Abstract
Recently, several attempts have been made to use the phytopharmaceuticals from plant extracts as reducing, capping and stabilizing agents for the biomimetic synthesis of various metal nanoparticles conjugated to the phytopharmaceuticals. These biogenic metal nanoparticles are non-toxic and can be used as contrast agents, drug delivery vehicles and photothermal agents for cancer therapy. Herein, we report the synthesis of both silver and gold nanoparticles using the pollen extract of Phoenix dactylifera (Date Palm), characterization using UV-visible spectroscopy, scanning electron microscopy and energy dispersive X-ray spectroscopy, quantitation of phytochemicals capping the nanoparticles using Folin – Ciocalteu’s method, cytotoxicity studies on MCF-7 breast cancer cells, cancer cell death analysis using fluorescent microscopy, and modulation of expression of the pro-apoptotic p53 and anti-apoptotic Bcl-2 proteins. The biosynthesis resulted in stable and poly-dispersed silver nanoparticles and gold nanoparticles, exhibiting strong and broad surface plasmon absorption peaks. The elemental analysis confirmed the presence of gold and silver of high purity and also the organic moieties from the plant extract acting as capping and stabilizing agents. The biogenic nanoparticles also exhibited dose-dependent cytotoxicity on MCF-7 cells and showed signs of apoptotic cell death. Immunoassays revealed the upregulation of the pro-apoptotic protein p53 and down-regulation of the anti-apoptotic protein Bcl-2 after the nanoparticle treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a condition in which a normal cell of the body transforms into an impaired cell exhibiting unregulated growth to form a mass of tissue called tumor that could be benign, which does not spread to other parts of the body, or could be malignant in which the tumor invades nearby tissues through the blood or lymph systems in a process called metastasis and destroys the other healthy living tissues. During the past few decades, cancer continues to remain as one of the leading cause of death worldwide and accounts for 8.2 million deaths in 2012. In women, breast cancer is the most frequent cause of cancer death in less developed countries (324,000 deaths) and the second cause of cancer death in more developed countries (198,000 deaths) after lung cancer [1]. Thus, treatment of cancer using a multi-disciplinary holistic approach that involves one or more tactics such as chemotherapy, immunotherapy, stem cell transplantation, hyperthermia, and complementarity and alternative medicine have been explored to identify the most promising, elaborate and systematic cancer management scheme.
Herbal medicine or phytomedicine is one of the approaches to prevent, manage, and treat cancer that focusses on the naturally occurring chemical substances from plants for improving health conditions, with fewer side effects in comparison with chemotherapy. Many of the allopathic drugs used today have pharmaceutically active substances derived from medicinal plants. These pharmaceutically active bioorganic phytochemicals modify various cellular processes associated with cancers and help in the cancer prevention and treatment. However, the limited bioavailability of the herbal drugs attributable to their poor lipid solubility, increased systemic clearance of the phytochemical agent demanding repeated administration or a higher dosage, restricted entry of the bioflavonoids across the epithelial barrier of the intestine because of their high molecular weight and due to action of drug efflux pumps like P-glycoprotein, makes the herbal drugs not very suitable for in vivo medical applications [2,3,4].
Nanotechnology is a modern field of science that has applications in the diverse fields of science. Particularly, nanotechnology appears to be a promising field in the area of medicine, where surface functionalized nanoparticles have been developed as a delivery vehicle to carry diagnostic and therapeutic agents directly to the specific cell. The emerging field of nanomedicine, which involves the usage of nanoparticles as drug delivery systems to potentiate the therapeutic action of a phytochemical agent, has been found to have myriad applications in cancer therapy.
In recent years, many efforts have been made to synthesize noble metal (particularly gold and silver) nanoparticles using plant extracts and to use these biogenic nanoparticles [5] to increase the bioavailability of the anti-cancerous plant metabolites in the tumor cells. Green synthesized silver nanoparticles (SNPs) using Podophyllum hexandrum leaf extract was found to initiate cancer cell death in human cervical cancer cell line (HeLa) by increasing intracellular ROS accumulation, DNA damage, and apoptosis [6]. Saponin rich leaf extracts of Albizia adianthifolia were used for the synthesis of SNPs, which was found to induce cell death in human lung carcinoma cell line via mitochondria mediated intrinsic apoptosis [7]. When compared to the extract of Phyllanthus emblica (Amla), the SNPs biosynthesized using Amla extract showed better ROS generation, DNA damage, signs of apoptosis, and oxidative stress on laryngeal carcinoma Hep2 cell line [8]. The gold nanoparticles (GNPs) synthesized using phytochemicals of tea had shown significant internalization of the non-toxic GNPs into prostate and breast cancer cells [9]. GNPs green synthesized from Bauhinia tomentosa Linn. showed significant in vitro anti-cancer activity when tested against Hep-2 laryngeal carcinoma cells [10]. Thus, nanotechnology can be used as an active tool for the delivery of anti-cancerous phytochemical substances into the cancer cells for effective destruction (Fig. 1).
