Abstract
Environmental pollution and exposure of people to heavy metals cause many bad obstetric outcomes. Our aim is to demonstrate the role of cadmium (Cd), lead (Pb), mercury (Hg), and selenium (Se) in preterm labor etiology with a case-control study. In this study, between November 2017 and April 2018, preterm delivery mothers and term delivery mothers were compared in Çorum, Turkey. All deliveries were performed with cesarean sections and there were 30 mothers in the control group and 20 in the study group. The maternal blood, maternal urine, umbilical cord blood, and heavy metal levels in the amnion fluid in both groups were studied. Graphite furnace atomic absorption spectrometry was used to determine the blood concentration of Cd, Pb, Hg, and Se. We found lower levels of selenium in blood and urine of preterm delivery mothers and umbilical cord and amnion fluids of preterm infants (p < 0.01). We found a statistically significant positive correlation at selenium levels between mother’s blood and umbilical cord blood (r (50) = 0.896, p < 0.001) and between maternal urine and amniotic fluid (r (50) = 0.841, p < 0.001). We have not found a similar correlation between mother and fetus of other metals (p > 0.05). We found that selenium levels were lower in mothers who were preterm birth in the light of the data in our study. We could not determine the positive or negative correlation of Cd, Pb, and Hg levels in blood, urine, and amniotic fluid samples with preterm birth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Preterm infants are defined as infants born before the 37th gestational week by the World Health Organization (WHO) and the International Federation of Gynecology and Obstetrics (FIGO). This diagnosis is clinically established and includes cervical dilatation, effacement, or both, associated with regular uterine contractions. In addition, patients with 2-cm cervical opening and regular uterine contractions at the time of first admission are diagnosed with preterm labor. There are many etiologic reasons underlying the pathophysiology of preterm labor. While intrauterine infections are often implicated in preterm labor in most studies, many complex causes such as maternal nutrition, genetic, and extracellular matrix regulation could be found at the basis of the pathophysiology [1].
Industrial environmental pollution, a threat to the whole world in the last century, affects all forms of life. Among these sources of environmental pollution are organic hydrocarbons, pesticides, and heavy metals. The most common heavy metals affecting the reproductive system are cadmium, mercury, arsenic, and lead [2]. There are reports that environmental pollutants cause oocyte maturation deterioration, ovulation defects, implantation/abortion failures, and birth defects [3]. Heavy metals can damage the fetus from weeks when fetal cell division and fetal cell differentiation begin to occur until weeks when tissues are formed and even organs become functional. Naturally, the fetus’ susceptibility to toxicity varies from week to week until birth [4]. The toxic effects of heavy metals vary according to the type. Cadmium (Cd) is one of the most common heavy metals found in human physiology with toxic effects. The toxic effects of Cd are observed even at low doses and the biological half-life is very long. The half-life in the human kidney lasts from 6 to 38 years, while the half-life in human liver lasts from 4 to 19 years [5]. In studies evaluating pregnancy outcomes with Cd, low birth weight, 5th minute APGAR (activity, pulse, grimace, appreciation, respiration) score changes, cognitive disorders, and epigenetic modifications were identified [6,7,8]. Lead (Pb) is a toxic heavy metal found naturally on earth, also found on petroleum products, local plants, water pipes, metal welding materials, and toys [9]. Lead toxicity has been shown to cause neurobehavioral deficits in children [10]. The immature blood-brain barrier in the early stage of fetal development causes further damage to the embryo or fetus in the case of lead exposure [11]. The placenta and blood-brain barrier allow the passage of Pb through passive diffusion. Pb negatively affects memory and learning ability as a cause of hippocampal destruction [12]. Lead affects fetal growth by disrupting normal cell functions as well as neurological development. Lead can slow down growth by toxicizing osteocytes responsible for bone growth. Furthermore, thyroid-stimulating hormone (TSH) secretion can be disturbed, slowing the development of soft tissues and reducing the birth weight [13]. Accumulation of lead in placenta can affect fetal growth by disturbing the transport of nutrients required for fetal growth [14]. Mercury (Hg) is one of the toxic heavy metals that have an adverse effect on tissue and organ functions and are frequently encountered in exposure. It can be found in nature, in soil, water, and air. It has elemental, organic, and inorganic forms. Mercury is spread due to volcanic activity, decomposition from the soil and human-induced environmental pollution [15]. Each form of mercury causes maternal and fetal toxicity. It can spread to all tissues in the organism and has neurotoxic, nephrotoxic, carcinogenic, teratogenic, and cardiotoxic effects [16]. The greatest risk for fetus is transplacental mercury transit. When the mercury levels in the mother’s blood were compared with the mercury levels in the cord blood, the mercury level in the cord blood was found to be 70% higher [17]. Severe neurological damage occurs in infants of mothers who are exposed to mercury. It disrupts the division of fetal neurons required for normal neurological development and the migration of nerve cells through microtubules. After these injuries, microcephaly, mental retardation, convulsions, blindness, hearing loss, and intelligence stress were detected [18].
