Abstract
This study aimed to investigate the effects of selenium on the ion profiles in the heart, liver, spleen, and kidney through the oral administration of hexavalent chromium. Approximately 22.14 mg/kg b.w. K2Cr2O7 was added to water to establish a chronic poisoning model. Different selenium levels (0.00, 0.31, 0.63, 1.25, 2.50, and 5.00 mg Na2SeO3/kg b.w.) around the safe dose were administered to the experimental group model. Ca, Mg, Mn, Fe, Cu, and Zn were detected in the organs through flame atomic absorption spectrometry after these organs were exposed to K2Cr2O7 and Na2SeO3 for 14, 28, and 42 days. Results showed that these elements exhibited various changes. Ca contents declined in the heart, liver, and spleen. Ca contents also decreased on the 28th day and increased on the 42nd day in the kidney. Mn contents declined in the heart and spleen but increased in the kidney. Mn contents also decreased on the 28th day and increased on the 42nd day in the liver. Cu contents declined in the heart and spleen. Cu contents increased on the 28th day and decreased on the 42nd day in the liver and kidney. Zn contents declined in the heart and spleen. Zn contents increased on the 28th day and decreased on the 42nd day in the liver and kidney. Fe contents decreased in the heart and liver. Fe contents increased on the 28th day and decreased on the 42nd day in the spleen and kidney. Mg contents did not significantly change in these organs. Appropriate selenium contents enhanced Mn and Zn contents, which were declined by chromium. Conversely, appropriate selenium contents reduced Ca, Fe, and Cu contents, which were increased by chromium. In conclusion, the exposure of chickens to K2Cr2O7 induced changes in different trace elements, and Na2SeO3 supplementation could alleviate this condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium is a common constituent of organic matter. This transition metal has been considered a trace element because it influences glucose, protein, and fat metabolism through an insulin-related mechanism [27]. It mainly exists in two valence states, namely, trivalent and hexavalent Cr [28]. Trivalent chromium can enhance insulin action, cellular glucose uptake, and intracellular carbohydrate and lipid metabolism [6]. Hexavalent chromium [Cr(VI)] can cause cancer, malformation, and mutation to nucleic acids and other cellular components in organisms. These toxic effects are due to the rapid penetration of Cr(VI) in cellular membranes and its subsequent interaction with proteins and nucleic acids in cells [38]. Some researchers suggested that the long-term intake of Cr(VI) may cause squamous cell carcinoma, adenocarcinoma, and other diseases [3, 9, 21, 32].
Selenium is an essential trace element for living organisms and closely related to animal health. Se can induce the synthesis of selenoprotein involved in the antioxidant defense mechanism of organisms [15]. The lack of Se mainly results in the decreased expression of selenoproteins and changes in biological processes in many organs and tissues [13, 22]. Se also presents a strong affinity to metal elements, and it can possibly reduce the toxicity of heavy metals, such as cadmium and mercury [14, 40, 41]. In various physiological and pathological conditions, complex antagonisms exist between Se and other heavy metals and multi-ions [35].
Trace elements are crucial in the development and growth of animals, although their contents are considerably small, that is, less than 0.1% [12]. Various kinds of trace elements maintain the metabolism in a dynamic balance [1]. Disorder in these elements may trigger alterations in physiological activities, induce pathological changes in the metabolism of normal cells, and cause dysfunctions in growth and reproductive abilities [20]. Therefore, trace elements, which are active in relative organs, should be balanced for animal growth. Ca, Mg, Cu, Zn, Fe, and Mg are related to body homeostasis [16]. The effects of trace element contents on mammalian organs have been investigated, and the toxicity of trace elements accumulated in these organs exposed to a certain element has been detected [7, 17, 34]. Therefore, further studies should be performed to provide detailed insights into the interaction of Cr(VI)–Se-induced changes in Ca, Mg, Cu, Zn, Fe, and Mg contents in chickens.
This study aimed to investigate the effects of the oral administration of Cr(VI) on the Ca, Mn, Cu, Zn, Fe, and Mg contents in the heart, liver, spleen, and kidney of Hyland chickens and to determine the alleviating role of Se in vivo.
