Abstract
In low-income and middle-income countries such as Iran, smoking is becoming increasingly popular, especially among young people. This has led to additional exposure to a variety of substances, including metals which may exert a toxic influence and lead to severe diseases. In order to evaluate the influence of smoking on metal concentrations, a case-control study of levels of metal in urine was carried out in smokers (n = 64) and non-smokers (n = 35) from the city of Birjand (Iran). They were divided according to their age and socioeconomic status. Concentrations of cadmium (Cd), cobalt (Co), chromium (Cr), copper (Cu), iron (Fe), nickel (Ni), lead (Pb), and zinc (Zn) were measured using ET-AAS. We found higher concentrations of Cd (0.03 vs. 0.12 μg/L), Co (0.6 vs. 1.22 μg/L), and Cr (14.00 vs. 18.17 μg/L) in the urine of smokers. Age and occupation are factors that also influence the levels of metals. Young smokers demonstrate higher Cd and Pb levels than other age groups. It would also appear that public sector workers and self-employed are the sectors most susceptible to high levels of metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to the World Health Organization, 40% of men and 9% of women admit to smoking, which gives in a total of 2.7 billion people worldwide [1]. By the year 2030, another billion will take up smoking by the age of 18 [2]. Smoking was responsible for 6 million deaths worldwide in 2015, and that number is set to rise to 8.3 million in 2030 [3]. A general trend that tobacco-attributable deaths tend to decline in high-income countries and to double in low-income and middle-income countries has been observed [4, 5].
Tobacco contains metals that were absorbed from soil by the plants during their growth. This transfer is influenced by a number of factors such as soil type, climate, and plant species [6,7,8]. Some metals that enter the body via smoking may, in conjunction with occupational exposure, increased air, food, and water pollution, lead to an increase in the development of a disease, such as cardiovascular disease, lung cancer, and COPD in humans [9,10,11,12]. The risk increases with age and poor occupational status [13].
Several studies have been conducted on the levels of essential and non-essential metals in cigarettes from Iran, but the levels in the blood, hair, and urine samples from smokers have barely been considered [14, 15]. This is a significant gap in the knowledge, since urine samples have already been identified as the best matrix (and the easiest to collect) for daily monitoring of the excretion of metals. They may thus reflect exposure [16,17,18,19]. There is a serious need to present data for human biomonitoring of that region and so to estimate the probability of the development of severe diseases according to the concentrations of metals, occupational exposure, and age.
The purpose of this paper is therefore to determine the concentrations of metals (Cd, Co, Cr, Cu, Fe, Ni, Pb, and Zn) in the urine of people from Iran. The comparison of the levels of metals was made with respect to smoking status, age group, and the occupation of subjects. The relationships between the levels of metals in urine were also studied.
Materials and Methods
Sample Collection
In this case-control study, 99 first morning urine samples were collected (between 6 a.m. and 7 a.m.) from male smokers and male non-smokers between March and July 2018 at Birjand Health Center (Birjand University of Medical Sciences). All subjects were selected via the snowball sampling method and gave informed consent to take part in that study. The smokers consisted of subjects who smoked > 10 cigarettes per day and had done so for at least 5 years. The control (non-smoking) group included people who had no history of smoking and had not been exposed to second-hand smoke. All subjects filled in a questionnaire in order to collect demographic information, e.g., age (all subjects were divided into three age groups: young (under 35), middle-aged (between 35 and 50), and old (over 50)) and occupational status (public sector worker, laborer, self-employed, home-maker, and retirees) (Table 1).
