
We investigated the effects of homogeneous SMF on subacutely exposed animals by primary studying the hematological system and the liver, but we also monitored the brain, kidney, and spleen. Since literature analysis showed limited data regarding the interaction of differently oriented moderate SMF on biological systems, the present study was designed to investigate the effects of subacute exposure to differently oriented 128 mT SMF on mice.
Materials and methods
Animals
Male Swiss-Webster mice, weighing on average 23 ± 3 g, 9–10 weeks old, obtained from the Military Medical Academy Animal Research Facility (Belgrade, Serbia) were used. Mice were housed at four or five animals per cage and offered regular mouse feed and drinking water ad libitum. All experimental protocols involving animals were reviewed and approved by the University of Belgrade, Faculty of Medicine Experimental Animals Ethics Committee. Furthermore, all experiments were conducted in accordance with procedures described in the National Institutes of Health Guide for Care and Use of Laboratory Animals (Washington, DC, USA), as well as in accordance with the US-NIH guidelines for conducting magnetic field experiments on animals.
Magnetic field
Customarily, experiments performed in accelerator facilities utilize ion beams. However, the VINCY Cyclotron located at the Vinča Institute of Nuclear Sciences is still under construction. Its ferromagnetic structure is fully assembled and operative but still accessible, allowing employment of a strong static magnetic field as an experimental tool.
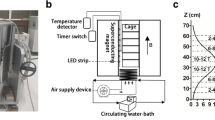
The VINCY Cyclotron is a sector-focused isochronous multipurpose machine (Nešković et al. 2003). Special attention was devoted to an extremely precise design of sectors on pole tips of the electromagnet to enable acceleration of the widest possible range of ion beams (Ćirković et al. 2009). Power consumption is minimal in the most frequent operating mode, while an appropriate choice of electrical currents of main coils and ten pairs of trim coils results in different isochronous magnetic fields that could be used for various modes of operation (Ćirković et al. 2008). The produced magnetic field between the two poles is highly homogeneous in the sector regions and in the valley regions; however, it abruptly changes in the vicinity of sector edges and the gradient of the magnetic field in that narrow area is strong. The main coil current can be set up to 1000 A, whereas the maximum mean magnetic field may reach 2 T. The electromagnet pole diameter measures 2 m, and it has four sectors per pole whose azimuthal width is 42°.
Magnetic flux density of 128 mT, desired for our experiment, was produced by setting the main coil current to 36.5 A, without necessity to use trim coils for further field adjustment. Field intensity is larger between the sectors and smaller in the valleys. For our experiment, it was convenient to center cages with experimental animals in the middle of the valley, well away from sector edges to avoid high magnetic field gradients in this region, as shown in Fig. 1. Cages, 19 cm high, 35.5 cm long, and 20.5 cm wide, were placed inside the circle of radius 80 cm (from the center of the cyclotron) to avoid the stray field components at radii larger than 84 cm. The desired magnetic field was achieved with less than 0.68 % variation throughout the above described area; therefore, SMF was considered highly homogeneous throughout the experimental volume. Note that the field produced by solenoids often used for exposure setups is about an order of magnitude less homogenous than the field we used.
The VINCY Cyclotron used as an experimental SMF exposure setup. a Photograph of the VINCY Cyclotron. b The lower pole of cyclotron magnet and position of the cage with animals in the middle of the cyclotron valley (drawn to scale). The produced magnetic field, depicted by the magnetic flux lines in the valley and above the sector, B, was vertical. The direction of the field was chosen to be directed upwards or downwards for the two groups of SMF exposed animals, denoted as the up group and down group, and is represented by the vectors B UP and B DOWN , respectively. The variation of the magnetic field intensity was smaller than 0.68 % everywhere in the experimental volume. Magnetic flux density used was 128 mT
The geomagnetic field is directed downward in the northern hemisphere and upward in the southern hemisphere, i.e., in the same and the opposite direction to gravity, respectively. Therefore, experiments in two sets of magnetic field exposures were performed; in the first set, we used the magnetic field oriented in the same direction as gravity (down group), and in the second, the magnetic field oriented upwards (up group). The third group of animals was control (sham exposed).
Experimental design
Mice were randomly divided into three groups (9 mice per group). All three groups were kept under same conditions. The first experimental group was exposed to the upward-oriented 128 mT SMF (up group) for 1 h/day over a 5-day period. The second experimental group was exposed to the downward-oriented 128 mT SMF (down group) for 1 h/day over a 5-day period. The exposure period was always between 8 and 12 a.m. on a consecutive day under standard light exposure and constant temperature. The control group was sham exposed. Following a 5-day period, all mice were sacrificed and the blood, spleen, liver, brain, and kidney were collected for further analyses. These analyses were performed blindly, with material marked just with a code and people conducting the analyses unaware of sample group origin.
Blood and spleen parameters
Blood parameters [red blood cells (RBS), lymphocytes, neutrophils, basophils, eosinophils, platelets (Plt), hemoglobin (Hgb), hematocrit (Hct), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC)] were determined using hematological counter ABX Pentra 80X (Montpellier, France) according to the manufacturer’s recommendations. The total number of granulocytes in blood and spleen samples was calculated by summing neutrophils with basophils and eosinophils. Total serum cholesterol, HDL, LDL, triglycerides, C-reactive protein (CRP), and alanine aminotransferase (ALT) were determined using BioSystems photometer type BTS-330 (Barcelona, Spain), according to manufacturer’s recommendations.
Spleen cellularity was prepared and analyzed by the previously explained procedure (Djordjevich et al. 2012).
Total lipid extraction from the liver
Obtained liver tissue was first homogenized using a 2:1 chloroform/methanol mixture and washed with a fivefold smaller volume of water or saline. The resulting mixture was separated into two phases. The lower phase was a total pure lipid extract. Liver tissue (1 g) was lyophilized and chloroform/methanol (2:1) and butylhydroxytoluene (BHT) as antioxidant were mixed. When the mixture was allowed to stand, a biphasic system was obtained. After evacuation, water was added. After centrifugation, the upper phase was put away until complete separation of the system was achieved. Further evacuation was done with 2:1 solvent systems: methanol/benzene, acetone/benzene, and ethanol/benzene. Addition and evacuation of chloroform and subsequent addition of hexane rendered the sample ready for thin liquid chromatography (TLC) (Tepšić et al. 2008).
