Abstract
This study aimed to investigate the role of purple tomato anthocyanin (PTA) in autophagy induced by chromium(VI) in a chicken hepatocellular carcinoma cell line (LMH cells). LMH cells were exposed to Cr(VI), PTA, and Cr(VI) + PTA. The changes in endoplasmic reticulum (ER) stress, autophagy, related proteins, and COX-2 were detected. Results showed that the cell viability was reduced after Cr(VI) treatment, and the decrease was also restrained by 3-MA or PTA. Levels of ER stress-related proteins (GRP78/Bip and PERK) and COX-2 increased after Cr(VI) treatment, which resulted in an increase in autophagy-related proteins (Beclin1 and LC3-II), inhibition of autophagy pathway protein mTOR, and degradation of autophagy-related protein p62, leading to excessive autophagy and cell damage. Meanwhile, the changes of these indicators induced by Cr(VI) were alleviated by PTA. In conclusion, our study suggested that Cr(VI) can induce excessive autophagy in LMH cells, while PTA can ameliorate Cr(VI)-induced autophagy by inhibiting ER stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chromium compounds are abundant in the earth’s crust and extensively used by humans in stainless steel manufacturing, leather tanning, and wood treatment. Cr predominantly occurs in the environment in two valence states, namely, Cr(VI) and Cr(III) [1]. Cr(VI) is detrimental to human health, biological resources, and ecosystems because of its carcinogenic, corrosive, and irritating effects. It is also more toxic and soluble than Cr(III). Excessive chromium can induce hepatotoxicity in various animal models [2, 3]. The target organs of chromium in the body mainly include liver, kidney, lung, brain, and intestine, and the liver is among the main targets of its accumulation [4, 5]. Cr(VI) can induce apoptosis and autophagy in L-02 hepatocytes, while Cr(VI)-induced autophagy protects L-02 hepatocytes from apoptosis via the ROS–AKT–mTOR pathway [6, 7]. Our previous study also showed that Cr(VI) can trigger autophagy in DF-1 cells [8]. The liver is the main detoxification organ for metal toxicity of machine weight and one of the main target organs of Cr(VI) toxicity. Therefore, this study investigated the damage of Cr(VI) on chicken hepatocytes, and used LMH cells as the test model. LMH cells are chemically mutagenized in vitro cultured chicken hepatocytes, enabling them to undergo unlimited passage proliferation, and retain many of the differentiated phenotypic characteristics of chicken hepatocytes, which have the characteristics of chicken liver epithelial cells [9] and therefore can be used as a cell model for chicken hepatocyte research. Gabis et al. reported the first measurable heat shock response of heme oxygenase-1 in CELC or LMH cells and show that LMH cells are a useful model for the study of heme oxygenase-1 regulation [10]. As such, our experiment determined the Cr(VI) concentration and treatment duration to verify the effect of Cr(VI) on autophagy in LMH cells.
Anthocyanin is one of the most extensive families of natural pigments in the plant kingdom and natural water-soluble pigments of flavonoids [11]. They can reduce the production of reactive oxygen species, thereby protecting cells from damage [12,13,14]. Purple tomato anthocyanin (PTA) used in this experiment was extracted from purple tomato fruits with anthocyanidins. Anthocyanin plays dual functions in autophagy [15, 16], but the regulatory effect of anthocyanin on autophagy caused by heavy metals is poorly studied. Xie et al. [17] reported the protective effects of anthocyanin against apoptosis and oxidative stress induced by arsanilic acid in DF-1 cells. And Zhang et al. reported that anthocyanin from Chinese bayberry extract negatively regulates oxidative stress-induced autophagy [18]. The concentration of PTA was determined based on these literatures, and the cell activity was tested to verify the correctness of the selection, and the regulatory effect of anthocyanin on autophagy caused by Cr(VI) was explored.
Autophagy is divided into three types in eukaryotic cells, namely, macroautophagy (hereafter referred to as autophagy), microautophagy, and chaperone-mediated autophagy [19]. Autophagy is a highly conserved large-scale protein degradation pathway in eukaryotes; in this process, unfolded proteins and damaged organelles in the cytoplasmic part are engulfed in double-membrane vesicles called autophagosomes, mature autophagosomes fuse with lysosomes to form autolysosomes, various lysosomal hydrolases degrade chelates, and amino acids become recycled for macromolecular synthesis and energy production [6, 20]. Under physiological conditions, autophagy is maintained at a relatively low level through the renewal of energy and cell construction materials, indicating an adaptive response to cell damage and a certain protective effect on intracellular homeostasis [21]. However, when excessive autophagy occurs, its protective mechanism changes into a damaging one. The excessive degradation of the basic proteins and organelles of cells leads to an impaired cellular function and even autophagic cell death. Excessive autophagy can be induced by multiple factors, such as oxidative stress, metabolic stress, and starvation [22]. Apoptosis and autophagy can also be triggered by heavy metals [23, 24] and other exogenous chemicals [25]. Autophagy is a prospective target for cancer therapy [26].