Materials and Methods
Reagents
Potassium dichromate (K2Cr2O7, AR, purity ≥ 99%) and sodium selenite (Na2SeO3, AR, purity ≥ 98%) were acquired from Putian Company Inc. (Tai’an, China). Nitric acid (HNO3, GR) and perchloric acid (HClO4, GR) were provided by the Chemical Institute of Shandong Agricultural University. All plastic and glassware materials used for the experiment were soaked in dilute HNO3 (1%) and washed with ultrapure water prior to their use. All other reagents were of analytical grade.
Animal Treatment and Experimental Design
The safe dose of Na2SeO3 for chicken was described previously [19]. A total of 105 Hyland male chickens (1 day old) were obtained from Tai’an Dongyue Poultry Breeding Company (Shandong, China). The chickens were randomly divided into seven groups (N = 15). Before the experiment was performed, all of the chickens were acclimatized to laboratory conditions for 7 days. All of them were provided with water and sufficient forage ad libitum and maintained at a suitable room temperature and a relative humidity with a 12-h/12-h light/dark cycle. All of the procedures were processed in accordance with the protocols of the Institutional Animal Care and Use Committee of Shandong Agricultural University (SDAU-2015-07).
Seven groups were treated as follows: The first group was given water only and considered the control group. Approximately 22.4 mg/kg b.w. K2Cr2O7 was added to the treatment for the six other groups. Subsequently, 0.00, 0.31, 0.63, 1.25, 2.50, and 5.00 mg/kg b.w. Na2SeO3 were supplied to the Cr-treated groups. Five chickens from each group were euthanized on the 14th, 28th, and 42nd days. After the chickens were euthanized with thiopental, their heart, liver, spleen, and kidney were quickly removed. The tissues were rinsed with ice-cold deionized water and stored at −20 °C until required.
Assay of Metal Concentrations
According to the method of Liu et al. [24, 25], the blood vessels, fat, and connective tissues around the heart, liver, spleen, and kidney were detached. Subsequently, each sample (1.0 g, wet body mass) was placed in Erlenmeyer flasks, which contained 25 mL of nitric acid and perchloric acid mixture (4:1), and allowed to digest for 24 h. The digested mixture was slowly heated in an electric hot plate in a well-ventilated room until the remaining liquid measured approximately 2–3 mL. Afterward, the system was slowly cooled to room temperature. Subsequently, 5 mL of ultrapure water was added into Erlenmeyer flasks and heated again. The excess acid was allowed to volatilize. A constant 2.5-mL diluted hydrochloric acid (1:1) was added to a volumetric flask until 25 mL was reached and then preserved at 4 °C. This solution was not exposed to light. The trace elements (Mn, Cu, Zn, Fe, Ca, Mg, and Cr) were detected through flame atomic absorption spectrometry (F-AAS) with Zeeman background correction (GFA-7000, Kyoto, Japan). The National Institute of Standards and Technology Standard Reference Materials® 1577c bovine liver was used for quality control. The Cr detection limit was 3 × 10−5 μg/mL. The actual contents of each trace element in the heart, liver, spleen, and kidney were presented for the measured value multiplied by the dilution ratio. The operating conditions of F-AAS to detect each element are listed in Table 1. The obtained results were in good agreement with certified values (Table 2). All of the experiments were conducted three times to ensure reproducibility.
Statistical Analysis
Statistical analyses were performed with Statistical Package for Social Sciences (SPSS) program version 19.0 (SPSS Inc., Chicago, IL, USA). One-way ANOVA was conducted to identify significant values (p < 0.05). Data were expressed as mean ± SD.
Results
Cr in the Heart, Liver, Spleen, and Kidney
The Cr(VI) contents in the heart, liver, spleen, and kidney tissues at different time points are presented in Table 3. In particular, the Cr(VI) contents distinctly accumulated (p < 0.05) in these organs. With K2Cr2O7 exposure, the Cr(VI) in these groups was significantly increased (p < 0.05) compared with that of the control group. Over time, the contents showed an upward trend in the heart, liver, and spleen but not in the kidney of the Cr(VI)-treated group. When the Cr(VI)-treated group was exposed to Se, the Cr(VI) content slightly decreased, but this observation was not significant.