Fatty acid analysis
The phospholipid fraction was isolated from the extracted lipids by one-dimensional (TLC) neutral lipid solvent system of hexane:diethyl ether:acetic acid (87:2:1) using Silica Gel GF plates (C. Merck, Darmstadt, Germany). The phospholipid fraction was scraped into glass tubes, and phospholipid fatty acids (FAs) methyl esters were prepared by transmethylation with sodium hydroxide (2 mol dm–3) in methanol (heated at 85 °C for 1 h) and after that with sulfuric acid (1 mol dm–3) in methanol (heated 85 °C for 2 h). After 30 min, samples of esters were centrifuged, upper phase were put into tubes and evaporated by technical nitrogen. FA methyl ester derivates formed from isolated plasma phospholipids fraction were separated by gas chromatography (GC) using Shimadzu GC 2014 (Kyoto, Japan) equipped with a flame ionization detector and DB-23 fused silica gel capillary column. The flame ionization detector was set at 250 °C, the injection port at 220 °C, and the oven temperature programmed from 130 to 190 °C at the heating rate of 3 °C/min (Folch et al. 1957). Comparing sample peak retention times with authentic standards (Sigma Chemical Company) and/or the (PUFA)-2 standard mixtures (Restec) identified individual FAs methyl esters.
Estimated activities of desaturase system
Several fatty-acid indices, reflecting desaturase and elongase activity, were derived from primary data (Cvetković et al. 2010). The ratios of 20:4/20:3, 20:3/18:2, and 22:6/22:5 were used as a measure of estimated Δ5-desaturase, Δ6-desaturase, and Δ4-desaturase activities, respectively, while 18:1/18:0 and 18:0/16:0 ratios represented estimated Δ9-desaturase and elongase activities.
Histology analysis
Brain, liver, spleen, and kidney tissues were prepared for histological analysis by the procedure explained earlier (Bancroft and Gamble 2001). In short, tissues were fixated in formalin (10 % formaldehyde-water solution) for 24 h and than embedded in paraffin. Generated 5-μm sections were mounted on slides and stained with hematoxylin (Bio-Optica, Milan, Italy) and eosin (MP Biomedicals LLC, Illkirch Cedex, France). Analysis of stained sections was conducted by two independent pathologists that used a light microscope Olympus BX41 (Tokyo, Japan) and made micrographs with Sony Exwave HAD digital camera, model SSC-DC50AP (Tokyo, Japan). Immunohistochemical (IHC) analysis was conducted with the CD3 (polyclonal rabbit anti-human CD3; Dako Denmark A/S; Glosturp, Denmark), CD15 (monoclonal mouse anti-human CD15 clone carb-3; Dako North America Inc., Camarillo, CA, USA), and CD20 (monoclonal mouse anti-human CD20cy clone L26; Dako Denmark A/S; Glosturp, Denmark) antibodies. After exposing tissue to primary antibodies for 1 h, slides were rinsed with water and then the secondary antibody was applied for 15 min. Detection was conducted by employing a horse radish peroxidase kit (UltraVision Large Volume Detection System Anti-Polyvalent, HRP (Ready-To-Use); Thermo Fisher Scientific, (Chestershire, UK). CD3 is a marker for T lymphocytes (Felgar et al. 1997), CD20 is a marker for B lymphocytes (Pinkus and Said 1988), and CD15 is a marker for granulocyte lineage (Barry et al. 2002).
Statistical analysis
Statistical analysis of obtained data was performed using software SPSS for Windows, version 16.0 (SPSS, Chicago, IL, USA). Differences among groups were evaluated by one-way ANOVA, followed by Fischer’s LSD test. Distribution of nonparametric data was analyzed by chi-square test. The level of significance was set at p < 0.05.
Results
Histological characteristics
Tissue morphology of the liver, spleen, kidney, and brain is presented in Fig. 2. The liver in the down group showed significant inflammation. Liver infiltrate localization was periportal and predominantly made of granulocytes and lymphocytes. Edematous hepatocytes were also present. The spleen did not show any pathological changes among groups, though congestive hyperemia was visible in all three groups, which is a usual finding following sacrifice. The kidney showed increased inflammation (nonspecific pyelonephritis) in exposed animals, but not in sham animals. Brain edema characterized by edematous neurons was present in the up group, but not in the down and sham groups.
Histological characteristics of the brain, liver, spleen, and kidney in mice exposed (up group, down group) or unexposed (sham-control) to SMF of different orientations. BC brain tissue in unexposed mice (brain control), BUp brain tissue in mice exposed to up-oriented SMF, BDw brain tissue in mice exposed to down-oriented SMF. LC liver tissue in unexposed mice (liver control), LUp liver tissue in mice exposed to up-oriented SMF, LDw liver tissue in mice exposed to down-oriented SMF. SC splenic tissue in unexposed mice (spleen control), SUp splenic tissue in mice exposed to up-oriented SMF, SDw splenic tissue in mice exposed to down-oriented SMF. KC kidney tissue in unexposed mice (kidney control), KUp kidney tissue in mice exposed to up-oriented SMF, KDw kidney tissue in mice exposed to down-oriented SMF. Arrow shows inflammatory cells
Histological analysis
Tissue characteristics of mice exposed to SMF of different orientations are presented in Table 1. The liver in the down group showed significant inflammatory characteristics when compared to up and sham groups (p < 0.05). The spleen did not show any pathological changes among groups (p > 0.05). The kidney showed increased inflammation in exposed animals (up and down group) when compared to control animals (p < 0.05). Brain tissue expressed significant edematous neurons in the up group when compared to the down and sham groups (p < 0.05).