Sample Preparation and Measurements
After collection, all the urine samples (10 mL each) were stored at − 20 °C until analysis. For the mineralization, the urine samples were mixed with 2 mL of nitric acid (HNO3, 65%, Merck, Germany) and 1 mL of perchloric acid (HClO4, 70%, Merck, Germany) and mineralized on a water bath (TW12, Julabo GmbH, Germany) at 100 °C. Mineralized solutions were diluted with ultrapure water (18.2 MΩ-cm at 25 °C, Fistreem, WSC044, UK) up to 25 mL. The levels of Cd, Co, Cr, Cu, Fe, Ni, Pb, and Zn were quantified with an electrothermal atomic absorption spectrometer (Analytik Jena AG, ContrAA 700). The limits of detection were expressed for raw urine samples. The validity of the whole procedure was checked against the certified reference material (Table 2). Spikes and control samples were run every 10 analyses and were adjusted to fit the mid-point of the working range of the methods.
Statistical Analysis
The general characteristics of participants in that study were expressed as a median with lower (Q1) and upper (Q3) quartiles and an interquartile range (IQR). Since the data was not always normally distributed, the Mann-Whitney and Kruskal-Wallis tests followed by Duncan’s post hoc were used to compare average metal concentrations in respect to smoking status, age, and occupation. Spearman’s correlation coefficient analysis and Ward’s method-based (and Euclidean distances) cluster analysis (CA) were also carried out to evaluate the relationships between metals in the urine. From all the correlations tested, only the strong (higher than 0.6) and significant are presented in the paper. All the analyses were performed with the SPSS 20 software (Chicago, IL, USA) with the significance level applied at 0.05.
Results
Metal Concentrations
The general scheme of the increase of metal concentrations in smokers was Cd < Co < Pb < Ni < Zn < Cr < Cu < Fe. In non-smokers, it was the same except for Cr and Zn, which switched their positions (Table 3). Smokers had significantly higher concentrations of Cd (0.12 vs. 0.03 μg/L), Co (1.22 vs. 0.6 μg/L), and Cr (18.17 vs. 14.00 μg/L) in the urine, while at the same time, they showed significantly lower levels of Cu (20.98 vs. 25.51 μg/L), Fe (32.56 vs. 61.48 μg/L), and Zn (17.96 vs. 24.25 μg/L) than non-smokers.
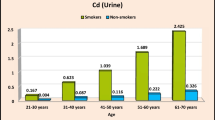
The age group affected only Zn concentrations among the smokers (p = 0.016; lowest among the middle-aged (15.9 μg/L) and highest among the old (21.8 μg/L)) and Cu concentrations among the non-smokers (p = 0.023; lowest among the young (19.6 μg/L) and highest among the old (29.9 μg/L); Table 4).
Among smokers, significant differences were found in the case of Cd (p = 0.003), Ni (p = 0.001), and Pb (p < 0.001) levels according to their occupation. In this respect, public sector workers had the highest Cd and Pb levels in their urine, while the self-employed presented the highest Ni levels (Table 5). Among the non-smokers, significant differences were found according to their occupation only in the case of Ni level (p < 0.001), which again was highest in the self-employed (22.5 μg/L).
Correlation between Metals in the Urine Samples from Smokers and Non-smokers
Among the smokers, we observed relationships between Fe and Zn (R = 0.73, p < 0.001), Fe and Cu (R = 0.63, p < 0.001), and Zn and Cu (R = 0.65, p < 0.001). Among the non-smokers, a clear, significant correlation was observed between Cr and Ni (R = 0.72, p < 0.001) in the urine.
In accordance with CA and the Sneath criterion, the metals were framed into different clusters in the groups studied (Fig. 1). In non-smokers, there were three clusters: Cr-Ni-Co-Cd-Pb, Cu-Zn, and Fe, while in smokers, there were four clusters: Cr-Ni-Cu-Zn, Pb, Cd-Co, and Fe. In both groups, only one cluster, Fe, was framed in the same way.