Materials and Methods
Materials
Potassium dichromate was purchased from Kaitong Chemical (Beichen, Tianjin, China). PTA was donated by Prof. Ding Xinhua of Shandong Agricultural University. After application of an efficient purification method, mainly including extraction with pH 1.0 distilled water and then desorption with pH 1.0 95% ethanol after a DM-130 resin adsorption step to obtain more pure anthocyanin extracts, the purity of anthocyanins extracted from purple tomato fruits reached 54%. Dulbecco’s modified Eagle’s medium (DMEM)/high glucose was obtained from HyClone Company (Logan, Utah, USA). Fetal bovine serum (FBS) was procured from Tianhang Biotechnology (Deqing, Zhejiang, China). Trypsin–EDTA solution and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) and Rapamycin (Rapa) were obtained from Solarbio Science and Technology (Beijing, China). 3-Methyladenine (3-MA) was obtained from MCE (USA). Thapsigargin (Tg), 4-phenyl butyric acid (4-PBA), and COX-2 inhibitor (NS-398) were procured from Sigma-Aldrich (USA). A CCK-8 assay kit was purchased from Dojindo Laboratories (Japan). Grp78/Bip (Cat: AB310) antibody and BCA assay kit were procured from Beyotime Institute of Biotechnology (Haimen, Jiangsu, China). LC3 II (ab229327) antibody was purchased from Abcam (Cambridge Science Park, Cambridge, UK). Beclin1 and P62 (Cat: 18420-1-AP) antibodies were obtained from Proteintech (Chicago, USA). Chicken COX-2 and chicken PERK enzyme-linked immunosorbent assay (ELISA) kits were bought from ML Biotech (Shanghai, China), and a chicken mTOR ELISA kit was procured from the Shanghai Fusheng Trizol Reagent Company (Shanghai, China).
Cell Culture
The LMH cells used in this study were obtained from the ATCC Agency Company (Beijing, China). The cells were cultured in DMEM with L-glutamine and glucose containing 10% heat-inactivated FBS and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) in 5% CO2 at 3 °C.
Cell Treatment
LMH cells undergoing experimental growth were cultured and incubated with Cr(VI) and PTA in 5% CO2 at 37 °C for 20 h. The six treatment groups in our study were as follows: (1) control: incubated in DMEM for 20 h; (2) Cr(VI) treatment: incubated in DMEM containing Cr (20 μM) for 20 h; (3) Cr(VI)+PTA treatment: incubated in DMEM containing Cr (20 μM)+PTA (50 μg/mL) for 20 h; (4) Cr(VI)+PTA treatment: incubated in DMEM containing Cr (20 μM)+PTA (100 μg/mL) for 20 h; (5) PTA treatment: incubated in DMEM containing PTA (50 μg/mL) for 20 h; and (6) PTA treatment: incubated in DMEM containing PTA (100 μg/mL) for 20 h. After 20 h of treatment, the cells were harvested for analyses.
Cell Viability Assay
Cell viability was detected by CCK-8 assay after treatment for 20 h. In brief, LMH cells were first seeded onto 96-well plates (1 × 105 cells/well) in 100 μL of DMEM. After 20 h of growth, the cells were treated with a series of Cr(VI) and PTA concentration for 20 h to detect the cytotoxic effect of Cr(VI) and the cytoprotective effect of PTA. The activity of living cells was measured using this assay in terms of dehydrogenase activity that reduces CCK-8 to an orange product. The absorbance of formazan at 450 nm was determined.
Western Blot Analysis
After treatment, the cells were washed with PBS (4 °C) and incubated on ice with RIPA lysis buffer supplemented with 1 mM phenylmethanesulfonyl fluoride for 10 min. Cell lysates were then clarified by centrifugation at 12,000×g for 15 min at 4 °C. The protein concentration was determined using a BCA protein assay kit (Beyotime Biotechnology, Jiangsu, China). Equal amounts of protein samples were diluted in 5× SDS-PAGE loading buffer and boiled for 5 min. Proteins (20 μg) were separated by SDS-PAGE and transferred onto 0.22-μm polyvinylidene fluoride membranes (Merck Millipore, Massachusetts, USA). The membranes were blocked with TBS with 0.1% Tween 20 (TBST) containing 5% nonfat milk powder for 1 h at room temperature and incubated with diluted primary antibodies against Beclin1 (1:1000, Proteintech, USA), LC3-II (1:1000, Abcam, UK), p62 (1:1000, Proteintech, USA), and GRP78/Bip (1:1000, Beyotime, China) at 4 °C overnight. Afterward, the membranes were washed thrice with TBST for 10 min. The membranes were incubated with appropriate secondary antibodies for 1 h at room temperature. Each target protein was measured using ECL Western detection reagents. Protein levels were then analyzed by ImageJ. The density of each band was normalized to its respective loading control (GAPDH).