Immunohistochemical analysis
Immunohistochemical (IHC) analysis of cell infiltrates in renal tissue is presented in Fig. 3. Initial determination of cell infiltrates in all affected organs was done by light microscopy. A confirmation of correct histological reading was conducted in a renal tissue by IHC analysis. In panel A, inflammatory cells are visible. In panel B, CD15 positive granulocytes are marked. In panel C, the CD3 positive T lymphocytes are shown. In panel D, the CD20 positive B lymphocytes are presented.
Immunohistochemical (IHC) analysis of cell infiltrates in renal tissue. a Renal tissue, magnification ×10, hematoxylin-eosin staining. Arrow shows inflammatory cells. b Renal tissue, magnification ×40, monoclonal mouse anti-human CD15 (granulocyte marker). Arrow shows CD15 positive granulocyte. c Renal tissue, magnification ×40, polyclonal rabbit anti-human CD3 (T lymphocyte marker). Arrow shows CD3 positive T lymphocyte. d Renal tissue, magnification ×40, monoclonal mouse anti-human CD20 (B lymphocyte marker). Arrow shows CD3 positive B lymphocyte
Blood parameters
Blood parameters in control mice and mice exposed to differently oriented SMF are shown in Table 2. Exposure of mice to differently oriented SMF did not alter the platelet count or Hgb, Hct, MCV, MCH, MCHC values among groups (p > 0.05). However, SMF of a different orientation influenced the serum lipids. There was a significant increase in serum values of HDL in exposed animals (up and down group) when compared to unexposed ones (61.50 ± 5.13 and 56.21 ± 2.38 vs. 41.77 ± 5.13; p < 0.01 and p < 0.05, respectively). Total serum cholesterol, LDL, and triglycerides did not change significantly among groups, but there was a trend of total cholesterol increase and LDL decrease in exposed groups (up and down group) (p > 0.05). C-reactive protein also did not change under the influence of differently oriented SMF (0.59 ± 0.09 and 0.64 ± 0.12 vs. 0.78 ± 0.32; p > 0.05), but we observed a significant increase of ALT in down group when compared to sham group (63.50 ± 8.17 vs. 42.33 ± 3.87; p < 0.05). In general, besides statistically significant results, the trend of increased values vs. sham group was more pronounced in the down group and the trend of decreased values vs. sham group was more pronounced in the up group.
Blood cellularity
Blood cellularity in mice exposed (up, down) or unexposed to SMF of different orientation is shown in Fig. 4. Total serum white blood cells (WBC) count was found to be significantly lower in exposed (up and down group) when compared to unexposed animals (3.49 ± 0.50 and 3.31 ± 0.30 vs. 6.61 ± 0.70; p < 0.01) (panel A). This decrease in exposed groups was mainly due to a reduction in serum lymphocytes (2.74 ± 0.36 and 2.97 ± 0.27 vs. 5.47 ± 0.61; p < 0.01) (panel D) and to a decrease in serum granulocytes, especially in down group (0.26 ± 0.03 vs. 0.87 ± 0.10; p < 0.01 compared to sham group and 0.26 ± 0.03 vs. 0.66 ± 0.14; p < 0.05 compared to up group) (panel C). While decrease in total WBC and serum lymphocytes were about the same in up and down groups, it is worth noting that statistically significant decrease in serum granulocytes occurred exclusively in the down group. This decrease in serum granulocytes was statistically significant not only versus the sham group but also versus the up group. SMF of a different orientation did not alter RBC count among groups (p > 0.05) (panel B).
Blood cellularity in mice exposed (up group, down group) or unexposed (sham-control) to SMF of a different orientation. Data are presented as mean ± SEM. a Total serum white blood cells in mice exposed (up group, down group) or unexposed (sham-control) to SMF of a different orientation; b serum red blood cells in mice exposed (up group, down group) or unexposed (sham-control) to SMF of a different orientation; c total serum granulocytes in mice exposed (up group, down group) or unexposed (sham-control) to SMF of a different orientation; d serum lymphocytes in mice exposed (up group, down group) or unexposed (sham-control) to SMF of a different orientation; **p < 0.01 compared to control; #p < 0.05 compared to up group
Spleen cellular parameters
Spleen cellular parameters in mice exposed to SMF of a different orientation are presented in Fig. 5. Total spleen cellularity in mice exposed to upward-oriented SMF was significantly higher when compared to those exposed to downward-oriented SMF (1.08 ± 0.05 vs. 0.69 ± 0.03; p < 0.01) and to control mice (1.08 ± 0.05 vs. 0.73 ± 0.05; p < 0.01) (panel A). However, there was no difference in spleen red blood cell count among groups (30.54 ± 3.26 vs. 34.51 ± 1.4 vs. 34.71 ± 1.05; p > 0.05, respectively) (panel B). Spleen granulocytes in the up group were significantly lower when compared to the sham group (2.2 ± 0.28 vs. 8.26 ± 1.30; p < 0.01) and spleen granulocytes in the down group were also significantly lower when compared to the sham group (1.64 ± 0.19 vs. 8.26 ± 1.30; p < 0.01) (panel C). In contrast, spleen lymphocytes did not alter among groups under the influence of SMF of different orientation (p > 0.05) (panel D).