Selenium is one of the essential minerals that can be obtained from foods and deficiency is responsible for various complications related to pregnancy [19]. It has been shown that low levels of selenium detected at 12th week of gestation cause preterm action [20]. Low selenium levels have also been shown to increase the possibility of developing preeclampsia [21]. Selenium with nutritional importance may cause toxic effects depending on the dose. Symptoms of selenium toxicity often begin in the form of loss of toe- and fingernails, loss of body and scalp hair, and muscle and joint pain [22]. Nausea, abdominal pain, diarrhea, peripheral neuropathy, fatigue, irritability, and hair and nail changes occurred in those exposed to high-dose selenium in various food supplements [23]. Various long-term high-dose exposures to the selenium were observed in various central nervous system manifestations. These are toxic symptoms in the form of convulsions, hyperreflexia, and increased delays in visual evoked potentials [24]. Selenium has various interactions with heavy metals. For example, in a rat study, concurrent exposure to mercury, lead, and cadmium increased selenium retention in the tissues [25]. But there is much evidence that selenium has a protective effect against heavy metal damage in the body. In the rat model, it was shown that cadmium-induced renal toxicity was prevented by reducing lipid peroxidation by selenium [26,27,28].
Although the sociodemographic, nutritional, medical, obstetric, and environmental factors increase the risk of spontaneous preterm labor, the precise etiology of preterm labor is still not fully understood [29]. Our aim is to demonstrate the role of cadmium (Cd), lead (Pb), mercury (Hg), and selenium (Se) in preterm labor etiology with a case-control study. The results of our study have clarified if there is a relationship between the concentration of studied heavy metals in the biological material and the occurrence of preterm delivery.
Material and Methods
Study Population and Selection of the Events
Thirty preterm delivery patients and 20 term delivery patients were included in the study among the patients who applied to the Hitit University Medical Faculty Obstetrics and Gynecology Policlinic and delivered by the cesarean section. Patients were selected among pregnant who delivered between November 2017 and April 2018. Before the start of the study, approval was obtained from the Ethics Committee of the Hitit University Medical Faculty in accordance with the Helsinki Declaration. Informed consent forms were obtained from the patients included in the study.
The study was designed as a case-control study. The patients were divided into two groups. The preterm delivery patients were in the first group and in the second group there were mothers who gave birth at term. The gestational weeks of the patients were based on the first day of the last menstrual period. Then, to evaluate the accuracy of the gestational weeks, ultrasonographic examination was performed before the cesarean section and compared with the first trimester ultrasonography. After all three evaluations, pregnancies smaller than the 37th gestational week were considered preterm delivery [30]. Thirty pregnancies were born with the preterm cesarean section (case group) and 20 were delivered with the term cesarean section (control group). Cesarean indications were limited to old cesarean and malpresentation when patients were selected.