ELISA Assay
A membrane and cytosol protein extraction kit (Beyotime Biotechnology, Jiangsu, China) was used to extract LMH cell proteins for detection. The serum levels of mTOR, PERK, and COX-2 were measured with ELISA kits, namely, mTOR (Shanghai Fusheng Trizol Reagent Co., Shanghai, China), PERK (ML Biotech, Shanghai, China), and COX-2 (ML Biotech, Shanghai, China), in accordance with the manufacturer’s instructions. Absorbance was read at 450 nm with an automatic enzyme standard instrument, and absorbance results were normalized via standard curves.
Statistical Analysis
Statistical analyses were performed with SPSS 19.0 (SPSS Inc., Chicago, IL, USA). One-way ANOVA was conducted to identify the significant values, and the Duncan method was used for post-test (P < 0.05). Data were expressed as mean ± SD, drawing with GraphPad Prism 7.0 software. All test data were repeated 3 times.
Results
Morphological Analysis and Viability Assay of LMH Cells
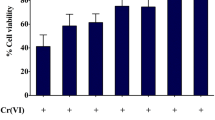
Chromium(VI) concentration and treatment time were determined by detecting cell activity (Fig. 1a, b). Therefore, 20 μM Cr(VI) treatment was determined for 20 h. Rapamycin (Rapa) is commonly used as an mTOR inhibitor to enhance the autophagic level. 3-MA, an autophagy inhibitor that suppresses phosphatidylinositol 3-kinase (PI3K) activity and blocks autophagosome formation, is one of the most classical inhibitors that inhibit the induction of autophagy. In our study, these drugs were used to explore the role of PTA in regulating the autophagy in LMH cells under Cr(VI) stress conditions. Cells were treated with an inducer (Tg) and inhibitor (4-PBA) of ER stress to verify the association between ER stress and autophagy. Morphological analysis was performed under a microscope. In Fig. 1c, the morphological characteristics of the LMH cells were normal in the control, PTA, Rapa, and 3-MA groups. Cr(VI) induced cell shedding and decreased the cell size and density after the cells were treated for 20 h. In comparison with the cells in the Cr(VI) group, the cells in the Cr (VI)+PTA group were less damaged and shrunken. In Fig. 1d, the cell activity of the Cr(VI) group decreased significantly, whereas the cell activity of the 3-MA group inhibited cell autophagy and increased cell viability. Anthocyanin had the same effect as 3-MA. At the same time, the results showed that the cell viability of the Tg+Cr(VI) group was significantly lower than that of the Cr(VI) group, while the cell viability of the 4-PBA+Cr(VI) group was significantly higher than that of the Cr(VI) treatment group. It is speculated that Cr(VI) can induce autophagy by activating ER stress, so the next measure of ER stress and autophagy is tested to verify this hypothesis.
Effect of PTA on Cr(VI)-induced morphology and cell viability in LMH cells after 20 h of treatment. a Cell viability under different Cr(VI) concentrations. b Cell viability under different time conditions of 20 μM Cr(VI). c Cell morphology. d Cell viability in different treatments. Cells were cultured for 20 h in DMEM/high glucose (Con) and DMEM/high glucose with 20 μM Cr(VI). For Cr(VI) treatment with PTA, PTA (50 or 100 μg/mL) was pretreated and cells were treated with Cr(VI) for 20 h. 3-MA (100 μM) and Rapa (1 μM) were set as autophagy negative and positive controls, respectively. Tg (10 μM) and 4-PBA (1 mM) were set as ER stress negative and positive controls, respectively. Data were presented as mean ± SD (n = 3). Bars without a shared common letter were significantly different (P < 0.05)
PTA Inhibited Cr(VI)-Induced ER Stress by the Altered Expression of PERK and GRP78 Proteins
The study of our group has shown that ER stress is induced by Cr(VI) in DF-1 cells [8]. Here, we investigated whether LMH cells exposed to Cr(VI) could induce ER stress and whether PTA could elicit a regulatory effect on them. GRP78/Bip and PERK are two essential indicators of ER stress. ER stress activation primarily relies on the exposure concentration and duration of stressors; thus, these two critical factors must first be determined in in vitro studies. Our findings (Fig. 2) demonstrated that Cr(VI) induced ER stress in LMH cells, and the expression of GRP78/Bip and PERK, which are well-known proteins of ER stress in the Cr(VI) group, was significantly higher than that in the control group. And the levels of GRP78/Bip and PERK in the PTA(100 μg/mL)+Cr(VI) group were significantly lower than those in the Cr(VI) group. Furthermore, the expression of GRP78/Bip and PERK in the PTA(100 μg/mL)+Cr(VI) group was significantly lower than that in the PTA(50 μg/mL)+Cr(IV) group. These results indicated that PTA could alleviate ER stress caused by Cr(VI), and this effect was dose related.