Spleen cellularity in mice exposed (up group, down group) or unexposed (sham-control) to SMF of a different orientation. Data are presented as mean ± SEM. a Total spleen cellularity in mice exposed (up group, down group) or unexposed (sham-control) to SMF of different orientation; b spleen red blood cells in mice exposed (up group, down group) or unexposed (sham-control) to SMF of a different orientation; c spleen granulocytes in mice exposed (up group, down group) or unexposed (sham-control) to SMF of different orientation; d spleen lymphocytes in mice exposed (up group, down group) or unexposed (sham-control) to SMF of different orientation; **p < 0.01 compared to control; #p < 0.01 compared to down group
Liver phospholipid profile
Liver phospholipid profile in mice exposed to SMF of a different orientation is presented in Table 3. There was no difference in total saturated fatty acids (SFA). However, in mice exposed to downward-oriented SMF, there was a significant increase in content of palmitic acid (16:0) when compared to control mice (24.54 ± 0.19 vs. 22.90 ± 0.70; p < 0.01). There was also an increase of palmitic acid in the upward-oriented group when compared to sham group, but this increase was on the border of significance (24.01 ± 0.13 vs. 22.90 ± 0.70; p = 0.05). Stearic acid (18:0) content showed tendency of decrease in exposed groups, but without statistical significance (p > 0.05). There was no difference in monounsaturated fatty acids (MUFA) in general. However, content of palmitoleic acid (16:1n-7) showed a significant, twofold increase in exposed animals (up and down group) when compared to sham group (0.82 ± 0.06 and 0.84 ± 0.07 vs. 0.40 ± 0.04; p < 0.01). On the other hand, oleic acid (18:1n-9) did not show alterations among the groups (p > 0.05). The amount of vaccenic acid (18:1n-7) significantly increased in the down group, but not in the up group, when compared to sham group (2.49 ± 0.14 vs. 1.97 ± 0.17) and also in the down group when compared to the up group (2.49 ± 0.14 vs. 1.96 ± 0.18). Not only that polyunsaturated fatty acids (PUFA) did not alter, but neither did the sum of omega-3 fatty acids (n-3), the sum of omega-6 fatty acids (n-6), or the ratio of omega-6 to omega-3 (n-6/n-3). However, dihomo-γ-linolenic acid (20:3n-6) was reduced in the exposed groups (up and down) when compared to sham group (1.06 ± 0.08 and 1.12 ± 0.02 vs. 1.40 ± 0.11; p < 0.01). In addition, α-linolenic acid (18:3n-3) was reduced in the down group when compared to both sham group (0.40 ± 0.04 vs. 0.62 ± 0.07; p < 0.05) and the up group (0.40 ± 0.04 vs. 0.67 ± 0.08; p < 0.01). At the same time, α-linolenic acid from the up group did not change when compared to sham group (p > 0.05). Overall, in both up and down groups, there was a statistically significant change in content of palmitoleic (16:1n-7) and dihomo-γ-linolenic acid (20:3n-6) that occurred in the same direction. Namely, both the amount of palmitoleic acid and the dihomo-γ-linolenic acid decreased when compared to sham group. However, in three situations, a change in the amount of fatty acids was observed exclusively in the down group. Namely, palmitic (16:0) and vaccenic (18-1n7) acids increased and α-linolenic acid (18:3n-3) decreased in the down group when compared to sham group.
Desaturase and elongase activities
Desaturase and elongase activities in the liver in mice exposed to SMF of a different orientation are presented in Table 4. Delta 9 (Δ9) desaturase activity did not change in groups of exposed or unexposed animals (p > 0.05). Elongase activity decreased in the liver of exposed animals (up and down groups) when compared to sham group (0.73 ± 0.03 and 0.70 ± 0.01 vs. 0.81 ± 0.03; p < 0.05 and p < 0.01, respectively). Delta 6 (Δ6) desaturase and elongase also showed significant decrease in livers of exposed animals (up and down groups) when compared to control animals (0.07 ± 0.01 and 0.07 ± 0.003 vs. 0.09 ± 0.01; p < 0.05). Delta 5 (Δ5) desaturase showed significant increase in the up group compared to sham group (13.52 ± 0.88 vs. 10.64 ± 1.02; p < 0.05) but did not differ when compared to the down group (13.52 ± 0.88 vs. 12.27 ± 0.32; p > 0.05).
Animal weight
There was no significant difference in animal weight or food intake among the groups at the beginning and at the end of the experiment (data not shown).
Discussion
In this study, we investigated the influence of a homogeneous, differently oriented static magnetic field (SMF) of 128 mT on hematological parameters, tissue characteristics, and lipid content in subacutely exposed mice. Aiming to single out the influence of field orientation, we used downward- as well as upward-oriented vertical homogeneous SMF (Fig. 1). SMF affected various organs and these effects were, to some degree, dependent on the SMF orientation. Since the field gradients were negligible, any possible biological effect of varying field gradients was excluded and all the observed effects were appropriated to the applied field intensity in the chosen direction.
Both upward- and downward-oriented magnetic field caused a decrease in the amount of total WBC and lymphocytes in serum, a decrease of granulocytes in the spleen, kidney inflammation, and an increase in the amount of HDL. In addition, upward-oriented SMF caused brain edema and increased spleen cellularity. In contrast, downward-oriented SMF induced liver inflammation and a decrease in the amount of serum granulocytes. It appears that SMF of 128 mT affected specific organs in the body, rather than simultaneously and equally influencing the entire body system.