Date palm (Phoenix dactylifera L.), which belongs to the Palmae family, is one of the oldest cultivated crop. Date palm trees have a great spiritual and cultural influence on the lives of large human population of the desert regions and had been referred to as “tree of life” that provided all the life necessities, including food, fuel, medicine, and construction material to the people [11, 12].
Bedouin Arabs in the Middle East had used various parts of date palm tree such as fruits, leaves, seeds, pollen, spathe, and bark as a folk remedy for ague, anemia, asthma, bronchitis, cancer, catarrh, condylomata, cough, diarrhea, fatigue, fever, flu, gonorrhea, endurations, longevity, piles, pterygia, splenitis, sterility, stomach ache, thirst, toothache, tuberculosis, urogenital ailments, vaginitis, virility, warts, and whitlows [13]. In his survey of plants used against cancer, Hartwell mentions that date palm tree has been used for the treatment of cancers or tumors of the abdomen, gum, liver, mouth, parotids, spleen, stomach, testicle, throat, uterus, and viscera [14]. Studies have shown that consumption of date fruits on a regular basis is associated with extremely low incidence rate of cancers and heart diseases [15]. Date palm is also found to possess antiviral, antifungal, antioxidant, anti-hyperlipedemic, and hepatoprotective activity [16]. The abovementioned medicinal properties are attributed to the rich contents of phytochemicals of date palm.
A preliminary phytochemical screening study of the different date palm tissues, including shoot tip, pollen grain, leaves, fruits, callus, embryos, and in vitro leaf tissue, revealed the presence of carbohydrates, alkaloids, steroids, flavonoids, and tannins [17]. The date palm fruit pulp is wealthy in phytochemicals like sterols, phenolics (p-coumaric, ferulic, and sinapic acids), carotenoids, anthocyanins, flavonoids (flavonoid glycosides of luteolin, quercetin, and apigenin), and procyanidins [18,19,20,21,22,23]. Numerous studies have shown the presence of secondary plant metabolites having medicinal value such as alkaloids, steroids, fatty acids, triterpenoids, flavanoids, and phenolic compounds in the date palm seed [24]. Phytochemical screening of dried date palm pollens revealed the presence of sterols, triterpenes, saponins, proteins, carbohydrates, and glycosides [25,26,27,28,29,30,31,32,33]. Abbas et al. [34] were able to isolate estradiol, esteriol, estrone, and novel flavonoids from date palm pollen extract using column chromatography technique.
Date palm tree is found to have numerous antioxidants and other cancer-fighting phytochemicals. Consequently, a number of attempts have been made to synthesize nanoparticles using extracts of various parts of antioxidant rich date palm tree, as these phytochemical coated nanoparticles facilitate the effective delivery of bioflavonoids directly into the cancer cells, overcoming the drawbacks of in vivo delivery of phytochemicals and without causing any toxicity to the healthy living cells [35,36,37,38,39]. Successful synthesis of metal nanoparticles has been achieved with extracts of date palm seeds [35, 37], leaves [36, 38], and pollen grains [39].
Our research is based on the green chemistry approach for the synthesis of both gold and silver metal nanoparticles using the extract of date palm pollen grain, which is rich in the phytochemicals. These phytochemicals acts as reducing agents and stabilizing agents for the bioreduction of ionic silver and gold to nanoparticles. The silver nanoparticles synthesized using the date palm pollen extract (DPP-SNPs) and the gold nanoparticles synthesized using the date palm pollen extract (DPP-GNPs) were characterized using UV-visible spectrophotometry, scanning electron microscopy (SEM), and energy dispersive X-ray spectroscopy (EDX); the phytochemicals capping the nanoparticles were quantitated using Folin–Ciocalteu’s method; and their anti-cancer activity was studied using MCF-7 breast cancer cell line. Furthermore, the mechanism of cell death and the modulation of levels of p53 and Bcl2 protein expressions in the cancer cells after treatment with the metal nanoparticles had been studied using fluorescent microscopy and immunoassays, respectively.