Effect of PTA on Cr(VI)-induced GRP78/Bip and PERK protein expression after 20 h of treatment. a GRP78/Bip detected by Western blot and quantitative analysis of GRP78/Bip level. b PERK level. Data were presented as mean ± SD (n = 3). Bars without a shared common letter were significantly different (P < 0.05)
PTA Inhibited Cr(VI)-Induced Autophagy by the Altered Expression of LC3 and P62 Proteins
LC3 is an autophagosomal ortholog of yeast Atg8. LC3-II, a lipidated form of LC3, is an autophagosomal marker in mammals. p62 is an ubiquitin-binding protein involved in the degradation of the ubiquitin–proteasome system and the autophagy–lysosomal system. It is also a protein marker of autophagy. LMH cells were exposed to Cr(VI); the expression level of LC3-II was higher than that in the control group (Fig. 3a), whereas the expression level of p62 was lower than that in the control group (Fig. 3b). However, this change could be mitigated by the treatment with Cr(VI)+PTA (100 μg/mL), indicating that PTA(100 μg/mL) could inhibit Cr(VI)-induced autophagy.
Effect of PTA on Cr(VI)-induced LC3 and p62 protein expression after 20 h of treatment. a LC3-I and LC3-II detected by Western blot and quantitative analysis of LC3-II/I protein level. b p62 detected by Western blot and quantitative analysis of p62 level. Data were presented as mean ± SD (n = 3). Bars without a shared common letter were significantly different (P < 0.05)
PTA Inhibited Cr(VI)-Induced Autophagy by the Altered Expression of Beclin1 and mTOR Proteins
Beclin1 is an essential molecule for autophagosome formation. As a molecular platform, it can mediate autophagy-associated proteins localized to phagocytic vacuoles and react with various proteins to regulate autophagosome formation and maturation. In Fig. 4a, the Beclin1 protein level of the Cr(VI) group increased compared with that of the control group. However, this change could be mitigated by the treatment with Cr(VI)+PTA(100 μg/mL), indicating that PTA(100 μg/mL) could inhibit Cr(VI)-induced autophagy. mTOR is a key protein found in the upstream pathway of autophagy and implicated in the regulation of cell growth, proliferation, and autophagy. The inhibition of the PI3K-AKT-mTOR pathway is effective in inducing autophagy. In this experiment, the mTOR level (Fig. 4b) was significantly weakened when Cr(VI) was supplied. When PTA was added, the mTOR level was significantly enhanced compared with that in the Cr(VI) group.
Effect of PTA on Cr(VI)-induced Beclin1 and mTOR protein expression after 20 h of treatment. a Beclin1 detected by Western blot and quantitative analysis of Beclin1 level. b mTOR level. Data were presented as mean ± SD (n = 3). Bars without a shared common letter were significantly different (P < 0.05)
PTA Attenuated Cr(VI)-Induced COX-2 in LMH Cells
The study of our group has elucidated a novel molecular mechanism of Cr(VI)-induced DF-1 cell autophagy and injury dependent on the ER stress-regulated COX-2 overexpression. COX-2 expression was assessed after the cells were exposed to Cr(VI) to explore the role of COX-2 in Cr(VI)-induced cell dysfunction. When Cr(VI) was added, the COX-2 level increased significantly. By contrast, after PTA treatment was administered, the COX-2 levels decreased compared with the Cr(VI) group (Fig. 5c). Thus, PTA could inhibit Cr(VI)-induced increase in the level of COX-2. Compared with chromium treatment, the expression of LC3-II decreased significantly and the degradation level of p62 increased significantly after adding NS-398 (COX-2 inhibitor), indicating that COX-2 played an important role in chromium-induced autophagy.
Effect of PTA (100 μg/mL) on Cr(VI)-induced COX-2 expression after 20 h of treatment. a LC3-I and LC3-II detected by Western blot and quantitative analysis of LC3-II/I protein level. b p62 detected by Western blot and quantitative analysis of p62 level. c COX-2 level. NS-398(20 μM) was COX-2 inhibitor. Data were presented as mean ± SD (n = 3). Bars without a shared common letter were significantly different (P < 0.05)
Discussion
At present, since chromium(VI) toxicity is significantly higher than chromium(III), research on chromium poisoning has focused on Cr(VI). The previous study of this group determined the dose and treatment time of Cr(VI) [8]. Therefore, the cells were treated with 20 μM Cr(VI) for 20 h. At the same time, the cell survival rate was tested by the CCK-8 method to verify whether the concentration and treatment time met the test requirements, and the verified results were true (Fig. 1).
The selection of PTA concentration was also based on the previous study of the research group [17], using PTA at concentrations of 50 μg/mL and 100 μg/mL, and the cell viability was measured by the CCK-8 method. The anthocyanin concentration was determined to meet the requirements of this test (Fig. 1). Therefore, the test was carried out by using PTA (50 μg/mL, 100 μg/mL) and a concentration of 20 μM of Cr(VI) which were used for 20 h.