We initially found inflammation in the liver after exposure to the down-oriented SMF of 128 mT (Fig. 2; Table 1). These histological findings were confirmed by an increase of serum ALT in the down group only (Table 2). Enzyme ALT is found in the highest concentration in the liver, and it is typically used to detect liver injury. Moreover, we have been the first to discover specific changes in fatty acid profile following SMF exposure. We observed exclusively in the down group an increase in pro-inflammatory palmitic fatty acid (16:0) (Soto-Vaca et al. 2013, Wu et al. 2014) and a decrease in anti-inflammatory α-linolenic fatty acid (18:3 n-3) (Ren et al. 2007, Erdinest et al. 2012) (Table 3). It has been shown that palmitic acid increases the level of pro-inflammatory TNF-alpha and IL-6 (Staiger et al. 2004, Zhou et al. 2013). An increase in palmitic fatty acid that we observed could be a consequence of a reduced activity of the enzyme elongase 18:0/16:0 that converts palmitic fatty acid into stearic fatty acid (18:0) (Table 4). In the case of α-linolenic acid, which is an essential fatty acid, we can speculate that employed SMF affected its metabolism in the liver. SMF of 128 mT may directly affect activity of enzymes by inducing their conformational changes. Alternatively, SMF may act through the rearrangement of membrane phospholipids, leading to a flux of ions (Rosen 1993) that alters enzyme functions. Observing that 128 mT SMF affected differently the two enzymes that contain the same metal, magnesium (enzyme Δ6 desaturase and elongase 20:3n-6/18:2n-6 and enzyme Δ5 desaturase 20:4n-6/20:3n-6), while at the same time, enzyme Δ9 desaturase 18:1n-9/18:0 that contains a different metal, iron, was not affected (Table 4), we conclude that SMF influence was probably at the membrane level, rather than at the level of enzymes’ conformational change. This explanation is based on the important role of the liver in synthesis and degradation of phospholipids that are a major component of cell membranes (Rigotti et al. 1994). Alteration of membrane fatty acids changes membrane fluidity and ion flux and, consequently, the function of various enzymes (McGarry 2002). The ratio of unsaturated to saturated fatty acids influences membrane fluidity in bacteria (Mihoub et al. 2012) and in mammals (Perona et al. 2007). Moderate intensity SMF influences cell membrane ratio of unsaturated versus saturated fatty acids in bacteria, representing an adaptive reaction (Mihoub et al. 2012). However, in our study, although some fatty acids increased and some decreased, the total ratio of MUFA/SFA and PUFA/SFA of liver phospholipids did not change (Table 3), probably because of different intensity and duration of SMF exposure.
Various studies have shown an increase in free radical production and degradation of lipid bilayers in liver cells in SMF exposed animals (Watanabe et al. 1997, Amara et al. 2007, Hashish et al. 2008). This disruption of lipid membranes causes cellular edema and loss of functional integrity, which leads to histological changes and decreased liver function. In our experiments, we observed a tendency of a decrease in direct cholesterol transport via LDL and significant increase in reverse cholesterol transport via HDL (Table 2). An explanation for these findings could be that cholesterol synthesis, uptake, and degradation in liver decreased under SMF influence. As a consequence, there was a tendency of serum total cholesterol increase in exposed animals, mainly due to HDL increase. HDL is a major serum lipoprotein in rodents and its fluctuation affects total serum cholesterol to a significant extent (Lizenko et al. 2008). In previously conducted experiments, increased serum total cholesterol was accompanied by an increase in serum triglycerides (Lahbib et al. 2010), which we did not observe. The increase in serum triglycerides observed in their experiment could be due to different duration of applied SMF.
In the kidney, as in the liver, an inflammatory infiltrate was present in SMF exposed animals, causing nonspecific pyelonephritis (Fig. 2). The extent of this infiltrate was more pronounced in the down group. Other studies showed that besides the liver, SMF of 128 mT can also cause decreased activity of anti-oxidative enzymes in the kidney that leads to increased lipid peroxidation and oxidative stress (Amara et al. 2007, Ghodbane et al. 2011). We reported here morphological changes in the kidney as reflected by infiltration of various inflammatory cells (Fig. 3). Furthermore, we showed that morphological changes, observed in the kidney and liver of exposed animals, were more profound in the down group (Table 1). The specificity of our findings in the liver and kidney was confirmed by histological analysis of spleen, where no inflammation was observed (Fig. 2, Table 1).
In contrast to the liver and kidney, the brain showed edematous changes in upward exposed animals (Table 1, Fig. 2). Various animal studies showed that SMF causes increased blood-brain permeability (Saunders 2005). SMF can also induce changes in Na/K pump redistribution especially in the cytoplasm of affected neurons (Nikolić et al. 2013). Increased permeability of the blood-brain barrier and functional changes in Na/K pump can cause neuron swelling. It was shown that 128 mT SMF induces hypoxia-like status in exposed rats (Elferchichi et al. 2007). When hypoxia develops, one of the first organs that are affected is the brain, with intraneuronal edema that we observed in the up group (Fig. 2).
Spleen cellularity is bound to the amount of RBC and WBC in serum. WBC consists of granulocytes and lymphocytes. Lymphocytes make the majority of WBC in mice (Green 1966). Analysis of the spleen has revealed a significant increase in spleen total cellularity (RBC and WBC together) in the up group (Fig. 5, panel A). This is a consequence of a fact that even a small increase in percentage of lymphocytes could cause a large increase in total spleen cellularity. We also observed a significant decrease in spleen granulocyte count in exposed animals (up and down) (Fig. 5, panel C) with concomitant decrease in serum granulocytes found mainly in the down group (Fig. 4, panel C). Previous research also showed a decrease in splenic granulocytes under the influence of SMF that was explained by increased phagocytosis and oxidative stress followed by death of granulocytes (Elferchichi et al. 2007). In our case, serum WBC and lymphocytes were also decreased in both exposed groups (Fig. 4, panel A and panel D). The total serum WBC reduction was probably a consequence of lymphocyte redistribution among the serum and various tissues. Namely, a decrease in serum lymphocytes in both groups of exposed animals (Fig. 4, panel D) was followed by an increase in lymphocyte content in the liver and kidney (Fig. 2 and Fig. 3).
Red blood cell count did not statistically change in the blood or in the spleen between exposed and unexposed animals. Additionally, there were no changes in hemoglobin content or in MCV, MCH, and MCHC values among groups (Table 2). This is in accordance with findings that rats exposed to a magnetic field of extremely low frequency for 50 and 100 days did not show alteration in RBC count (Cakir et al. 2009). Additionally, a previous study also showed that SMF of moderate intensity do not influence plasma red blood cell count (Elferchichi et al. 2007). We concluded that various intensities of magnetic fields and different exposure times do not influence RBC count in the blood and spleen of experimental animals. This is probably due to diamagnetic property of RBC and fast recovery of RBC after exposure to SMF (Schenck 2005).