Patients were selected from non-smoking volunteers aged 18–40 years. All the pregnancies taken for labor had single pregnancy and none of the patients had amniotic membrane rupture before the cesarean section. Information on the sociodemographic characteristics of the patients was obtained by face-to-face interviews. Patients who smoke and drink alcohol and use intrauterine devices at any stage of their life were excluded from the study. The mothers of newborn who were diagnosed with intrauterine fetal anomaly or born with neonatal intensive care need or born without spontaneous breathing were excluded from the study. None of the patients included in the study had diabetes, preeclampsia, metabolic disease, malabsorption syndrome, and proven infection. All pregnant women in the study have been living in Corum/Turkey. None of the participants live in the industrial zone. The first-degree industrial zone nearest to the city is approximately 14 km away. No patient was exposed to heavy metal exposure at any time during pregnancy. The history of dental treatment was obtained. Pregnant women with dental amalgam were not included in the study. Participants received only 40–60 mg of elementary iron and 1200 IU of vitamin D daily in line with the country’s health policies. During the follow-up of pregnancies, selenium-containing support treatments and multivitamins were not used. Liver and kidney function tests were normal for all cases in the case and control group.
All of the patients included in the study received regional anesthesia during the cesarean section. No blood or blood product transfusions were performed in any patient. Patients in the case group were treated with nifedipine for preterm labor and medication for fetal lung maturation with betamethasone. None of the patients received magnesium sulfate treatment.
Collection of Materials and Measurement of Metal Concentrations
Preoperative venous blood for the cesarean section was taken from the patients and 10 mL of blood was separated, taken into spray-coated tubes with sodium heparin, and stored at − 80 °C in the freezer. When the cesarean section was taken, 10 mL of amniotic fluid was removed from the amnion sac. After the umbilical cord was clamped and the baby was delivered to the neonatal team, 5 mL of blood was collected from the placental side of the placenta before the placenta medical waste was thrown. All samples were stored in the Eppendorf tube, − 80 °C freezer until working day.
Reagents
All chemicals were of analytical grade and all of them are useful for trace element analysis. Sixty-five percent (v/v) nitric acid (HNO3) (Sigma-Aldrich Corp, St Louis, MO, USA), 70% (m/v) perchloric acid (HClO4) (Merck, Darmstadt, Germany), and Triton X-100 (Sigma-Aldrich Corp, St Louis, MO, USA) were used for the sample preparation. Working each solution for Cd (CL01.0306), Hg (CL00.1333), Pb (CL01.1226), and Se (CL01.1927) elements from Chem-Lab, Zedelgem, Belgium, was used as standards in concentrations below:
-
Pb: 3–5–10–15–20 μg/L (ppb)
-
Cd: 1–5–10–15–20 μg/L (ppb)
-
Se: 5–10–30–50–80 μg/L (ppb)
-
Cu: 2–5–10–15–20 μg/L (ppb)
Digestion and Element Analysis
The following sample preparation procedures were tested:
For measurement of Cd and Se levels, open digestion system was used; 1 mL of each sample (amnion floods and urines) was accurately measured with graduated cylinder, whole bloods measured with automatic pipettes (pipette head was washed with solvent) then added into a propylene tube, and 9 mL of a 4:1 HNO3:HClO4 mixture was added. Shaked the mixture carefully or stirred with a clean glass bar. Waited at least 10 min before putting water bath. The mixture was digested in a water bath at 80 °C for 2 h. The digestion was continued until the samples became colorless. After cooling, colorless solutions were transferred into 10-mL volumetric flasks and made up to the volume with 5% HNO3. For measurement of Hg and Pb levels, 1 mL of each sample and 6 mL HNO3 solution and 3 mL Triton X-100 solution were mixed and then shaked the mixture carefully or stirred then putting water bath. The mixture was digested in a water bath at 80 °C for 2–3 h. The digestion was continued until the samples became colorless. After cooling, colorless solutions were transferred into 10-mL volumetric flasks and made up to the volume with 5% HNO3 [31].