Results
Biosynthesis of DPP-GNPs and DPP-SNPs Evident by Visually Observed Color Change
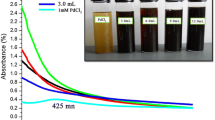
After the addition of the prepared extract from date palm pollen to hydrogen tetrachloroaurate solution, the biosynthesis of DPP-GNPs was apparent as the color of the solution quickly began to change to reddish purple (Fig. 2a), indicating the formation of DPP-GNPs. Similarly, the addition of the prepared date palm pollen extract to silver nitrate solution resulted in the change of the colorless solution to yellowish brown color (Fig. 2b) within a short period of time due to the conversion of silver ions to SNPs [40, 41].
a Synthesis of DPP-GNPs using date palm pollen extract: The figure represents DPP-GNPs exhibiting reddish purple color in solution due to excitation of surface plasmon vibrations, indicating the formation of DPP-GNPs. b Biosynthesized DPP-SNPs: formation of SNPs indicated by the yellowish brown color originating from their surface plasmon resonance characteristics
Analysis of Absorption Spectra of DPP-GNPs and DPP-SNPs by Characterization Using UV-Visible Spectroscopy
In general, the absorption spectra of the synthesized DPP-GNPs can range from 500 nm to the near infrared part of the spectrum for the GNPs of different shapes [25]. The UV-visible absorption spectrum, measured after 2 days of the biosynthesis, showed a characteristic surface plasmon resonance peak at 659 nm showing the presence of large GNPs (Fig. 3a). In addition to the surface plasmon resonance peak at 659 nm, the GNP solution also showed an additional peak at 289 nm after 2 days of nanoparticle synthesis, indicating the presence of unreduced chloroaurate ions in the solution [42, 43]. However, the measurement of absorption spectra after 4 weeks resulted in a single-absorption peak at 659 nm, indicating the complete reduction of gold ions into DPP-GNPs.
a Ultraviolet-visible spectra of DPP-GNPs, biosynthesized using date palm pollen extracts, measured after 2 days [A] and after 4 weeks [B] of synthesis. Both, the UV-visible spectra [A] and [B] has the strong and broad absorption peaks at wavelengths 659 nm, corresponding to the surface plasmon resonance peak of the DPP-GNPs of increased particle size. The absorbance peak of the DPP-GNPs around 289 nm in spectrum [A] denotes the presence of unreduced chloroaurate ions in the solution; and the absence of this peak in spectrum [B] signifies the complete reduction of chloroaurate ions to GNPs. b UV-visible absorption spectra of DPP-SNPs, biosynthesized using date palm pollen extracts, measured after 2 days [A] and after 4 weeks [B] of synthesis. Both the UV-visible spectra [A] and [B] have the strong and broad absorption peaks at wavelengths 470 nm, attributable to the surface plasmon resonance peak of the formed DPP-SNPs. The absorbance peak of the DPP-GNPsat around 301 nm in spectrum [A] denotes the presence of unreduced silver ions in the solution; and the absence of this peak in spectrum [B] signifies the complete reduction of silver ions to DPP-SNPs
The silver nanoparticle solution when subjected to UV spectrophotometric analysis, after 2 days of synthesis, exhibited an observable peak at 470 nm corresponding to the surface plasmon resonance peak of the formed colloidal SNPs as represented in Fig. 3b [44]. The sharp peak obtained at 470 nm is representative of the formed SNPs, and the second peak observed at 301 nm is due to purely ionic silver that is unreduced, which eventually disappeared after 4 weeks of synthesis due to complete reduction of ionic silver to SNPs [45].
Size, Shape, and Morphological Study of Gold and Silver Nanoparticles by SEM
The results of SEM analysis confirmed the presence of high-density silver and gold nanoparticles in the nanoparticle suspensions. SEM images clearly indicated the presence of poly-dispersed and largeDPP-GNPs as shown in Fig. 4a; and extremely small, irregularly shaped and poly-dispersed DPP-SNPs as shown in Fig. 4b.
a The analysis of size and morphology of the biosynthesized DPP-GNPs by SEM. The SEM micrograph confirmed the presence of poly-dispersed and largegold nanoparticles. All the biosynthesized DPP-GNPs seemed to be almost spherical in shape with an average size of about 95 nm in diameter. b The biosynthesized DPP-SNPs, which were extremely small in size, irregularly shaped and poly-dispersed. All the biosynthesized DPP-SNPs seemed to be almost spherical in shape with an average size of about 27 nm in diameter
Most of the synthesized gold and silver nanoparticles seemed to be almost spherical in their morphology and the average size of the gold and silver nanoparticles measured using the SEM image was about 95 and 27 nm in diameter, respectively. The size distribution of the synthesized metal nanoparticles was determined by analyzing the diameters of about 460 DPP-GNPs (Fig. 5a) and 414 DPP-SNPs (Fig. 5b) from the SEM micrograph. The size distribution curve also illustrates the steric stabilization of the nanoparticles due to the chemisorption of the macromolecules of the plant extract, which acts as surface capping agents.