Cluster analysis of the levels of metals in urine among smokers and non-smokers. Boxes around elements indicate clusters framed according to the Sneath criterion at 33%. Vertical dashes present the Sneath criterion at 33% and 66%. Significant correlations according to Spearman’s analysis are presented in the right corners
Discussion
We found that Cd, Co, and Cr concentrations in the urine were higher in smokers and Cu, Fe, and Zn concentrations were higher in non-smokers. We also observed that smokers in the public sector had significantly elevated levels of Cd and Pb in the urine when compared with other occupations among both the smokers and the non-smokers. The age factor was a significant influence only regarding concentrations of Zn and Cu in smokers and non-smokers.
The Levels of Metals in the Urine of Smokers and Non-smokers
Smoking is regarded as the main source of Cd in humans and a major source of Pb exposure for humans, which explains increased Cd and Pb levels in the smokers we studied. While the increase was statistically insignificant for Pb, probably due to high variance, the increasing tendency was clear to see [8, 18, 20]. The levels of Cr we measured in both smokers and non-smokers were in turn several times higher than those observed by other scientists [21]. This suggests our subjects’ constant exposure to some Cr source, possibly water. High levels of toxic metals lead to metal-induced oxidative stress and to a subsequent decrease in the levels of trace elements, also noted in smokers [22]. This is crucial, since an increased Fe level improves the rate of proliferation of lymphocytes and determines the body’s sensitivity to immunotoxicity from xenobiotic metals [23]. Smoking is also related to an increase in the concentrations of Cu and the intensification of lipid peroxidation [24]. However, we noted a higher level of Cu in non-smokers, suggesting other sources of Cu exposure in that group.
In our study, the concentrations of Ni and Pb among both smokers and non-smokers were the only ones we found to exceed the reference values (4.4 μg/L and 1.9 μg/L, respectively) for these metals’ levels in urine [25]. Even levels we measured in non-smokers were higher than observed by other scientists [26, 27]. We did find no reference values, however, for levels of Co, Cr, and Fe in the urine.
Relationship between Urine Metals Levels and Age
Available data indicated that homeostasis of Zn is relatively stable throughout life until the age of 65, when the aging process leads to the nutritional deficiency of micronutrients [28, 29]. Our data are consistent with that and additionally indicate that smoking habits have a greater influence than age on levels of Zn, since only among smokers we did observe statistically significant differences in levels of Zn. An inverse relationship between Zn and smoking-derived Cd also supports the above-mentioned dependence [30]. Statistically significant differences in Cu levels were observed in non-smokers of different ages and are consistent with studies on human nutrition and metabolism [31]. Generally, the absorption of Cu ions occurs mainly in the small intestine, thus lowering the metabolic rate as observed in older people and may reduce absorption intensity and simultaneously increase Cu excretion in urine [31]. We may also suspect that older people consume more vitamin C, which inhibits the absorption of Cu [32]. We also observed that levels of Pb tend to be highest among young smokers. This is consistent with the US study, which determined that youth, low BMI, and low PIR (poverty to income ratio) lead to an increase in levels of Pb [33].
Relationship Between Levels of Metal in Urine and Occupation
Our analysis of smokers with regard to their professions indicated public sector workers were the most vulnerable to high levels of Cd and Pb. Smoking is implicated in inducing significantly high levels of Cd [34]. In addition to smoking, traffic fumes and the incineration of urban waste were, however, mentioned as sources of Cd; thus, those we observed may also have come from different places [35]. That group also presented significantly higher levels of Pb, which may be due to smoking, but may also be linked to a different diet and water sources than in those with other kinds of jobs [36, 37]. The self-employed also appear to be susceptible to toxic metals, since that group demonstrated significantly higher levels of Ni in urine among both smokers and non-smokers. This suggests sources of exposure to Ni other than smoking and may include diet (e.g., a high consumption of nickel-rich products such as spinach, lettuce, or cocoa powder) and frequent use of stainless-steel kitchen utensils or cheap jewelry [38].