The optimize furnace parameters wizard in the SOLAAR (Thermo Scientific, Cambridge, England) software was used to determine the most suitable temperatures and flow rate for analyses of the digested samples for each elements. Autosampler was used to optimize the position of the injection capillary and to observe the deposition of the sample into the cuvette. The 10 μg/L of each element solution was used as the master standard for the method. The autosampler was programmed to automatically generate calibration standards at useful range. All samples, blanks, and standards were injected at a constant fixed volume of 10 μL, alongside an additional aliquot of 10 μL of matrix modifier into an electrographite cuvette. Cd was analyzed at 228.8 nm, Hg was analyzed at 253.7 nm, Pb was analyzed at 217.0 nm, and Se was analyzed at 196.0 nm and Zeeman background correction was used throughout. Peak areas were measured for the production of the calibration and subsequent determination of the sample concentrations.
The detection limit (LOD) of each element: Cd, 0.01 ppb; Pb, 1.24 ppb; Se, 4.25 ppb; and Hg, 0.12 ppb. The precision for inter-day and intra-day determinations for all elements ranged under 4%. In addition, the certified reference materials supplied from Seronorm were used to perform daily quality control (LOT, 1309438).
Apparatus
An atomic absorption spectrophotometer’s graphite furnace (Thermo Scientific iCE 3000 Series, Thermo Scientific, Cambridge, England) was used to measure total concentrations of Cd, Hg, Pb, and Se in all samples. Analytical lines of Cd 228.8 nm, Hg 253.7 nm, Pb 217.0 nm, and Se 196.0 nm were measured [32].
Statistical Method
Statistical analyses were performed with SPSS (version 22.0, SPSS Inc., Chicago, IL, USA). The distribution of normality was examined by the Shapiro-Wilk test. Descriptive statistics were presented as mean ± standard deviation or median (min-max) according to data distribution for continuous variables. The median comparisons of Cd, Hg, Pb, and Se between the control and preterm groups were made by the Mann Whitney U test. Relationships between mother blood, fetus blood, and urine amnion were assessed by Spearman’s correlation coefficient. Regression analysis was applied to determine the cause-and-effect relationship between the variables with significant correlation. The statistical significance was accepted as p < 0.05.
Results
When the ages of the study groups were compared, the mean age of the control group (28.0 ± 5.19) was higher than the preterm delivery group (25.15 ± 2.78). This difference between the averages of age was statistically significant (p = 0.015). There was a significant difference between the groups in the maternal birth weeks (p < 0.001). The mean birth week of the control group (39.08 ± 0.71) was higher than that of the preterm delivery group (35.11 ± 0.87). There was a significant difference between the weights of newborns (p < 0.001). The mean weight of newborns in the control group (3422 ± 224) was higher than that of the preterm delivery group (2445 ± 238) (Table 1).
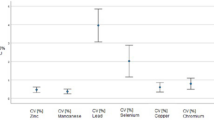
Pb levels in maternal urine were higher in the control group than in the preterm labor group when heavy metal levels were compared between the two groups (p < 0.001). However, there was no elevation in maternal blood, fetal blood, and amniotic fluid Pb levels in the control group (p = 0.357, p = 0.285, p = 0.789, Table 2). Se levels were significantly different between maternal blood, fetal blood, maternal urine, and amnion fluid groups (p < 0.001). There was no statistically significant difference in Cd and Hg levels between the groups of blood, urine, and amnion fluid (p > 0.05).
There was a statistically significant positive correlation between selenium levels in the mother’s blood and selenium levels in the umbilical cord blood (r (50) = 0.896, p < 0.001). There was also a statistically significant positive correlation between selenium levels between maternal urine and amnion fluid (r (50) = 0.841, p < 0.001). No significant correlation was found between maternal vs fetal blood values and urine vs amnion fluid values for other heavy metals (p > 0.05).