Elemental Analysis of Gold, Silver, and Other Elements Using EDX
The EDX spectra of the gold and silver nanoparticle suspensions showed prominent characteristic peaks at around 2.1 keV (Fig. 6a) and at around 3 keV (Fig. 6b), corresponding to the elemental gold and silver, respectively [46, 47]. Additionally, both the silver and gold EDX spectra exhibit weaker signal peaks at C, N, and O representing the elements from the organic moieties from the date palm pollen extract.
a The EDX analysis of the GNP suspension revealed a strong signal for gold at about 2.1 KeV and weaker signals for C, N, and O, representing the elements from the organic moieties from the date palm pollen extract. b The EDX analysis of the biosynthesized DPP-SNPs showed a strong signal for silver at about 3 keV and weaker signals for C, N, and O, representing the elements from the organic moieties from the date palm pollen extract
Quantitation of Date Palm Pollen Phytochemicals
Capping the Nanoparticles Using Folin–Ciocalteu’s Method
The bioflavonoids from the date palm pollen extract, acting as reducing, capping, and stabilizing agents on the surface of the metal nanoparticles, were quantitated by the Folin–Ciocalteu’s method, using the bioflavanoid quercetin as standard. The concentrations of bioflavonoids of date palm pollen capping the gold and silver nanoparticles were found to be 0.674 and 0.317 μg/ml quercetin equivalents, respectively (Fig. 7).
In Vitro Anti-Cancer Studies of Biosynthesized Gold and Silver Nanoparticles on Breast Cancer Cell Line
The MTT results demonstrated a marked reduction in the rate of cell viability with the increase in the concentration of metal nanoparticles after 48 h of nanoparticle exposure. The cell viability was significantly reduced, after the metal nanoparticle treatment, in a dose-dependent manner, i.e., 100, 85.80, 66.71, 44.96, 29.24, and 21.58% cell viability for the gold nanoparticle at a concentration of 0, 1.25, 2.50, 5, 10, and 20 μg/ml (Fig. 8a), and 100, 73.86, 60.77, 46.0, 37.28, and 24.17% cell viability for the DPP-SNPs at a concentration of 0, 2.50, 5, 10, 20, and 40 μg/ml (Fig. 8b).The median inhibitory concentrations of the gold and silver nanoparticles were found to be 4.76 and 9.06 μg/ml, respectively. The experimental values represent the average of three duplicates.
a The DPP-GNPs exhibited a significant dose-dependent cytotoxicity on the breast cancer cell lines having a percentage cell viability of 100, 85.80, 66.71, 44.96, 29.24, and 21.58 at a concentration of 0, 1.25, 2.50, 5, 10, and 20 μg/ml, respectively. b The DPP-SNPs also exhibited cytotoxic effects on the MCF-7 breast cancer cells in a dose-dependent manner with a cell viability of 100, 73.86, 60.77, 46.0, 37.28, and 24.17% at a concentration of 0, 2.50, 5, 10, 20, and 40 μg/ml, respectively
Analysis of Signs of Apoptosis/Necrosis Using Fluorescent Microscopy
After the exposure of MCF-7 breast cancer cells to the metal nanoparticles for about 48 h, the morphological alterations in the stained cancer cells were analyzed to examine the mechanism of cancer cell death. The fluorescent microscopic images after GNP treatment (Fig. 9a) and after SNP treatment (Fig. 9b), revealed distinct morphological changes including signs of apoptosis such as cell shrinkage, depolymerization of plasma membrane and plasma membrane blebbing.