Correlation Between Levels of Metals in Urine Samples from Smokers and Non-smokers
Fe and Zn are found in similar food sources and thus have the potential to be positively correlated [39]. We observed this relation only in smokers and this may be related to a different diet in the groups studied, as some dietary compounds reduced absorption [40]. An Fe-deficient diet generates Cu deficiency, which may explain the clear correlation between those metals we noted [41]. In our study, Cu was found to be correlated with Zn and this suggests common sources of exposure to them [42]. Ni tends to correlate with Cr in smokers, which is pivotal since their accumulation was observed in lung cancer tumors suggesting that both metals may play a role in the development of lung cancer [6, 43]. We found that correlation to be stronger in non-smokers and that may be related to second-hand smoke [44].
CA analysis revealed relationships between essential metals as well as between essential and xenobiotic metals. The first of these relationships indicates the participation of essential metals in maintaining homeostasis, while the second suggests antagonism between the above-mentioned groups of metals [45, 46]. This is crucial, since it indicates that regardless of smoking habits, the metabolism is still working to reduce the adverse health effects of toxic metals [47]. Clusters of metals may suggest their similar metabolic pathways [48] or their mutual origin, e.g., environmental pollution or occupational exposure [49]. For both issues, age and diet, however, may have an impact on the relationships [50].
Our study is one of only a few carried out in Eastern Iran to assess the levels of metals in human samples and at the same time to take into consideration several exposure factors. We managed to match the study and the control groups with the same basic characteristic to facilitate unbiased comparisons. As a limitation, however, we need to point out the study population, while sufficient to discuss Birjand’s population, should be increased if the results are to be applied to the general population of Iran [51]. The specific approach of this study is to use urine as a biomonitoring tool. This matrix through non-invasive, easy, and abundant collection has its advantages over popular in vivo human studies (blood, hair, and nails which are harder to collect as well as the fact that samples are smaller and often contaminated), but it should be used in inferences on short-term exposure, since it is associated with a rapid metabolism and element and compound turnover [52, 53]. This limitation may be minimized by using timed urine samples, which allow us to observe changes in metabolites concentrations in each sample collected in the following 24, 48, or 72 h, or by combining urine studies with blood content analysis, for instance.
Conclusions
Smoking leads to an increase in levels of Cd, Co, and Cr in smokers’ urine samples compared with the samples supplied by non-smokers. Age and occupation were also shown to influence the levels of metals in the urine. Age, however, affects the concentrations of Cd, Ni, and Pb, while profession only affects the concentrations of Cu and Zn. A growing body of scientific evidence shows that smoking is connected with the development of cancer, even if subjects are passive smokers. It is thus necessary to monitor the levels of metals in smokers and non-smokers and to identify the relationships between smoking habits and carcinogenesis, especially in those countries in which smoking is still in fashion and popular.
References