When the amount of Se in the mother’s blood increased by 1 unit in control group, the amount of Se in the fetal blood increased by 0.936 (0.624–1.248) units (p < 0.001). According to the regression analysis expression coefficient (R2), 57.4% of the amount of Se in cord blood can be explained by the amount of selenium in the mother’s blood. When the amount of Se in the mother’s blood increased by 1 unit, the amount of Se in the fetal blood increased by 0.306 (0.004–0.608) units (p = 0.047) for the preterm group. According to the regression analysis expression coefficient (R2), 20.1% of the amount of Se in cord blood can be explained by the amount of selenium in the mother’s blood (Table 3).
When the amount of selenium in the mother’s urine increased by 1 unit, the amount of Se in the amnion fluid increased by 0.47 (0.06–0.87) units (p = 0.027) for the control group. According to the regression analysis expression coefficient (R2), 16.4% of the amount of Se in the amnion fluid can be explained by the amount of selenium in the mother’s urine. When the amount of selenium in the mother’s urine increased by 1 unit, the amount of Se in the amnion fluid increased by 0.25 (0.01–0.49) units (p < 0.001) for the preterm group. According to the regression analysis expression coefficient (R2), 20.6% of the amount of Se in the amnion fluid can be explained by the amount of selenium in the mother’s urine (Table 4).
Discussion
The aim of our research was to determine the role of heavy metal levels in the etiology of preterm delivery. Selenium levels were found to be lower in preterm delivery mothers when the heavy metal levels of the groups of term and preterm delivery mothers included in the study were compared. There was no significant difference between the two groups in cadmium and mercury levels. It was observed that the lead was higher in the urine of the mothers who gave birth to the term.
There are many studies in literature that show the effects of high heavy metal levels on pregnancy outcomes. However, a case-control study evaluating preterm birth and heavy metal levels is limited. In our study, we found a positive correlation in selenium levels between maternal blood and cord blood. A similar correlation was found in another study in which the selenium level was assessed with birth weight [33]. In our study, there was a relationship between preterm labor and low selenium levels, whereas in another case-control study in which preterm labor was evaluated, there was no relationship between selenium levels and the week of birth [34].
In our study, no statistically significant difference was found between the levels of cadmium, mercury, and lead of the control group with the preterm action group. In a large retrospective study conducted in Japan, a relationship was found between maternal blood cadmium levels and preterm birth at early weeks [35]. Cadmium levels were also investigated in ectopic pregnancies. There was no significant association between blood cadmium levels and ectopic pregnancy [36]. In a study where maternal blood lead levels were measured, a correlation was found between premature birth risk and high lead levels [37]. We found a higher level of lead in the urine of the control group mothers. There are also publications in literature where higher levels of lead are detected in the urine of preterm delivery mothers [38]. We did not find a relationship between mercury levels and preterm labor in our study, but there are studies in the literature that indicate that mercury accumulation is higher in the hair of prematurely delivered mothers [39]. In addition to these studies, there was another study from Myanmar, a cohort study of the effects of arsenic, cadmium, and lead urine levels on pregnancy outcomes showed that heavy metal exposure was associated with low birth weight, but not with preterm labor [40].
The heavy metal levels obtained in our study are similar when compared to previous studies. However, there is no consensus on the limits of heavy metal levels during pregnancy [41]. However, when we compared our results with previous studies, we found no difference in Pb blood levels in the preterm birth and term birth group. The levels of Pb that we found in the blood were lower the maximum limits by the USA, Germany, and Austria. Pb levels were found to be low in our study because the patients in our study live in areas where there is no industrial production.