Ca in the Heart, Liver, Spleen, and Kidney
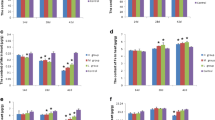
The Ca content presented different trends in these organs with increasing time and dose (Fig. 1). The Ca contents decreased in the heart, liver, and spleen at all time points. By contrast, the Ca content decreased on the 28th day and increased on the 42nd day in the kidney. After Cr(VI) was administered, the Ca content was higher than that in the control group (p < 0.05). By comparison, the Ca content decreased when Se was added. The Ca content can be restored to normal level by Se treatment. However, the content did not significantly change when the Se dose increased (p > 0.05) in the heart and liver. In the spleen, the contents in the Se-supplemented group were apparently lower than those in the Cr(VI)-treated group (p < 0.05). In the kidney, the Ca contents in the Se-supplemented groups were lower than those in the Cr (VI)-treated group. The Ca contents decreased as Se was added.
a–d Ca contents in different organs of chickens with different levels of selenium supplement with increasing time. The bars represent arithmetic means, and the upper tiny strokes represent SD from n = 5. Bars without a shared common letter are significantly different (p < 0.05). Data are means ± SDs, n = 5
Mn in the Heart, Liver, Spleen, and Kidney
In the heart, liver, spleen, and kidney, the Mn contents in the Cr(VI)-treated group were lower than those in the control group (p < 0.05; Fig. 2). After the time was extended and the dose was increased, the Mn contents in the heart and spleen were slightly decreased. The Mn contents decreased on the 28th day and increased on the 42nd day in the liver. By comparison, the Mn contents increased in the kidney. In these organs, the Mn contents constantly increased when Se was supplied. In the Se-treated groups, the low-Se-treated group with 0.31 mg/kg Se (p < 0.05) recovered to nearly the normal level.
a–d Mn contents in different organs of chickens with different levels of selenium supplement with increasing time. The bars represent arithmetic means, and the upper tiny strokes represent SD from n = 5. Bars without a shared common letter are significantly different (p < 0.05). Data are means ± SDs, n = 5
Cu in the Heart, Liver, Spleen, and Kidney
In these organs, the Cu contents in the Cr(VI)-treated group were higher than those in the control group. In the heart and spleen, the contents presented a decreasing trend, which was lower than those of the initial values and increased dosage (Fig. 3). In the low-Se-treated (0.31 and 0.63 mg/kg) groups, the contents were not significantly different from those in the control group (p < 0.05). On the contrary, the Cu content increased again as Se dose increased. In the liver and kidney, the Cu contents showed a similar trend, that is, the Cu contents were higher on the 28th day but were lower after 42 days of exposure to Cr(VI).
a–d Cu contents in different organs of chickens with different levels of selenium supplement with increasing time. The bars represent arithmetic means, and the upper tiny strokes represent SD from n = 5. Bars without a shared common letter are significantly different (p < 0.05). Data are means ± SDs, n = 5
Zn in the Heart, Liver, Spleen, and Kidney
In the heart, the Zn contents in the Cr(VI)-treated group were lower than those in the control group at different time points (p < 0.05) but were higher in the liver, spleen, and kidney of the Cr(VI)-treated group than in these organs of the control group (Fig. 4). In the heart, the Zn contents increased when low Se levels (0.31 and 0.63 mg/kg) were supplied. By contrast, the Zn contents decreased as Se content increased. In the liver, the Zn contents decreased to normal levels when low-dose Se (0.31 mg/kg) was added. At increasing Se dose, the Zn contents remarkably declined. In the spleen and kidney, the Zn contents decreased when low Se level was added. Likewise, the Zn contents increased and finally reached the Cr(VI)-treated level as the Se dose increased.