Related studies have reported that Cr(VI) could cause autophagy in L-02 hepatocytes and BEAS-2B-Cr cells [7, 27]. Therefore, this experiment investigated the intrinsic link between Cr(VI) and autophagy. Autophagy is required in many physiological and pathological processes and implicated in maintaining cellular metabolic balance and homeostasis [28]. Anthocyanin is a large class of polyphenols and water-soluble pigments that produce a reddish blue appearance in various plant tissues [29]. Anthocyanins have several health benefits, such as anti-atherosclerotic activity, visual improvement, and anticancer and anti-inflammatory activities [11, 13, 30, 31]. Zhang et al. found that anthocyanin protects INS-1 cells from exogenous H2O2-induced autophagic cell death and anthocyanin pretreatment to reduce the degree of autophagy in early β-cell transplants after transplantation [16]. And Zhao et al. indicate that PSTA can interact with Cr(VI) in advance to protect the secondary and tertiary structures of BSA [32]. As such, the effect of anthocyanin on the Cr(VI)-induced autophagy of LMH cells was investigated in our study.
Autophagy protects the body in most cases, but when autophagy is over-activated, it can cause damage to cells and even induce autophagic cell death [33]. Autophagy could be inhibited by the inhibition of autophagy by genetic or chemical methods, thus confirming the autophagic death of cells [33, 34]. Therefore, this experiment involved the determination of cell viability to observe cell death progression accurately. In Fig. 1, the cell viability of the 3-MA+Cr(VI) group was significantly higher than that of the Cr(VI) group, and PTA had the same effect as 3-MA. The inhibition of autophagy could alleviate cell damage and death caused by Cr(VI), indicating that Cr(VI) treatment caused excessive autophagy in LMH cells, and PTA could reduce the level of autophagy caused by Cr(VI).
Zhang et al. reported that Cr(VI) can lead to ER stress in L-02 hepatocytes [35]. ER stress activation also induces autophagy [8, 36,37,38]. In Fig. 1, the cell viability of the 4-PBA+Cr(VI) group was significantly higher than that of the Cr(VI) group, and the cell viability of the Tg+Cr(VI) group was significantly lower than that of the Cr(VI) group. GRP78, as the molecular chaperone of ER cavity, is involved in the folding and processing of ER proteins and the binding of calcium ions to maintain ER homeostasis [39]. During ER stress occurs, an unfolded protein activates GRP78, releases sensors, and transduces unfolded protein signals to the cytoplasm and the nucleus [40]; in addition, GRP78 dissociates from PERK, which is activated by phosphorylation [41]. Our results (Fig. 2) showed that Cr(VI) could increase the expression of GRP78 and PERK proteins and induce ER stress, whereas PTA could effectively relieve ER stress.
This study showed the effects of Cr(VI) and PTA on ER stress in LMH cells, and previous studies revealed that ER stress could activate autophagy. As such, autophagy-related proteins were examined to explore their effect on autophagy. Beclin1, LC3-II, and p62 are important proteins during autophagy. Beclin1 can participate in autophagosome formation at the initial stage of autophagy [42]. At the maturation stage of autophagy, LC3 acts as an LC3-II by binding to phosphatidylethanolamine (PE, LC3–PE) to form autophagic vacuoles via a ubiquitination-like enzymatic reaction [43]. LC3-II is located on the autophagic membrane. Therefore, the detection of the LC3-II protein expression can be used as a classical indicator of autophagy. At the late stage of autophagy, the polyubiquitin-binding protein P62/SQSTM1 degrades the contents in autophagosomes by directly binding to LC3 [7]. In Figs. 3 and 4, Cr(VI) treatment increased the LC3-II and Beclin1 levels and decreased the p62 protein level in LMH cells, whereas PTA treatment reduced the effects of Cr(VI) treatment. This finding indicated that Cr(VI) induced autophagy in cells, whereas PTA attenuated the degree of autophagy caused by Cr(VI). Autophagy is important for cell survival, and autophagy dysregulation is the cause of many diseases; excessive autophagy activation can induce cell death and pathological changes through overactive degradation processes [44,45,46].
Chen et al. found that blocking the autophagic upstream signaling, such as ER stress and COX-2 signaling, improves cell growth, indicating that the ER stress–COX-2 pathway may contribute to autophagy-related growth [8]. Therefore, the changes in the expression of COX-2 were detected after the cells were treated with Cr(VI) and PTA. The result (Fig. 5c) showed that Cr(VI) increased the expression of COX-2, whereas anthocyanin significantly decreased the change in the Cr(VI)-induced expression. In addition, the effect was strengthened as the concentration increased. When COX-2 was inhibited, Cr(VI)-induced autophagy was significantly inhibited (as shown in Fig. 5a, b). However, the intrinsic relationship between autophagy, ER stress, and COX-2 should be further investigated.