In one of the previous studies, the blood lead levels of pregnant women who had preterm labor were high. In our study, no statistical difference was found between the groups [42]. In addition, high blood Cd levels were correlated with low birth weight in one of the previous studies. In our study, no statistically significant difference was found between the groups in terms of maternal blood and fetal blood Cd levels [43]. The effect of cadmium exposure on pregnancy outcomes was investigated in another study. The levels of development and intelligence were found to be lower in children born over 0.6 μg/L of Cd levels in cord blood. In our study, all cord Cd values were found to be less than 0.3 μg/L [44].
One of the limitations of working is the number of volunteers included in the groups. Reasons for the low number of volunteers included in the study were rigid exclusion criteria used to form homogeneous groups, the number of volunteers who are informed about the study is low, and finally to obtain four biological materials such as peripheral blood, cord blood, urine, and amnion fluid from a volunteer. Another limitation of the study is that we can not explain the fact that the lead level is higher in the healthy volunteer group, although we have checked the same time and same medical and laboratory materials at least 3 times for all groups.
Conclusion
Our study found that selenium levels in preterm delivery mothers and infants were lower. There was a positive correlation between selenium levels between maternal blood and fetal blood and between maternal urine and amniotic fluid. Heavy metal levels were not different between the groups and it was concluded that there was no relationship with preterm birth.
References
Savitz DA, Blackmore CA, Thorp JM (1991) Epidemiologic characteristics of preterm delivery: etiologic heterogeneity. Am J ObstetGynecol 164:467–471
Flora SJS, Pachauri V, Saxena G. (2011) Arsenic, cadmium and lead. In: Gupta RC, editor. Reproductive and developmental toxicology Elsevier,London,pp. 415–439
Foster WG, Neal MS, Han MS, Dominguez MM (2008) Environmental contaminants and human infertility: hypothesis or cause for concern? J Toxicol Environ Health 11:162–176
Rogers J(2013) Developmental toxicology. In: Klaassen C.D., editor. Casarett and Doull’s toxicology: the basic science of poisons. McGraw-Hill, Health Professions Division; New York, NY, USA
Kjellstrom T, Nordberg GF (1978) A kinetic model of cadmium metabolism in the human being. Environ Res 16:248–269. https://doi.org/10.1016/0013-9351(78)90160-3
Al-Saleh I, Shinwari N, Mashhour A, Rabah A (2014) Birth outcome measures and maternal exposure to heavy metals (lead, cadmium and mercury) in Saudi Arabian population. Int J Hyg Environ Health 217:205–218. https://doi.org/10.1016/j.ijheh.2013.04.009
Sanders AP, Claus Henn B, Wright RO (2015) Perinatal and childhood exposure to cadmium, manganese, and metal mixtures and effects on cognition and behavior: a review of recent literature. Curr Environ Health Rep 2:284–294. https://doi.org/10.1007/s40572-015-0058-8
Vilahur N, Vahter M, Broberg K (2015) The epigenetic effects of prenatal cadmium exposure. Curr Environ Health Rep 2:195–203. https://doi.org/10.1007/s40572-015-0049-9
Rischitelli G, Nygren P, Bougatsos C, Freeman M, Helfand M (2006) Screening for elevated lead levels in childhood and pregnancy: an updated summary of evidence for the US Preventive Services Task Force. Pediatrics 118:e1867–e1895