WHO (2010) The facts on gender and tobacco. http://www.who.int/gender/documents/10facts_gender_tobacco_en.pdf. Accessed 3 Dec 2018
GYTSC (The Global Youth Tobacco Survey Collaborative Group) (2002) Tobacco use among youth: a cross country comparison. Tob Control 11:252–270
WHO (2015) WHO global report on trends and prevalence of tobacco smoking 2015
https://apps.who.int/iris/bitstream/handle/10665/156262/9789241564922_eng.pdf;jsessionid=9A9133D12FE0419B1BEF7585C31A1C1C?sequence=1. Accessed 27 Mar 2019
Mathers CD, Loncar D (2006) Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 3(11):2011–2030
WHO (2017) WHO report on the global tobacco epidemic? http://www.who.int/tobacco/global_report/2017/en. Accessed 3 Dec 2018
Caruso RV, O’Connor RJ, Stephens WE, Cummings KM, Fong GT (2014) Toxic metal concentrations in cigarettes obtained from U.S. smokers in 2009: results from the International Tobacco Control (ITC) United States survey cohort. Int J Environ Res Public Health 11Ł:202–217
Saha N, Rahman MS, Jolly YN, Rahman A, Sattar MA, Hai MA (2016) Spatial distribution and contamination assessment of six heavy metals in soils and their transfer into mature tobacco plants in Kushtia District, Bangladesh. Environ Sci Pollut Res 23:3414–3426
Pinto E, Cruz M, Ramos P, Santos A, Almeida A (2017) Metals transfer from tobacco to cigarette smoke: evidences in smokers’ lung tissue. J Hazard Mater 325:31–35
Burke GM, Genuardi M, Shappell H, D'Agostino RB, Magnani JW (2017) Temporal associations between smoking and cardiovascular disease, 1971 to 2006 (from the Framingham Heart Study). Am J Cardiol 120(10):1787–1791
Bhatt SP, Kim Y, Harrington KF, Hokanson JE, Lutz SM, Cho MH, Bailey WC (2018) Smoking duration alone provides stronger risk estimates of chronic obstructive pulmonary disease than pack-years. Thorax 73(5):414–421
Japuntich SJ, Kumar P, Pendergast JF, Juarez-Caballero GY, Malin JL, Wallace R, Chrischilles E, Keating NL, Park EL (2014) Smoking status and survival among a national cohort of lung and colorectal cancer patients. Nicotine Tob Res 21(4):497–504
Omrane F, Gargouri I, Khadhraoui M, Elleuch B, Zmirou-Navier D (2018) Risk assessment of occupational exposure to heavy metals mixtures: a study protocol. BMC Public Health 18:314–324
Forum of International Respiratory Societies (2017) The global impact of respiratory disease – second edition. Sheffield, European Respiratory Society
Abdolahnejad A, Ebrahimi A, Jafari N, Vahid Dastjerdi M, Nourmoradi H (2013) Determining the heavy metals contents in some highly-used samples of cigarettes and aromatic tobaccos in Iranian market. J Toloo-E-Behdasht 12(3):116–127 (in Persian)
Yousefinejad V, Mansouri B, Ramezani Z, Mohammadzadeh N, Akhlaghi M (2017) Evaluation of heavy metals in tobacco and hookah water used in coffee houses in Sanandaj city in 2017. Sci J Kurdistan Univ Med Sci 22:96–106
Gil F, Hernández AF, Márquez C, Femia P, Olmedo P, López-Guarnido O, Pla A (2011) Biomonitorization of cadmium, chromium, manganese, nickel and lead in whole blood, urine, axillary hair and saliva in an occupationally exposed population. Sci Total Environ 409:1172–1180
Zhang X, Cui X, Lin C, Ma J, Liu X, Zhu Y (2016) Reference levels and relationships of nine elements in first-spot morning urine and 24-h urine from 210 Chinese children. Int J Hyg Environ Health 220:227–234
Wongsasuluk P, Chotpantarat S, Siriwong W, Robson M (2018) Using urine as a biomarker in human exposure risk associated with arsenic and other heavy metals contaminating drinking groundwater in intensively agricultural areas of Thailand. Environ Geochem Health 40:323–348