Conclusion
In summary, Cr(VI) could induce autophagy by activating ER stress in LMH cells, and PTA(100 μg/mL) could attenuate Cr(VI)-induced cell dysfunction via autophagy by regulating ER stress and COX-2. Therefore, PTA might be a potential natural active substance that alleviated the cytotoxicity of Cr(VI)-induced LMH cells.
References
Chen H, Cao J, Li L, Wu X, Bi R, Klerks PL, Xie L (2016) Maternal transfer and reproductive effects of Cr(VI) in Japanese medaka (Oryzias latipes) under acute and chronic exposures. Aquat Toxicol 171:59–68. https://doi.org/10.1016/j.aquatox.2015.12.011
Chen P, Zhu Y, Wan H, Wang Y, Hao P, Cheng Z, Liu Y, Liu J (2017) Effects of the oral administration of K2Cr2O7 and Na2SeO3 on Ca, Mg, Mn, Fe, Cu, and Zn contents in the heart, liver, spleen, and kidney of chickens. Biol Trace Elem Res 180(2):285–296. https://doi.org/10.1007/s12011-017-0999-x
Zhu Y, Chen P, Wan H, Wang Y, Hao P, Liu Y, Liu J (2018) Selenium-chromium(VI) interaction regulates the contents and correlations of trace elements in chicken brain and serum. Biol Trace Elem Res 181(1):154–163. https://doi.org/10.1007/s12011-017-1038-7
Wang Y, Liu Y, Wan H, Zhu Y, Chen P, Hao P, Cheng Z, Liu J (2017) Moderate selenium dosing inhibited chromium (VI) toxicity in chicken liver. J Biochem Mol Toxicol 31(8). https://doi.org/10.1002/jbt.21916
das Neves RP, Santos TM, De Lourdes Pereira M, de Jesus JP (2016) Comparative histological studies on liver of mice exposed to Cr(VI) and Cr(V) compounds. Hum Exp Toxicol 21(7):365–369. https://doi.org/10.1191/0960327102ht243oa
Xie Y, Xiao F, Luo L, Zhong C (2014) Activation of autophagy protects against ROS-mediated mitochondria-dependent apoptosis in L-02 hepatocytes induced by Cr(VI). Cell Physiol Biochem: international journal of experimental cellular physiology 33(3):705–716. https://doi.org/10.1159/000358646
Liang Q, Xiao Y, Liu K, Zhong C, Zeng M, Xiao F (2018) Cr(VI)-induced autophagy protects L-02 hepatocytes from apoptosis through the ROS-AKT-mTOR pathway. Cell Physiol Biochem 51(4):1863–1878. https://doi.org/10.1159/000495713
Chen P, Geng N, Zhou D, Zhu Y, Xu Y, Liu K, Liu Y, Liu J (2019) The regulatory role of COX-2 in the interaction between Cr(VI)-induced endoplasmic reticulum stress and autophagy in DF-1 cells. Ecotoxicol Environ Saf 170:112–119
Kawaguchi T, Nomura K, Hirayama Y, Kitagawa T (1987) Establishment and characterization of a chicken hepatocellular carcinoma cell line. LMH Cancer Res 47(16):4460–4464
Gabis KK, Gildemeister OS, Pepe JA, Lambrecht RW, Bonkovsky HL (1996) Induction of heme oxygenase-1 in LMH cells. Comparison of LMH cells to primary cultures of chick embryo liver cells. Biochim Biophys Acta Gen Subj 1290(1):113–120. https://doi.org/10.1016/0304-4165(96)00009-8
Xia X, Ling W, Ma J, Xia M, Hou M, Wang Q, Zhu H, Tang Z (2006) An anthocyanin-rich extract from black rice enhances atherosclerotic plaque stabilization in apolipoprotein E-deficient mice. J Nutr 136(8):6. https://doi.org/10.1093/jn/136.8.2220
Guerra MC, Galvano F, Bonsi L, Speroni E, Costa S, Renzulli C, Cervellati R (2007) Cyanidin-3-O-β-glucopyranoside, a natural free-radical scavenger against aflatoxin B1- and ochratoxin A-induced cell damage in a human hepatoma cell line (Hep G2) and a human colonic adenocarcinoma cell line (CaCo-2). Br J Nutr 94(02):211–220. https://doi.org/10.1079/bjn20051425
Chun OK, Kim DO, Lee CY (2003) Superoxide radical scavenging activity of the major polyphenols in fresh plums. J Agric Food Chem 51(27):6. https://doi.org/10.1021/jf034740d
Galvano F, La Fauci L, Lazzarino G, Fogliano V, Ritieni A, Ciappellano S, Battistini NC, Tavazzi B, Galvano G (2004) Cyanidins: metabolism and biological properties. J Nutr Biochem 15(1):10. https://doi.org/10.1016/s0955-2863(03)00133-5
Chanoca A, Kovinich N, Burkel B, Stecha S, Bohorquez-Restrepo A, Ueda T, Eliceiri KW, Grotewold E, Otegui MS (2015) Anthocyanin vacuolar inclusions form by a microautophagy mechanism. Plant Cell 27(9):2545–2559. https://doi.org/10.1105/tpc.15.00589