Greig J, Thurtle N, Cooney L, Ariti C, Ahmed AO, Ashagre T, Ayela A, Chukwumalu K, Criado-Perez A, Gómez-Restrepo C, Meredith C, Neri A, Stellmach D, Sani-Gwarzo N, Nasidi A, Shanks L, Dargan PI (2014) Association of blood lead level with neurological features in 972 children affected by an acute severe lead poisoning outbreak in Zamfara State, northern Nigeria. PLoS One 9:–e93716
Freedman R, Olson L, Hoffer BJ (1990) Toxic effects of lead on neuronal development and function. Environ Health Perspect 89:27–33
Neal A, Guilarte T (2012) Mechanisms of heavy metal neurotoxicity: lead and manganese. J Drug Metab Toxicity S5:002
Hernandez-Avila M, Peterson KE, Gonzalez-Cossio T, Sanin LH, Aro A, Schnaas L, Hu H (2002) Effect of maternal bone lead on length and head circumference of newborns and 1-month-old infants. Arch Environ Health 57:482–488
Llanos MN, Ronco AM (2009) Fetal growth restriction is related to placental levels of cadmium, lead and arsenic but not with antioxidant activities. ReprodToxicol 27:88–92
WHO (2013) Mercury and health. Fact sheet. Geneva, Switzerland: WHO; September
Clarkson TW, Magos L (2006) The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36:609–662
Mahaffey KR, Clickner RP, Bodurow CC (2004) Blood organic mercury and dietary mercury intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environ Health Perspect 112:–562
Yorifuji T, Tsuda T, Takao S, Harada M (2008) Long-term exposure to methylmercury and neurologic signs in Minamata and neighboring communities. Epidemiology 19:3–9
Barrington JW, Lindsay P, James D et al (1996) Selenium deficiency and miscarriage: a possible link? Br J ObstetGynaecol 103:130–132
Rayman MP, Wijnen H, Vader H, Kooistra L, Pop V (2011) Maternal selenium status during early gestation and risk for preterm birth. CMAJ 183(5):549–555. https://doi.org/10.1503/cmaj.101095
Rayman MP, Bode P, Redman CW (2003) Low selenium status is associated with the occurrence of the pregnancy disease preeclampsia in women from the United Kingdom.Br J Obstet Gynaecol 189:1343–1349
Morris JS, Crane SB (2013) Selenium toxicity from a misformulated dietary supplement, adverse health effects, and the temporal response in the nail biologic monitor. Nutrients 5(4):1024–1057. https://doi.org/10.3390/nu5041024
Helzlsouer K, Jacobs R, Morris JS (1985) Acute selenium intoxication in the United States. Fed. Proc. (FASEB). 44, 7366
Vinceti M, Mandrioli J, Borella P, Michalke B, Tsatsakis A, Finkelstein Y (2014) Selenium neurotoxicity in humans: bridging laboratory and epidemiologic studies. Toxicol Lett 230(2):295–303
Grosicki A, Kowalski B (2002) Lead, cadmium and mercury influence on selenium fate in rats. Bull Vet İnst Pulawy 46:337–343
Whanger PD (1992) Selenium in the treatment of heavy metal poisoning and chemical carcinogenesis. J Trace Elem Electrolites Health Dis 6:209–221
Ganther HE (1980) Interactions of vitamin E and selenium with mercury and silver. Ann N Y Acad Sci 355:212–226
El-Sharaky AS, Newairy AA, Badreldeen MM, Eweda SM, Sheweita SA (2007) Protective role of selenium against renal toxicity induced by cadmium in rats. Toxicology 235(3):185–193
Vogel JP, Lee AC, Souza JP (2014) Maternal morbidity and preterm birth in 22 lowand middle-income countries: a secondary analysis of the WHO Global Survey dataset. BMC Pregnancy Childbirth 14:56
Howson CP, Kinney MV, Lawn J. (2012) Born too soon: the global action report on preterm birth. March of Dimes, PMNCH, Save the Children, WHO
Matusiewicz H (2003) Wet digestion methods. In: Mester Z, Sturgeon R (eds) Comprehensive analytical chemistry. Volume 41: sample preparation for trace element analysis. Elsevier, Netherlands, pp 193–233
Sharma T, Dev Banerjee B, Yadav CS, Gupta P, Sharma A (2014) Heavy metal levels in adolescent and maternal blood: association with risk of hypospadias. ISRN Pediatr 4:714234