a After the GNP treatment, morphological alterations in the cancer cells with the fluorescent microscopic images showing signs of apoptosis such as depolymerisation of plasma membrane. b The fluorescent microscopic images of the cancer cells after being treated with DPP-SNPs showing morphological alterations such as cell blebbing, which are signs of apoptosis
Modulation of p53 and Bcl-2 Protein Expressions After Combination Treatment
The alteration in the levels of gene expression of the apoptosis associated proteins p53 and Bcl-2 was studied in MCF-7 breast cancer cell line in response to the gold and silver nanoparticle treatments. In response to the GNP treatment, the levels of expression of pro-apoptotic protein p53 were found to rise from 9.60 to 24.67 U/ml (Fig. 10a), whereas the levels of expression of the anti-apoptotic protein Bcl-2 was found to drop from 18.51 to 13.30 ng/ml (Fig. 10b). Similarly, in response to the silver nanoparticle treatment, the levels of expression of pro-apoptotic protein p53 was found to rise from 9.60 to 15.73 U/ml (Fig. 10a), whereas the levels of expression of the anti-apoptotic protein Bcl-2 was found to drop from 18.51 to 17.16 ng/ml (Fig. 10b).
a The gene expression levels of the pro-apoptotic protein p53 was found to increase about 2.57-folds and 1.64-folds in response to the biosynthesized GNP and SNP treatments, respectively. b The biosynthesized DPP-GNPs and DPP-SNPs were found to reduce the gene expression of the anti-apoptotic protein Bcl-2 in MCF-7 cells by about 1.40-folds and 1.08-folds, respectively
Discussion
The antioxidant and antiproliferative activities exhibited by various parts of the date palm, such as fruits, seeds, and pollen, makes them very attractive for cancer prevention and treatment. Numerous studies have been performed on the antioxidant property of date palm fruits from various parts of the world and date fruit is found to be a potent chemo-preventive agent [20, 48,49,50,51]. Al-Shoaibi et al. [52]recommends the consumption of date palm fruits as they report evident in vivo antioxidant and hepatoprotective actions after the administration of palm date syrups (rich in phenolics and flavonoids) to rabbits having liver injuries. Date seed oil is found to possess chemoprotective effect as it can limit oxidative injuries induced by hydrogen peroxide in human skin organ culture [53]. Date palm pollen extract has been found to exhibit anti-inflammatory and antiproliferative activities when tested on experimentally-induced atypical prostatic hyperplasia in rats [54].
In our study, we have used the phytochemicals from the date palm pollen water extract to synthesize gold and silver nanoparticles for use in cancer treatment. We believe that the exposure of gold and silver ions to the bioorganic compounds present in the date palm pollen extract is responsible for the reduction of metal ions to their corresponding metal nanoparticle, as the date palm pollen is known to possess several phytochemical substances that can act as biological reducing agents [25, 34, 54]. The DPP-GNPs when formed quickly changed the yellow color of the gold chloride solution to reddish purple upon addition of the colorless date palm pollen water extract indicating the formation of GNPs. Likewise, the silver nanoparticle formation was indicated by the transformation of the colorless silver nitrate solution to yellowish brown. In the case of silver and gold nanoparticles, the conduction and the valence bands lie very close to each other, where the freely moving electrons in the conduction band leads to the formation of surface plasmon resonance that is associated with the shape, size, and the dielectric constant of the synthesized nanoparticles. The observed color change of the solution occurs on account of the plasmon resonance absorption of visible light spectrum [40].
After 2 days of biosynthesis, the GNP solution during the UV spectrophotometric analysis exhibited peaks at 659 and 515 nm. The peak at 659 nm corresponds to the plasmon absorption of large DPP-GNPs which are formed. Mie theory states that the surface plasmon peak of the large nanoparticles is red shifted that occurs towards the infrared region with the increase in the particle size [55]. Therefore, the increased particle size of the GNP could be the reason behind the peak at 659 nm, which is found to be almost red shifted to the infrared region. Moreover, the shape of the nanoparticle is also known to have an influence on the surface plasmon peak. Spherical nanoparticles are found to exhibit absorption peak at 515–570 nm, whereas irregular-shaped particles have absorption peak in the near infrared region of the spectra. Thus, the strong NIR absorption peak at 659 nm indicates the formation of DPP-GNPs with increased particle size and irregular shape.
The additional peak at 289 nm exhibited by the GNP solution is mainly due to the unreduced chloroaurate ions in the solution, as the absorption peak at wavelength range of 200–300 nm denotes residues of unreacted gold chloride [42, 43]; and this peak apparently disappeared when the absorption spectra is measured after 4 weeks of biosynthesis, indicating the complete reduction of gold ions into GNPs.