Zhang B, Buya M, Qin W, Sun C, Cai H, Xie Q, Xu B, Wu Y (2013) Anthocyanins from Chinese bayberry extract activate transcription factor Nrf2 in β cells and negatively regulate oxidative stress-induced autophagy. J Agric Food Chem 61(37):8765–8772. https://doi.org/10.1021/jf4012399
Xie N, Geng N, Zhou D, Xu Y, Liu K, Liu Y, Liu J (2018) Protective effects of anthocyanin against apoptosis and oxidative stress induced by arsanilic acid in DF-1 cells. Mol Biol Rep 46:301–308. https://doi.org/10.1007/s11033-018-4472-5
Zhang B, Buya M, Qin W, Sun C, Cai H, Xie Q, Xu B, Wu Y (2013) Anthocyanins from Chinese bayberry extract activate transcription factor Nrf2 in beta cells and negatively regulate oxidative stress-induced autophagy. J Agric Food Chem 61(37):8765–8772. https://doi.org/10.1021/jf4012399
Huang J, Klionsky DJ (2007) Autophagy and human disease. Cell Cycle 6(15):1837–1849
Cuervo AM (2004) Autophagy: in sickness and in health. Trends Cell Biol 14(2):70–77. https://doi.org/10.1016/j.tcb.2003.12.002
Yang F, Liao J, Pei R, Yu W, Han Q, Li Y, Guo J, Hu L, Pan J, Tang Z (2018) Autophagy attenuates copper-induced mitochondrial dysfunction by regulating oxidative stress in chicken hepatocytes. Chemosphere 204:36–43. https://doi.org/10.1016/j.chemosphere.2018.03.192
Wang Z, Li S, Ren R, Li J, Cui X (2015) Recombinant buckwheat trypsin inhibitor induces mitophagy by directly targeting mitochondria and causes mitochondrial dysfunction in Hep G2 cells. J Agric Food Chem 63(35):7795–7804. https://doi.org/10.1021/acs.jafc.5b02644
Lee YH, Su SB, Huang CC, Sheu HM, Tsai JC, Lin CH, Wang YJ, Wang BJ (2014) N-Acetylcysteine attenuates hexavalent chromium-induced hypersensitivity through inhibition of cell death, ROS-related signaling and cytokine expression. PLoS One 9(9):e108317
Wang SH, Shih YL, Ko WC, Wei YH, Shih CM (2008) Cadmium-induced autophagy and apoptosis are mediated by a calcium signaling pathway. Cell Mol Life Sci 65(22):3640–3652. https://doi.org/10.1007/s00018-008-8383-9
Hussain S, Al-Nsour F, Rice AB, Marshburn J, Yingling B, Ji Z, Zink JI, Walker NJ, Garantziotis S (2012) Cerium dioxide nanoparticles induce apoptosis and autophagy in human peripheral blood monocytes. ACS Nano 6(7):5820–5829. https://doi.org/10.1021/nn302235u
Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, Han W, Lou F, Yang J, Zhang Q, Wang X, He C, Pan H (2013) Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis 4:e838. https://doi.org/10.1038/cddis.2013.350
Dai J, Ji Y, Wang W, Kim D, Fai LY, Wang L, Luo J, Zhang Z (2017) Loss of fructose-1,6-bisphosphatase induces glycolysis and promotes apoptosis resistance of cancer stem-like cells: an important role in hexavalent chromium-induced carcinogenesis. Toxicology & Applied Pharmacology 331:164–173. https://doi.org/10.1016/j.taap.2017.06.014
Matthias J, Messling S, Eichinger L (2016) The two Dictyostelium autophagy eight proteins, ATG8a and ATG8b, associate with the autophagosome in succession. Eur J Cell Biol 95(1):15–25. https://doi.org/10.1016/j.ejcb.2015.10.007
Clifford M (2004) Diet-derived phenols in plasma and tissues and their implications for health. Planta Med 70(12):1103–1114. https://doi.org/10.1055/s-2004-835835
Hitoshi M, Yuko N, Shuji T, Satoru K, Masao H (2003) Stimulatory effect of cyanidin 3-glycosides on the regeneration of rhodopsin. J Agric Food Chem 51(12):3560–3563. https://doi.org/10.1128/IAI.01773-14
Wang H, Nair MG, Strasburg GM, Chang YC, Booren AM, Gray JI, Dewitt DL (1999) Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J Nat Prod 62(2):294–296. https://doi.org/10.1021/np980501m
Xingchen Z, Feng S, Jianli Z, Rutao L (2011) Composition and stability of anthocyanins from purple Solanum tuberosum and their protective influence on Cr(VI) targeted to bovine serum albumin. J Agric Food Chem 59(14):7902–7909