Dos Santosa M, Soaresa MCF, Baischa PRM, Baischa ALM, Silva RMR (2018) Biomonitoring of trace elements in urine samples of children from a coal-mining region. Chemosphere 197:622–626
Richter PA, Bishop EE, Wang J, Swahn MH (2009) Tobacco smoke exposure and levels of urinary metals in U.S. youth and adult population: the national health and nutrition examination survey (NHANES) 1999-2004. Int J Environ Res Public Health 6:1930–1946
Interdonato M, Bitto A, Pizzino G, Irrera N, Pallio G, Mecchio A, Cuspilici A, Minutoli L, Altavilla D, Squadrito F (2014) Levels of heavy metals in adolescent living in the industrialized area of Milazzo-Valle del Mela (northern Sicily). J Environ Public Health 2:326845
Viroonudomphol D, Suwanton L, Pinyosirikul U, Satsue S, Harnroongroj T (2016) Effect of active and passive smoking on heavy metals toxic and antioxidant trace elements. J Biomed Bioeng 5:58–62
Jung D, Bolm-Audorff U, Faldum A, Hengstler JG, Ismail Attia D, Janssen K, Reifenrath M, Bienfait HG, Mayer-Popken O, Konietzko J (2003) Immunotoxicity of co-exposures to heavy metals: in vitro studies and results from occupational exposure to cadmium, cobalt and lead. EXCLI J 2:31–44
Bernhard D, Rossmann A, Wick G (2005) Metals in cigarette smoke. Life 57(12):805–809
Saravanabhavan G, Werry K, Walker M, Haines D, Malowany M, Khoury C (2017) Human biomonitoring reference values for metals and trace elements in blood and urine derived from Canadian Health Measures Survey 2007-2013. Int J Hyg Environ Health 220:189–200
Hoet P, Jacquerye C, Deumer G, Lison D, Haufroid V (2013) Reference values and upper reference limits for 26 trace elements in urine of adults living in Belgium. Clin Chem Lab Med 51:839–849
Wilhelm M, Ewers U, Schulz C (2004) Revised and new reference values for some trace elements in blood and urine for human biomonitoring in environmental medicine. Int J Hyg Environ Health 207(1):69–73
Goodnough LT, Schrier SL (2014) Evaluation and management of anemia in the elderly. Am J Hematol 89(1):88–96
Cabrera AJR (2015) Zinc, aging, and immunosenescence: an overview. Pathobiol Aging Age Relat Dis 5:25592–25601
Collins JF, Klevay LM (2011) Copper. Adv Nutr 2(6):520–522
Fu S, Jiang W, Zheng W (2015) Age-dependent increase of brain copper levels and expressions of copper regulatory proteins in the subventricular zone and choroid plexus. Front Mol Neurosci 8:1–22
Jasińska-Starczewska M, Szydłowska I, Mroczek B, Laszczyńska M, Chlubek D, Kemicer-Chmielewska E, Chełstowski K, Karakiewicz B, Ciećwież S, Starczewski A (2017) The influence of cigarette smoke exposure on the copper concentration in the serum depending on the use of menopausal hormone therapy. Biomed Res Int 2017:5732380
Apostolou A, Garcia-Esquinas E, Fadrowski JJ, McLain P, Weaver VM, Navas-Acien A (2012) Secondhand tobacco smoke: a source of lead exposure in US children and adolescents. Am J Public Health 102(4):714–722
Zhou Z, Lu Y, Pi H, Gao P, Li M, Zhang L, Pei L, Mei X, Liu L, Zhao Q, Chen Y, Jiang Y, Zhang Z, Yu Z (2016) Cadmium exposure is associated with the prevalence of dyslipidemia. Cell Physiol Biochem 40:633–643
Valerio F, Pala M, Piccardo MT, Lazzarotto A, Balducci D, Brescianini C (1995) Exposure to airborne cadmium in some Italian urban areas. Sci Total Environ 172(1):57–63
Zhang R, Wilson V, Hou A, Meng G (2015) Source of lead pollution, its influence on public health and the countermeasures. Int J Health Anim Sci Food Saf 2(1):18–31
Ancona C, Bauleo L, Biscotti G, Bocca B, Caimi S, Cruciani F, Di Lorenzo S, Petrolati M, Pino A, Piras G, Pizzabiocca A, Rabbiosi S, Ruggieri F, Salatino C, Alimonti A, Forastiere F (2016) A survey of a lifestyle and level of biomarkers of environmental exposure in residents of Civitavecchia (Italy). Ann Ist Super Sanita 52(4):488–494