The absorption peaks exhibited by DPP-SNPs at 470 and 301 nm corresponds to the formed colloidal SNPs and unreduced ionic silver, respectively. Alike the GNP solution, the additional peak disappeared after 4 weeks of the biosynthesis due to the complete reduction of silver ions into SNPs.
To give an insight into the morphology of biosynthesized metal nanoparticles, the gold and silver nanoparticle suspensions were subjected to SEM studies at various magnifications. The SEM analysis showed the presence of high-density gold and silver nanoparticles, which were poly-dispersed and irregularly shaped. However, both the gold and silver nanoparticle solutions were found to have particles, which were mostly spherical in appearance. When compared to the DPP-GNPs, the DPP-SNPs were comparatively very smaller in size.
The elemental composition analysis of the nanoparticle suspension was performed using EDX to determine its purity in terms of weight% and atom% of the metal, and to detect the presence of elements from organic moieties present in the date palm pollen extract. The results of the EDX spectra confirm that the reaction products were composed of high purity silver and DPP-GNPs without any impurities. The weaker signals for C, N, and O represent the organic moieties from the date palm pollen extract, and this is indicative of the functionalization of metal nanoparticles with the bioorganic phytochemical compounds present in the date palm pollen extract that acted as reducing, stabilizing, and capping agents of the metal nanoparticles [56].
The metal nanoparticle suspensions were stable for more than about 4 weeks without any measurable color change or any observable aggregation. The UV-visible absorption spectra of the metal nanoparticles showed no significant shift in the surface plasmon resonant peaks even after 4 weeks of nanoparticle synthesis. Altogether, the metal nanoparticle suspensions did not exhibit any signs of destabilization and flocculation. The long-term stability of the metal nanoparticles could be attributed to the fact that the phytochemicals present in the date palm pollen extract acts as capping and stabilizing agents preventing the agglomeration of the metal nanoparticles. The EDX spectra also supports this fact as weaker signal for C, N, and O elements from the organic moieties was detected in the sample, indicating the presence of stabilizing and capping bioorganic phytochemicals from the date palm pollen extract on the surface of metal nanoparticles. The prolonged stability could also be due to the surface charge of the metal nanoparticles, as the nanoparticles that are highly charged do not agglomerate as that of nanoparticles having low surface charges due to strong Coulumb repulsion [57, 58].
MTT assay was performed to study the dose-dependent cytotoxic effect of metal nanoparticles on MCF-7 breast cancer cells. The relationship between the concentration of metal nanoparticles and cell viability after 48 h was analyzed. Exposure of cell culture to higher concentrations of metal nanoparticles for a longer incubation period of 48 h generated more serious cytotoxicity in a dose-dependent manner. The DPP-GNPs on MCF-7 cancer cells exhibited a lower median inhibitory concentration when compared to the silver nanoparticle treatment. Date palm pollen extract was also directly tested for its cytotoxicity against the MCF-7 cell line and no significant cytotoxicity was observed, whereas the metal nanoparticles, having the conjugated bioorganic compounds from the date palm pollen extract, had a significant dose-dependent cytotoxicity on the breast cancer cell lines.
Numerous researches have been conducted to study the toxicity of various kinds of nanomaterials on cancer cells and stem cells, and the results have shown a dose-dependent cytotoxicity and genotoxicity. In vitro toxicity studies of reduced graphene oxide nanoplatelets (rGONPs) on human mesenchymal stem cells have shown cell destructions at concentrations of even 1 μg/ml, whereas the reduced graphene oxide sheets of comparatively bigger sizes can mediate significant cytotoxic effect only at a higher concentration of 100 μg/ml. Also, the DNA fragmentations and chromosomal aberrations were noticed at exposures of even a low concentration of 0.1 μg/mL of rGONPs [59]. Similar toxicity studies on graphene oxide and reduced graphene oxide have been carried out on spermatogonial stem cells and the results have shown that the graphene oxide increased the oxidative stress at 100 and 400 μg/ml in comparison to low concentrations that did not show any significant effect. Also, the presence of high concentrations of graphene nanosheets in cell culture medium resulted in the reduction of number of colonies and colony forming cells [60]. Likewise, the cytotoxicity and genotoxicity studies of silicon carbide nanoparticles on A549 lung epithelial cells have shown reactive oxygen species production, glutathione depletion, inactivation of some antioxidant enzymes and significant genotoxicity [61]. In a similar manner, several researches on carbon, silver, and gold nanoparticle mediated cellular response have shown a dose-dependent reduction of viability of cancer cells [62,63,64,65,66].