a–d Zn contents in different organs of chickens with different levels of selenium supplement with increasing time. The bars represent arithmetic means, and the upper tiny strokes represent SD from n = 5. Bars without a shared common letter are significantly different (p < 0.05). Data are means ± SDs, n = 5
Fe in the Heart, Liver, Spleen, and Kidney
In these organs, the Fe contents in the Cr-treated group were higher (p < 0.05) than those in the control group at different time points (Fig. 5). These contents showed various trends among different organs. In the heart and liver, the contents decreased as treatment time was extended and dose increased. In the spleen and kidney, the contents were higher on the 28th day and reduced considerably on the 42nd day. After Se was supplied, the Fe contents changed. In the low-dose Se-treated (0.31 mg/kg) group, the contents recovered to an improved state. No change was observed with increasing dose and time. The results indicated that low Se dose (0.31 mg/kg) was not significantly different from that in the control group (p < 0.05).
a–d Fe contents in different organs of chickens with different levels of selenium supplement with increasing time. The bars represent arithmetic means, and the upper tiny strokes represent SD from n = 5. Bars without a shared common letter are significantly different (p < 0.05). Data are means ± SDs, n = 5
Mg in the Heart, Liver, Spleen, and Kidney
In general, the Mg changes in these groups were not obvious (Fig. 6). On the 14th day, the Mg contents were slightly lower in the Cr(VI)-treated group than those in the control group in the heart. However, they presented an opposite trend in the liver, spleen, and kidney. In particular, the Mg content was higher in the Cr(VI)-treated group than in the control group (p < 0.05), but it showed no significant difference among all of the groups on the 28th and 42nd days in each organ.
a–d Mg contents in different organs of chickens with different levels of selenium supplement with increasing time. The bars represent arithmetic means, and the upper tiny strokes represent SD from n = 5. Bars without a shared common letter are significantly different (p < 0.05). Data are means ± SDs, n = 5
Discussion
Trace elements are essential for many physiological progress, and adverse changes in their contents are often associated with many diseases [26]. The present study revealed that a specific dose of orally administered Cr(VI) significantly altered the contents of trace elements in the heart, liver, spleen, and kidney of chickens in comparison to control levels. This condition could be alleviated by the supplementation of low-dose Se to some extent.
Ca is an essential element in many physiological processes. Some researchers suggested that large amounts of Ca intake can adversely affect cardiovascular outcomes [2, 30], cardiomyocyte apoptosis [5, 39], and kidney stone formation [4]. Fe is an essential element for erythropoiesis and cellular bioenergetics. Cr(VI) and Fe are both transported by β-globulin but combined in different loci [18], which can express the synergistic effect of Cr(VI) and Fe. Furthermore, Mn is an essential trace element for the growth of animals, and it participates in cellular homeostasis, bone formation, and calcium absorption [8]. Liu et al. [23] demonstrated the antagonistic effects of Mn and Fe. Ca can limit the bioavailability and retention of Mn [37]. In our results, the Ca contents in the heart and kidney were increased when Cr(VI) was added. Excess Fe may cause the generation of reaction oxygen species, and Se is a component of glutathione peroxidase, which produces an antioxidant effect [29]. Our results also revealed that treatment with Cr(VI) could enhance the Fe content. The Mn content decreased when the Ca and Fe contents increased, and our findings were consistent with previous results. The Ca, Fe, and Mn levels normalized when low-dose Se was added.
Cu and Zn provide protection against the progression of some diseases [11, 31]. Cu plays roles on some metalloproteins, which participate in many vital functions. Schafer et al. [33] found that Cu enhances immune responses and Zn strengthens immunity [10, 36]. When pathological changes occurred in the body, the enhanced immune function is considerably attributed to Cu and Zn. In our experiment, the Cu and Zn contents increased when the chickens were treated with Cr(VI). This observation was consistent with that described in previous studies. This condition could be improved with low Se dose.
Our study confirmed that low-dose Se could resolve the Cr(VI)-induced changes in the ion contents of the heart, liver, spleen, and kidney. Se could form a complex with Cr(VI) to inhibit the function of Cr(VI) that accumulated in the organs. Moreover, these ions interacted with one another, but these interactions were undetected.
Conclusions
Cr(VI) can accumulate in the body of chickens, and Se can alleviate Cr(VI)-induced changes in the profile of related ions. Complex interactions among ions elicit synergistic and antagonistic effects, and these interactions maintain homeostasis.