Previous research showed that SMF influences the biological system by causing pro-inflammatory changes and an increase in production of reactive oxygen species (Sahebjamei et al. 2007, Hashish et al. 2008, Zhao et al. 2011, Todorović et al. 2015). Namely, these studies stated that SMF increased phagocytosis and death of granulocytes, associated with production of free radicals. Our experiments showed a redistribution of granulocytes and lymphocytes that was dependent upon SMF orientation. In addition, previous studies (Bras et al. 1998, Chionna et al. 2003) demonstrated cytoskeleton reorganization and modulation of orientation of lymphocytes following exposure to moderate static magnetic field. They hypothesized that some sublethal damage persists in the cells, even when the cell morphology still seems to be normal, since it is known that SMF interferes with DNA repair processes (Ghodbane et al. 2013). Our observed inflammatory changes in the liver and kidney of exposed animals may not be connected to the previously observed oxidative stress (Amara et al. 2007). It has been suggested that SMF increases the level of acetylcholine by enhancing its release and by decreasing its degradation (Xu et al. 2011). Increased level of acetylcholine induces blood vessel dilatation and permeability that leads to granulocyte and lymphocyte infiltration of the surrounding tissue. However, another study showed that inhomogeneous SMF could inhibit the release of pro-inflammatory cytokines IL-6, IL-8, and TNF-α from leukocytes (Vergallo et al. 2013). The inhibition of release of pro-inflammatory cytokines from granulocytes and lymphocytes could be the factor explaining the absence of CRP increase in our experiment despite inflammatory changes in the observed tissue (Pepys and Hirschfield 2003). Here, presented results have been obtained in a very controlled environment with the outbred strain, which renders them even more significant.
Conclusion
We investigated the influence of downward- and upward-oriented homogeneous SMF of 128 mT on subacutely exposed mice. Our results suggested that SMF of moderate intensity produced pro-inflammatory effects that depended on its orientation. We discovered that upward-oriented SMF caused changes in the serum, spleen, kidney, and brain, while downward-oriented SMF influenced the serum, kidney, and liver. Observed changes varied from brain edema and alterations in blood total WBC count and spleen granulocyte count to inflammation of the liver and kidney. Mechanisms that led to inflammation of affected organs were direct infiltration of inflammatory cells, whereas, in the case of liver, we also found an increase of pro-inflammatory palmitic fatty acid and a decrease in anti-inflammatory α-linolenic fatty acid. Further studies are needed to fully understand these processes.
References
Abdelmelek H, Molnar S, Servais S, Cottet-Emard JM, Pequignot JM, Favier R, Sakly M (2006) Skeletal muscle HSP72 and norepinephrine response to static magnetic field in rat. J Neural Transm 113:821–827
Amara S, Abdelmelek H, Garrel C, Guiraud P, Douki T, Ravanat J, Favier A, Sakly M, Ben Rhouma K (2007) Zinc supplementation ameliorates static magnetic field-induced oxidative stress in rat tissues. Environ Toxicol Pharmacol 23:193–197
Amara S, Douki T, Garel C, Fevier A, Sakly M, Ben Rhouma K, Abdelmelek H (2009) Effects of static magnetic field exposure on antioxidative enzymes activity and DNA in rat brain. Gen Physiol Biophys 28:260–265
Bancroft JD, Gamble M (2001) Theory and practice of histological techniques. 5th ed. Churchill Livingstone, Edinburgh
Barry SM, Condez A, Johnson MA, Janossy G (2002) Determination of bronchoalveolar lavage leukocyte populations by flow cytometry in patients investigated for respiratory disease. Cytometry 50(6):291–297
Bras W, Diakun GP, DíazJF MG, Kramer H, Bordas J, Medrano FJ (1998) The susceptibility of pure tubulin to high magnetic fields: a magnetic birefringence and X-ray fiber diffraction study. Biophys J 74:1509–1521
Cakir DU, Yokus B, Akdag MZ, Sert C, Mete N (2009) Alterations of hematological variations in rats exposed to extremely low frequency magnetic fields (50 Hz). Arch Med Res 40:352–356
Chater S, Abdelmelek H, Douki T, Garrel C, Favier A, Sakly M, Ben Rhouma K (2006) Exposure to static magnetic field of pregnant rats induces hepatic GSH elevation but not oxidative DNA damage in liver and kidney. Arch Med Res 37:941–946
Chionna A, Dwikat M, Panzarini E, Tenuzzo B, Carlà EC, Verri T, Pagliara P, Abbro L, Dini L (2003) Cell shape and plasma membrane alterations after static magnetic fields exposure. Eur J Histochem 47(4):299–308
Colbert AP, Wahbeh H, Harling N, Connelly E, Schiffke HC, Forsten C, Gregory WL, Markov MS, Souder JJ, Elmer P, King V (2009) Static magnetic field therapy: a critical review of treatment parameters. Evid-Based Complement Alternat Med 6:133–139
Csillag A, Kumar BV, Szabó K, Szilasi M, Papp Z, Szilasi ME, Pázmándi K, Boldogh I, Rajnavölgyi É, Bácsi A, László JF (2014) Exposure to inhomogeneous static magnetic field beneficially affects allergic inflammation in a murine model. J R Soc Interface 11:20140097
Cvetković Z, Vučić V, Cvetković B, Petrović M, Ristić-Medić D, Tepšić J, Glibetić M (2010) Abnormal fatty acid distribution of the serum phospholipids of patients with non-Hodgkin lymphoma. Ann Hematol 89:775–782
Ćirković S, Ristić-Djurović JL, Ilić AŽ, Vujović V, Nešković N (2008) Comparative analysis of methods for isochronous magnetic field calculation. IEEE Trans Nucl Sci 55(6):3531–3538