Researches have shown that the DPP-GNPs synthesized from non-toxic reducing agents are entirely harmless to the cells and did not show any cytotoxicity on normal healthy living cell as well as in the cancerous cells [67, 68]. Likewise, several reports have identified that SNPs when used at low levels does not cause any cytotoxicity, whereas the high concentration of SNPs is found associated with toxicity on cancerous cells [69, 70]. Therefore, the cytotoxicity associated with the DPP-GNPs could be solely attributed to the anti-cancerous bioflavanoids available on the surface of metal nanoparticles as stabilizing and capping agents, which are delivered to the cancerous cell using GNP as a vehicle, whereas the cytotoxic effect observed with the DPP-SNPs might be due to the nanoparticle surface bound bioflavonoids and also due to the toxicity of the silver nanoparticle as such. We understand that the direct date palm pollen extract treatment on cancer cells did not exhibit any significant cytotoxic activity, as the entry and bioavailability of the anti-cancerous phytochemicals of the extract into the cancer cells was very much restricted in the absence of nanoparticle as a drug delivery vehicle.
Apoptosis has been characterized by a number of evident morphological changes in a cancer cell. The apoptotic potential of the biogenic DPP-GNPs on the MCF-7 cells was analyzed using fluorescent microscopy to identify the morphological changes associated with apoptosis. After 48 h, evident signs of apoptosis such as cell shrinkage, depolymerization of plasma membrane, cell blebbing, and chromatin condensation indicated the apopotic cancer cell death [71, 72].
In order to understand more on the apoptotic cell death, the key regulatory genes associated with cancer, namely the p53 and Bcl-2 proteins, were studied. The protein p53 is pro-apoptotic in nature as the transcriptional transactivation of p53 is found to be associated with the induction of apoptosis and this protein is also believed to have an influence on the expression of other apoptosis associated proteins such as Fas and Bax [73]. The Bcl-2 protein is an anti-apoptotic protein that is over-expressed in many of the cancerous conditions. The Bcl-2 protein prevents the mitochondrial membrane permeabilization to release the pro-apoptotic molecules and inhibit the intrinsic mitochondria mediated cell death pathway [74]. The variation in the expression of the p53 and Bcl-2 protein after treatment with biogenic metal nanoparticles surface capped with bioorganic molecules of date palm pollen extract was studied. The DPP-GNPs were found to increase the levels of the p53 protein in the MCF-7 cancer cells by 2.57-folds and also result in the reduction of expression of the Bcl-2 protein by 1.64-folds. Likewise, the DPP-SNPs were found to increase the levels of the p53 protein by 1.40-folds and result in the reduction of expression of the Bcl-2 protein by 1.08-folds in the cancer cells. Thus, we understand that the mechanism of cancer cell destruction by the biogenic metal nanoparticles is by the induction of apoptosis and the modulation of gene expression of the apoptosis associated genes p53 and Bcl-2.
Experimental Procedure
Materials Used
The chemical reagents silver nitrate (> 99%), hydrogen tetrachloroaurate (III) trihydrate (> 99%), high glucose Dulbecco’s Modified Eagle’s Medium (DMEM) with l-glutamine, sodium pyruvate and sodium bicarbonate, phosphate buffered saline, 1% penicillin (100 U/ml), 1% streptomycin (100 mg/ml), 10% fetal bovine serum, trypsin EDTA, trypan blue, (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT), dimethyl sulfoxide, and high-performance liquid chromatography (HPLC) grade water were all purchased from Sigma Aldrich, USA. For measuring the human p53 and Bcl2 levels, Bcl-2 ELISA kit (catalog no. NBP1-91188) and p53 ELISA kit (catalog no. NBP1-91257), purchased from Novus Biologicals, USA were used. All of the chemicals and reagents used for the experimentation were of analytical reagent grade and used as received without further purification. HPLC grade water was used for all the reagent preparations.
Preparation of Plant Extract
Date palm pollen was identified and arranged by Dr. N.K. Rao from the International Center for Biosaline Agriculture (ICBA). Date palm pollen was collected from dried spikelets (Fig. 11), which have the male date palm flowers, by dusting and stored at 25 °C in an airtight container until further use. The date palm pollen powder (2 g) was weighed and added to 10 ml of HPLC grade water. The mixture was heated for 30 min at 60 °C. The contents were then filtered through a muslin cloth and the filtrate was centrifuged at 10,000 rpm for 10 min. The resulting supernatant was collected and stored at 4 °C until use. The clear extract of date palm pollen was used for the biosynthesis of both SNPs and GNPs.