References
Aaseth J, Boivin G, Andersen O (2012) Osteoporosis and trace elements—an overview. J Trace Elem Med Biol 26:149–152
Bolland MJ, Barber PA, Doughty RN et al (2008) Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ (Clinical research ed) 336:262–266
Braver ER, Infante P, Chu K (1985) An analysis of lung cancer risk from exposure to hexavalent chromium. Teratogenesis Carcinogenesis & Mutagenesis 5:365
Curhan GC, Willett WC, Rimm EB et al (1993) A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 328:833–838
D'souza-Li L (2006) The calcium-sensing receptor and related diseases. Arq Bras Endocrinol Metab 50:628–639
Dkhil MA, Abdel-Baki AS, Al-Quraishy S et al (2013) Hepatic oxidative stress in Mongolian gerbils experimentally infected with Babesia divergens. Ticks and Tick-borne Diseases 4:346–351
Dogukan A, Sahin N, Tuzcu M et al (2009) The effects of chromium histidinate on mineral status of serum and tissue in fat-fed and streptozotocin-treated type II diabetic rats. Biol Trace Elem Res 131:124
Erikson KM, Syversen T, Aschner JL et al (2005) Interactions between excessive manganese exposures and dietary iron-deficiency in neurodegeneration. Environmental Toxicology & Pharmacology 19:415–421
Fan Y, Ovesen JL, Puga A (2012) Long-term exposure to hexavalent chromium inhibits expression of tumor suppressor genes in cultured cells and in mice. J Trace Elem Med Biol 26:188–191
Gaby AR (2006) Natural remedies for scleroderma. Alternative Medicine Review A Journal of Clinical Therapeutic 11:188–195
Goldhaber SB (2003) Trace element risk assessment: essentiality vs. toxicity. Regulatory Toxicology & Pharmacology 38:232–242
Haenlein GFW, Anke M (2011) Mineral and trace element research in goats: a review. Small Rumin Res 95:2–19
Jiang ZH, Khoso PA, Yao HD et al (2015) SelW regulates inflammation-related cytokines in response to H2O2 in Se-deficient chicken liver. RSC Adv 5:37896–37905
Kalisinska E, Gorecki J, Okonska A et al (2014) Mercury and selenium in the muscle of piscivorous common mergansers (Mergus merganser) from a selenium-deficient European country. Ecotoxicology & Environmental Safety 101:107–115
Kieliszek M, Błażejak S (2016) Current knowledge on the importance of selenium in food for living organisms: a review. Molecules 21:609
Kim MH, Choi MK (2013) Seven dietary minerals (Ca, P, Mg, Fe, Zn, Cu, and Mn) and their relationship with blood pressure and blood lipids in healthy adults with self-selected diet. Biol Trace Elem Res 153:69–75
Kuriwaki J, Nishijo M, Honda R et al (2005) Effects of cadmium exposure during pregnancy on trace elements in fetal rat liver and kidney. Toxicol Lett 156:369–376
Lal CS, Kumar S, Ranjan A et al (2013) Comparative analysis of serum zinc, copper, magnesium, calcium and iron level in acute and chronic patients of visceral leishmaniasis. Journal of Trace Elements in Medicine & Biology Organ of the Society for Minerals & Trace Elements 27:98–102
Li JL, Jiang CY, Li S et al (2013) Cadmium induced hepatotoxicity in chickens (Gallus domesticus) and ameliorative effect by selenium. Ecotoxicology & Environmental Safety 96:103–109
Lin CC, Huang HH, Hu CW et al (2014) Trace elements, oxidative stress and glycemic control in young people with type 1 diabetes mellitus. Journal of Trace Elements in Medicine & Biology Organ of the Society for Minerals & Trace Elements 28:18–22
Linos A, Petralias A, Christophi CA et al (2011) Oral ingestion of hexavalent chromium through drinking water and cancer mortality in an industrial area of Greece—an ecological study. Environ Health 10:1–8
Liu C, Fu J, Liu C et al (2015) The role of nitric oxide and autophagy in liver injuries induced by selenium deficiency in chickens. RSC Adv 5:50549–50556