Klionsky D, Abdelmohsen K, Abe A, Abedin M, Abeliovich A, AA A, Adachi H et al (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12(1):222. https://doi.org/10.1080/15548627.2015.1100356
Fang CY, Chen JS, Chang SK, Shen CH (2018) Reversine induces autophagic cell death through the AMP-activated protein kinase pathway in urothelial carcinoma cells. Anti-Cancer Drugs 29(1):29–39. https://doi.org/10.1097/CAD.0000000000000563
Zhang Y, Xiao F, Liu X, Liu K, Zhou X, Zhong C (2017) Cr(VI) induces cytotoxicity in vitro through activation of ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction via the PI3K/Akt signaling pathway. Toxicol in Vitro 41:232–244. https://doi.org/10.1016/j.tiv.2017.03.003
Wang Z, Yin F, Xu J, Zhang T, Wang G, Mao M, Wang Z, Sun W, Han J, Yang M, Jiang Y, Hua Y, Cai Z (2019) CYT997(Lexibulin) induces apoptosis and autophagy through the activation of mutually reinforced ER stress and ROS in osteosarcoma. J Exp Clin Cancer Res 38(1):44. https://doi.org/10.1186/s13046-019-1047-9
Fouillet A, Levet C, Virgone A, Robin M, Dourlen P, Rieusset J, Belaidi E, Ovize M, Touret M, Nataf S, Mollereau B (2014) ER stress inhibits neuronal death by promoting autophagy. Autophagy 8(6):915–926. https://doi.org/10.4161/auto.19716
Periyasamy P, Guo ML, Buch S (2016) Cocaine induces astrocytosis through ER stress-mediated activation of autophagy. Autophagy 12(8):1310–1329. https://doi.org/10.1080/15548627.2016.1183844
Louessard M, Bardou I, Lemarchand E, Thiebaut AM, Parcq J, Leprince J, Terrisse A, Carraro V, Fafournoux P, Bruhat A, Orset C, Vivien D, Ali C, Roussel BD (2017) Activation of cell surface GRP78 decreases endoplasmic reticulum stress and neuronal death. Cell Death Differ 24(9):1518–1529. https://doi.org/10.1038/cdd.2017.35
Cubillos-Ruiz JR, Bettigole SE, Glimcher LH (2017) Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in cancer. Cell 168(4):692–706. https://doi.org/10.1016/j.cell.2016.12.004
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334(6059):1081–1086. https://doi.org/10.1126/science.1209038
Wargasetia T, Shahib N, Martaadisoebrata D, Dhianawaty D, Hernowo B (2015) Characterization of apoptosis and autophagy through Bcl-2 and Beclin-1 immuno expression in gestational trophoblastic disease. Iran J Reprod Med 13(7):8. https://doi.org/10.1128/IAI.01773-14
Yang Z, Wilkie-Grantham RP, Yanagi T, Shu C-W, Matsuzawa S-i, Reed JC (2015) ATG4B (autophagin-1) phosphorylation modulates autophagy. J Biol Chem 290(44):26549–26561. https://doi.org/10.1074/jbc.M115.658088
Li H, Song Y, He Z, Chen X, Wu X, Li X, Bai X, Liu W, Li B, Wang S, Han Y, Xu L, Zhang D, Li J, Chai R, Wang H, Fan Z (2018) Meclofenamic acid reduces reactive oxygen species accumulation and apoptosis, inhibits excessive autophagy, and protects hair cell-like HEI-OC1 cells from cisplatin-induced damage. Front Cell Neurosci 12. https://doi.org/10.3389/fncel.2018.00139
Ryter SW, Mizumura K, Choi AMK (2014) The impact of autophagy on cell death modalities. Int J Cell Biol 2014:1–12. https://doi.org/10.1155/2014/502676
Czaja MJ, Ding W-X, Donohue TM, Friedman SL, Kim J-S, Komatsu M, Lemasters JJ, Lemoine A, Lin JD, Ou JH, Perlmutter DH, Randall G, Ray RB, Tsung A, Yin X-M (2014) Functions of autophagy in normal and diseased liver. Autophagy 9(8):1131–1158. https://doi.org/10.4161/auto.25063
Funding
The project was supported by the National Nature Science Foundation of China (No. 31872535), National Key R&D Program (2016YFD0501208), Shandong Natural Science Foundation of China (ZR2018MC027, ZR2016CQ29), and Funds of Shandong “Double Tops” Program. PTA was donated by Prof. Ding Xinhua of Shandong Agricultural University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, K., Chen, P., Lu, J. et al. Protective Effect of Purple Tomato Anthocyanidin on Chromium(VI)-Induced Autophagy in LMH Cells by Inhibiting Endoplasmic Reticulum Stress. Biol Trace Elem Res 194, 570–580 (2020). https://doi.org/10.1007/s12011-019-01795-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01795-3