Nazemi L, Shariat M, Chamari M, Chahardoli R, Asgarzadeh L, Seighali F (2015) Comparison of maternal and umbilical cord blood selenium levels in low and normal birth weight neonates. J Family Reprod Health 9(3):125–128
Iranpour R, Zandian A, Mohammadizadeh M, Mohammadzadeh A, Balali-Mood M, Hajiheydari M (2009) Comparison of maternal and umbilical cord blood selenium levels in term and preterm infants. Zhongguo Dang Dai Er Ke Za Zhi 11(7):513–516
Tsuji M, Shibata E, Morokuma S, Tanaka R, Senju A, Araki S, Sanefuji M, Koriyama C, Yamamoto M, Ishihara Y, Kusuhara K, Kawamoto T, Japan Environment & Children’s Study Group (2018) The association between whole blood concentrations of heavy metals in pregnant women and premature births: the Japan Environment and Children’s Study (JECS). Environ Res 166:562–569. https://doi.org/10.1016/j.envres.2018.06.025
Karaer A, Tuncay G, Tanrıkut E, Ozgul O (2018) Blood cadmium concentrations in women with ectopic pregnancy. Biol Trace Elem Res 184:42–46
Li J, Wang H, Hao JH et al (2017) Maternal serum lead level during pregnancy is positively correlated with risk of preterm birth in a Chinese population. Environ Pollut 227:484–489. https://doi.org/10.1016/j.envpol.2017.05.009
Zhang B, Xia W, Li Y et al (2015) Prenatal exposure to lead in relation to risk of preterm low birth weight: a matched case-control study in China. Reprod Toxicol 57:190–195. https://doi.org/10.1016/j.reprotox.2015.06.051
Xue F, Holzman C, Rahbar MH, Trosko K, Fischer L (2007) Maternal fish consumption, mercury levels, and risk of preterm delivery. Environ Health Perspect 115(1):42–47
Wai KM, Mar O, Kosaka S, Umemura M, Watanabe C (2017) Prenatal heavy metal exposure and adverse birth outcomes in Myanmar: a birth-cohort study. Int J Environ Res Public Health. 14(11). doi: https://doi.org/10.3390/ijerph14111339
Taylor CM, Golding J, Emond AM (2014) Lead, cadmium and mercury levels in pregnancy: the need for international consensus on levels of concern. J Dev Orig Health Dis 5(1):16–30
Vigeh M, Yokoyama K, Seyedaghamiri Z, Shinohara A, Matsukawa T, Chiba M, Yunesian M (2011) Blood lead at currently acceptable levels may cause preterm labour. Occup Environ Med 68(3):231–234
Menai M, Heude B, Slama R, Forhan A, Sahuquillo J, Charles MA, Yazbeck C (2012) Association between maternal blood cadmium during pregnancy and birth weight and the risk of fetal growth restriction: the EDEN mother–child cohort study. Reprod Toxicol 34:622–627
Tian LL, Zhao YC, Wang XC, Gu JL, Sun ZJ, Zhang YL, Wang JX (2009) Effects of gestational cadmium exposure on pregnancy outcome and development in the offspring at age 4.5 years. Biol Trace Elem Res 132(1–3):51–59. https://doi.org/10.1007/s12011-009-8391-0
Acknowledgments
We would like to thank Çorum Municipality. Our work was supported by the program “I have a project for Çorum” initiated by the Hitit University and the Municipality of Çorum. We would also like to thank Hitit University Scientific Technique Application and Research Center for their help in analysis on samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Before the start of the study, approval was obtained from the Ethics Committee of the Hitit University Medical Faculty in accordance with the Helsinki Declaration. Informed consent forms were obtained from the patients included in the study.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yıldırım, E., Derici, M.K., Demir, E. et al. Is the Concentration of Cadmium, Lead, Mercury, and Selenium Related to Preterm Birth?. Biol Trace Elem Res 191, 306–312 (2019). https://doi.org/10.1007/s12011-018-1625-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1625-2