Liu X, Zuo N, Guan H et al (2013) Manganese-induced effects on cerebral trace element and nitric oxide of Hyline cocks. Biol Trace Elem Res 154:202–209
Liu Y, Pan H, Xiao Z et al. (2016) Effects of excess Cr3+ on trace element contents in the brain and serum in chicken. Biol Trace Elem Res. doi:10.1007/s12011-016-0875-0
Liu Y, Zhao X, Zhang X et al (2016) Effects of oral administration of CrCl3 on the contents of Ca, Mg, Mn, Fe, Cu, and Zn in the liver, kidney, and heart of chicken. Biol Trace Elem Res 171:1–9
Loguercio C, De GV, Federico A et al (1997) Trace elements and chronic liver diseases. J Trace Elem Med Biol 11:158–161
Mertz W (1993) Chromium in human nutrition: a review. J Nutr 123:626–633
O’brien TJ, Ceryak S, Patierno SR (2003) Complexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanisms. Mutation Research/Fundamental & Molecular Mechanisms of Mutagenesis 533:3–36
Paolicchi F, Perea J, Cseh S et al (2013) Relationship between paratuberculosis and the microelements copper, zinc, iron, selenium and molybdenum in beef cattle. Braz J Microbiol 44:153–160
Pentti K, Tuppurainen MT, Honkanen R et al (2009) Use of calcium supplements and the risk of coronary heart disease in 52–62-year-old women: the Kuopio Osteoporosis Risk Factor and Prevention Study. Maturitas 63:73–78
Raouf AA, Radwan et al (2013) Serum zinc, copper, and iron in children with chronic liver disease. Egyptian Liver Journal 3:63–72
Rupp ME (2004) Diagnostic test for elemental imbalances [P]. In:US 6,821,786 B2
Schafer AS, Leal MLR, Molento MB et al (2014) Immune response of lambs experimentally infected with Haemonchus contortus and parenterally treated with a combination of zinc and copper. Small Rumin Res 123:183–188
Staniek H, Rhodes NR, Bona KRD et al (2013) Comparison of tissue metal concentrations in Zucker lean, Zucker obese, and Zucker diabetic fatty rats and the effects of chromium supplementation on tissue metal concentrations. Biol Trace Elem Res 151:373
Sun L, Yu Y, Huang T et al (2012) Associations between ionomic profile and metabolic abnormalities in human population. PLoS One 7:e38845
Tipu MK, Saleem U, Hussain K et al (2012) The role of zinc on anti-Newcastle disease virus specific antibody response and agranulocytes count in rabbits treated with methotrexate and prednisolone. Pak J Pharm Sci 25:845–849
Trumbo P, Yates AA, Schlicker S et al (2001) Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc 101:294–301
Valko M, Izakovic M, Mazur M et al (2004) Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem 266:37–56
Zhang WH, Fu SB, Lu FH et al (2006) Involvement of calcium-sensing receptor in ischemia/reperfusion-induced apoptosis in rat cardiomyocytes. Biochemical & Biophysical Research Communications 347:872–881
Zhao J, Li Y, Li Y et al (2014) Selenium modulates mercury uptake and distribution in rice (Oryza sativa L.), in correlation with mercury species and exposure level. Metallomics 6:1951–1957
Zhao W, Liu W, Chen X et al (2014) Four endoplasmic reticulum resident selenoproteins may be related to the protection of selenium against cadmium toxicity in chicken lymphocytes. Biol Trace Elem Res 161:328–333
Acknowledgements
This work was supported by the National Key R&D Program (2016YFD0501208) and the Shandong Modern Agricultural Technology & Industry System (No. SDAIT-11-04).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Chen, P., Zhu, Y., Wan, H. et al. Effects of the Oral Administration of K2Cr2O7 and Na2SeO3 on Ca, Mg, Mn, Fe, Cu, and Zn Contents in the Heart, Liver, Spleen, and Kidney of Chickens. Biol Trace Elem Res 180, 285–296 (2017). https://doi.org/10.1007/s12011-017-0999-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-0999-x