Das KK, Chandramouli Reddy R, Bagoji IB, Das S, Bagali S, Mullur L, Khodnapur JP, Biradar MS (2018) Primary concept of nickel toxicity – an overview. J Basic Clin Physiol Pharmacol 30(2):141–152
Cole CR, Grant FK, Swaby-Ellis ED, Smith JL, Jacques A, Northrop-Clewes CA, Caldwell KL, Pfeiffer CM, Ziegler TR (2010) Zinc and iron deficiency and their interrelations in low-income African American and Hispanic children in Atlanta. Am J Clin Nutr 91:1027–1034
Lim K, Booth A, Szymlek-Gay EA, Gibson RS, Bailey KB, Irving D, Nowson C, Riddell L (2015) Associations between dietary iron and zinc intakes, and between biochemical iron and zinc status in women. Nutrients 7:2983–2999
Arredondo M, Núnez MT (2005) Iron and copper metabolism. Mol Asp Med 26:313–327
Bárány E, Bergdahl IA, Bratteby LE, Lundh T, Samuelson G, Schütz A, Skerfving S, Oskrasson A (2002) Relationships between trace element concentrations in human blood and serum. Toxicol Lett 134:177–184
Kuo CY, Wong RH, Lin JY, Lai JC, Lee H (2006) Accumulation of chromium and nickel metals in lung tumors from lung cancer patients in Taiwan. J Toxicol Environ Health A 69:1337–1344
Li L, Guo L, Chen X, Xiang M, Yang F, Ren JC, Zhang GH (2018) Secondhand smoke is associated with heavy metal concentrations in children. Eur J Pediatr 177(2):257–264
Goyer R, Golub M, Choudhury H, Hughes M, Kenyon E, Stifelman M (2004) Issue paper on the human health effects of metals. U.S. Environmental Protection Agency – Risk Assessment Forum. 19th August 2004, Washington DC. USA
Mehra R, Thakur AS (2012) Relationship between lead, cadmium, zinc, manganese, and iron in hair of environmentally exposed subjects. Arab J Chem 9:1214–1217
Goyer R, Golub M, Chounhury H, Hughes M, Kenyon E, Stifelman M (2004) Issue paper on the human health effects of metals. 19th August 2004, Risk Assessment Forum, U.S. EPA, Washington DC, USA
Wang Y, Ou YL, Liu YQ, Xie Q, Liu QF, Wu Q, Fan TQ, Yan LL, Wang JY (2012) Correlations of trace element levels in the diet, blood, urine, and faces in the Chinese male. Biol Trace Elem Res 145:127–135
Richter P, Faroon O, Pappas RS (2017) Cadmium and cadmium/zinc ratios and tobacco-related morbidities. Int J Environ Res Public Health 14(10):1154–1173
Becker K, Seiwert M, Casteleyn L, Joas R, Joas A, Biot P, Aerts D, Castaño A, Esteban M, Angerer J, Koch HM, Schoeters G, Den Hond E, Sepai O, Exley K, Knudsen LE, Horvat M, Bloemen L, Kolossa-Gehring M, DEMOCOPHES consortium (2014) A systematic approach for designing a HBM pilot study for Europe. Int J Hyg Environ Health 217:312–322
Esteban M, Castaño A (2009) Non-invasive matrices in human biomonitoring: a review. Environ Int 35:438–449
Calafat AM (2016) Contemporary issues in exposure assessment using biomonitoring. Curr Epidemiol Rep 3(2):145–153
Acknowledgments
The authors would like to appreciate S. Nakhaee and H. Ataei, who provided logistic for chemicals and samples.
Funding
The research was supported by the Elite Researcher Grant Committee of the Iranian Ministry of Health via the Omid Mehrpour award (Ethics code: IR.NIMAD.REC.1396.115) from the National Institutes for Medical Research Development (NIMAD), Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mansouri, B., Błaszczyk, M., Binkowski, L.J. et al. Urinary Metal Levels with Relation to Age, Occupation, and Smoking Habits of Male Inhabitants of Eastern Iran. Biol Trace Elem Res 195, 63–70 (2020). https://doi.org/10.1007/s12011-019-01848-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01848-7