Ćirković S, Ristić-Djurović JL, Vorozhtsov AS, Ilić AŽ, Nešković N (2009) Method for fine magnet shaping in cyclotrons. IEEE Trans Nucl Sci 56(5):2821–2827
Dini L, Abbro L (2005) Bioeffects of moderate-intensity static magnetic fields. Micron 36:195–217
Djordjevich DM, De Luka SR, Milovanovich ID, Janković S, Stefanović S, Vesković-Moračanin S, Ćirković S, Ilić AŽ, Ristić-Djurović JL, Trbovich AM (2012) Hematological parameters’ changes in mice subchronically exposed to static magnetic fields of different orientations. Ecotoxicol Environ Saf 81:98–105
Elferchichi M, Abdelmelek H, Sakly M (2007) Effects of sub-acute exposure to static magnetic field on iron status and hematopoiesis in rats. Turk J Hematol 24:64–68
Elferchichi M, Mercier J, Coisy-Quivy M, Metz L, Lajoix A, Rene Gross R, Belguith H, Abdelmelek H, Sakly M, Lambert K (2010) Effects of exposure to a 128-mT static magnetic field on glucose and lipid metabolism in serum and skeletal muscle of rats. Arch Med Res 41:309–314
Erdinest N, Shmueli O, Grossman Y, Ovadia H, Solomon A (2012) Anti-inflammatory effects of alpha linolenic acid on human corneal epithelial cells. Invest Ophtalmol Vis Sci 53(8):4396–406
Felgar RE, Macon WR, Kinney MC, Roberts S, Pasha T, Salhany KE (1997) TIA-1 expression in lymphoid neoplasms. Identification of subsets with cytotoxic T lymphocyte or natural killer cell differentiation. Am J Pathol 150:1893–1900
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226(1):497–509
Ghodbane S, Amara S, Garrel C, Arnaud J, Ducros V, Favier A, Sakly M, Abdelmelek H (2011) Selenium supplementation ameliorates static magnetic field-induced disorders in antioxidant status in rat tissues. Environ Toxicol Pharmacol 31:100–106
Ghodbane S, Lahbib A, Sakly M, Abdelmelek H (2013) Bioeffects of static magnetic fields: oxidative stress, genotoxic effects and cancer studies. Biomed Res Int 2013:602987. doi:10.1155/2013/602987
Ghodbane S, Amara S, Lahbib A, Louchami K, Sener A, Sakly M, Abdelmelek H (2014) Vitamin E prevents glucose metabolism alterations induced by static magnetic field in rats. Environ Sci Poll Res 21(22):12731–12738
Green EL (1966) Biology of the laboratory mouse. Dover publications, New York
Hashish AH, El-Missiry MA, Abdelkader HI, Abou-Saleh RH (2008) Assessment of biological changes of continuous whole body exposure to static magnetic field and extremely low frequency electromagnetic fields in mice. Ecotoxicol Environ Saf 71:895–902
Heinrich A, Szostek A, Nees F, Meyer P, Semmler W, Flor H (2011) Effects of static magnetic fields on cognition, vital signs, and sensory perception: a meta analysis. J Magn Reson Imaging 34(4):758–763
Kiss B, László JF, Szalai A, Pórszász R (2015) Analysis of the effect of locally applied inhomogeneous static magnetic field-exposure on mouse ear edema—a double blind study. PLoS ONE 10(2), e0118089
Kremmyda L-S, Tvrzicka E, Stankova B, Zak A (2011) Fatty acids as biocompounds: their role in human metabolism, health and disease—a review. Part 2: fatty acid physiological roles and applications in human health and disease. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 155(3):195–218
Lahbib A, Elferchichi M, Ghodbane S, Belguith H, Chater S, Sakly M, Abdelmelek H (2010) Time-dependent effects of exposure to static magnetic field on glucose and lipid metabolism in rat. Gen Physiol Biophys 29:390–395
László J, Reiczigel J, Székely L, Gasparics A, Bogár I, Bors L, Rácz B, Gyires K (2007) Optimization of static magnetic field parameters improves analgesic effect in mice. Bioelectromagnetics 28:615–627. doi:10.1002/bem.20341
László J, Gyires K (2009) 3 T homogeneous static magnetic field of a clinical MR significantly inhibits pain in mice. Life Sci 84:12–17
László JF, Hernádi L (2012) Whole body static magnetic field exposure increases thermal nociceptive threshold in the snail, Helix pomatia. Acta Biol Hung 63(4):441–452
László JF, Farkas P, Reiczigel J, Vágó P (2012) Effect of local exposure to inhomogeneous static magnetic field on stomatological pain sensation—a double-blind, randomized, placebo-controlled study. Int J Radiat Biol 88(5):430–438
Lizenko MV, Regerand TI, Bakhirev AM, Lizenko EI (2008) Comparative biochemical analysis of lipids of blood serum-lipoproteins of human and some animal species. J Evol Biochem Physiol 44(5):581–590
McGarry JD (2002) Lipid metabolism I. In: Devlin TM (ed) Texbook of biochemistry with clinical correlations, 5th edn. Wiley-Liss, New York, pp 693–726
Mihoub M, El May A, Aloui A, Chatti A, Landoulsi A (2012) Effects of static magnetic fields on growth and membrane lipid composition of Salmonella typhimurium wild-type and dam mutant strains. Int J Food Microbiol 157:259–266
Nešković N, Ristić-Djurović J, Vorojtsov SB, Beličev P, Ivanenko IA, Ćirković S, Vorozhtsov AS, Bojović B, Dobrosavljević A, Vujović V, Čomor JJ, Pajović SB (2003) Status report of the VINCY Cyclotron, Nukleonika vol. 48 suppl. 2 (2003),—Special Issue—Proc. of the XXXIII European Cyclotron Progress Meeting, Warsaw and Krakow, Poland, September, 17–21, 2002, S135-S139
Nikolić L, Bataveljić D, Andjus PR, Nedeljković M, Todorović D, Janać B (2013) Changes in the expression and current of the Na+/K+ pump in the snail nervous system after exposure to a static magnetic field. J Exp Biol 216(18):3531–3541
Oguzhan B, Sancho V, Acitores A, Villanueva-Peñacarrillo M-L, Portois L, Chardigny J-M, Sener A, Carpentier YA, Malaisse WJ (2006) Alteration of adipocyte metabolism in ω3 fatty acid-depleted rats. Horm Metab Res 38(12):789–798
Pepys MB, Hirschfield GM (2003) C-reactive protein: a critical update. J Clin Invest 111:1805–1812
Perona JS, Vögler O, Sánchez-Domínquez JM, Montero E, Escribá PV, Ruiz-Gutierrez V (2007) Consumption of virgin olive oil influences membrane lipid composition and regulates intracellular signaling in elderly adults with type 2 diabetes mellitus. J Gerontol A Biol Sci Med Sci 62(3):256–263
Pinkus GS, Said JW (1988) Hodgkin’s disease, lymphocyte predominance type, nodular-further evidence for a B cell derivation. L & H variants of Reed-Sternberg cells express L26, a pan B cell marker. Am J Pathol 133:211–217
Poniedzialek B, Rzymski P, Karczewski J, Jaroszyk F, Wiktorowicz K (2013) Reactive oxygen species (ROS) production in human peripheral blood neutrophils exposed in vitro to static magnetic field. Electromagn Biol Med 32(4):560–568
Ren J, Han EJ, Chung SH (2007) In vivo and in vitro anti-inflammatory activities of alpha-linolenic acid isolated from Actinidia polygama fruits. Arch Pharm Res 30(6):708–714
Rigotti A, Marzolo MP, Nervi F (1994) Lipid transport from the hepatocyte into the bile. Curr Top Memb 40:579–615
Rosen AD (1993) A proposed mechanism for the action of strong static magnetic fields on biomembranes. Int J Neurosci 73:115–119
Rosen AD (2003) Mechanism of action of moderate-intensity static magnetic fields on biological systems. Cell Biochem Biophys 39(2):163–173
Sahebjamei H, Abdolmaleki P, Ghanati F (2007) Effects of magnetic field on the antioxidant enzyme activities of suspension-cultured tobacco cells. Bioelectromagnetics 28:42–47
Saunders R (2005) Static magnetic fields: animal studies. Prog Biophys Mol Biol 87:225–239
Schenck JF (2005) Physical interactions of static magnetic fields with living tissues. Prog Biophys Mol Biol 87:185–204
Soto-Vaca A, Losso JN, McDonough K, Finley JW (2013) Differential effect of 14 free fatty acids in the expression of inflammation markers on human arterial coronary cells. J Agric Food Chem 61(42):10074–10079
Staiger H, Staiger K, Stefan N, Wahl HG, Machicao F, Kellerer M, Häring HU (2004) Palmitate-induced interleukin-6 expression in human coronary artery endothelial cells. Diabetes 53:3209–3216
Tepšić V, Pavlović M, Ristić-Medić D, Ristić V, Lekić N, Tepšić J, Debeljak-Martačić J, Milićević M, Glibetić M (2008) Influence of dietary fats on serum phospholipid fatty acid composition and its relation to obesity in animals. Acta Vet 58:33–41
Todorović D, Perić-Mataruga V, Mirčić D, Ristić-Djurović J, Prolić Z, Petković B, Savić T (2015) Estimation of changes in fitness components and antioxidant defense of Drosophila subobscura (Insecta, Diptera) after exposure to 2.4 T strong magnetic field. Environ Sci Pollut Res 22:5305–5314. doi:10.1007/s11356-014-3910-8
Vergallo C, Dini L, Szamosvölgyi Z, Tenuzzo BA, Carata E, Panzarini E, László JF (2013) In vitro analysis of the anti-inflammatory effect of inhomogeneous static magnetic field-exposure on human macrophages and lymphocytes. PLoS ONE 8(8), e72374
Watanabe Y, Nakagawa M, Miyakoshi Y (1997) Enhancement of lipid peroxidation in the liver of mice exposed to magnetic field. Industrial Health 35:285–230
Wu D, Liu J, Pang X, Wang S, Zhao J, Zhang X, Feng L (2014) Palmitic acid exerts pro-inflammatory effects on vascular smooth muscle cells by inducing the expression of C-reactive protein, inducible nitric oxide synthase and tumor necrosis factor-α. Int J Mol Med 34(6):1706–12
Xu S, Okano H, Tomita N, Ikada Y (2011) Recovery effects of a 180 mT static magnetic field on bone mineral density of osteoporotic lumbar vertebrae in ovariectomized rats. eCAM 2011:ID620984:1–8
Zhao G, Chen S, Wang L, Zhao Y, Wang J, Wang X, Zhang WW, Wu R, Wu L, Wu Y, Xu A (2011) Cellular ATP content was decreased by a homogeneous 8.5 T static magnetic field exposure: role of reactive oxygen species. Bioelectromagnetics 32:94–101
Zhou BR, Zhang JA, Zhang Q, Permatasari F, Xu Y, Wu D, Yin ZQ, Luo D (2013) Palmitic acid induces production of proinflammatory cytokines interleukin-6, interleukin-1β, and tumor necrosis factor-α via a Nf-κB-dependent mechanism in HaCaT keratinocytes. Mediators Inflamm 2013:530429. doi:10.1155/2013/530429
Acknowledgment
This study was supported by the grant numbers III-41013, III-41019, III41030, III-45003, and III-45006 from the Ministry of Education, Science and Technological Development, Government of Serbia. The experiment was performed at the Vinča Institute of Nuclear Sciences, Laboratory of Physics (010) to whose staff we thank for enabling access to the Cyclotron, as well as for their assistance and support.
Conflict of interest
The authors declare that they have no competing interests.
Role of funding source
No funding body had any involvement in the preparation or content of this article or in the decision to submit it for publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Ivan D. Milovanovich and Saša Ćirković contributed equally to this work.
Rights and permissions
About this article
Cite this article
Milovanovich, I.D., Ćirković, S., De Luka, S.R. et al. Homogeneous static magnetic field of different orientation induces biological changes in subacutely exposed mice. Environ Sci Pollut Res 23, 1584–1597 (2016). https://doi.org/10.1007/s11356-015-5109-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5109-z