Biosynthesis and Purification of Metal Nanoparticles
As metal nanoparticles are photosensitive, dark conditions were maintained throughout the biosynthesis experiments. To maintain the dark conditions, the entire biosynthesis was carried in a semi-dark room with the overhead lights turned off and the chemicals were prepared and stored in brown bottles. Silver nitrate (10 ml; 5 mM) was thoroughly mixed with 3 ml of date pollen extract and the mixture was then left undisturbed for 24 h at 25 °C under dark conditions to form the DPP-SNPs. DPP-GNPs were prepared by thoroughly mixing 10 ml of 1 mM hydrogen tetrachloroaurate (III) trihydrate with 1 ml of date pollen extract and keeping the mixture undisturbed for 24 h at 25 °C under dark conditions. The synthesized metal nanoparticles were centrifuged at 15,000 rpm for 10 min. They were then washed three times using HPLC grade water by repeated centrifugation and stored in dark at 4 °C until use.
Characterization of Metal Nanoparticles Using UV Spectrophotometry, SEM and EDX
In order to study the formation of metal nanoparticles, the UV-visible absorption spectra of the metal nanoparticles were measured using Shimadzu UV-1700, Pharmaspec UV-visible spectrometer; and the absorbance measurements were made over the wide wavelength range of 250–700 nm against HPLC grade water as blank. High-resolution SEM analysis of the synthesized metal nanoparticles was done using a Nova Nano SEM machine, operated at an accelerating voltage of about 5 to 15 kV. The SEM characterization was performed to analyze the structure, composition and average size of the synthesized metal nanoparticle samples after diluting, dispersing by ultrasonication for about 15 min, air drying, and mounting on silica specimen stubs. The data from the UV-visible spectroscopy and SEM were analyzed to characterize the morphology of the formed metal nanoparticles such as size, shape, and polydispersity. EDX was used to determine the elemental composition of the synthesized metal nanoparticle and the compositional data from the above experiment was analyzed to study the purity of the elements silver and gold, and to detect the presence of elements from organic moieties present in the extract.
Quantitation of Bioflavanoids
The bioflavanoids capping the metal nanoparticles were estimated by the Folin-Ciocalteu’s method. The concentration of the phytochemicals was expressed in terms of the quercetin equivalents.
Cell Line and Cell Culture
The human breast cancer cell line MCF-7 was used to test the cytotoxic effects of the synthesized metal nanoparticles. They were cultured using high glucose DMEM, which was supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin. The cell culture was maintained in a humidified atmosphere at 37 °C in the presence of 5% carbon dioxide. The cell lines were sub-cultured twice a week after 85% confluency was achieved, and the flasks having more than 85% of these confluent cells were used for the experimentation.
Screening Cytotoxic Effect of Metal Nanoparticles on Cancer Cells
The cytotoxic effect of the biosynthesized metal nanoparticles on cancer cells can be examined using the quantitative MTT calorimetric assay, which determines the cell viability by measuring the purple formazan product formed by the reduction of tetrazolium dye by the living cells [75]. The cultured MCF-7 cells were trypsinized and seeded on a 96 well microtitre plate with a seeding density of 5000 cells per well. The plate was then left to incubate for 24 h at 37 °C for attachment of cell lines to the plate. Then, 100 μl of the media having the DPP-GNPs at a concentration of 1.25, 2.50 and 5.0 μg/ml, and DPP-SNPs at a concentration of 5, 10, and 20 μg/ml were added to the wells and incubated. After 48 h, the viability of the cells was tested using the MTT assay following the standard protocol. Briefly, 20 μl of MTT was added to each well and incubated for about 4 h and 100 μl of DMSO was added after decanting the contents of the well to solubilize the formed formazan crystals. The absorbance of the samples was recorded at 570 nm to calculate the cell viability.
Fluorescent Microscopic Study to Analyze Mechanism of Cell Death
After the treatment of the MCF-7 cancer cells with the GNP, the fluorescence microscopic investigations were performed to study the alterations in the morphology of the cancer cells in response to the nanoparticle treatment. Briefly, the MCF-7 cells that were cultured for about 24 h were exposed to the DPP-GNPs at a concentration of 5 μg/ml and DPP-SNPs at a concentration of 20 μg/ml; and were stained with 1.5% Nile blue followed by incubation at 37 °C for 45 min, PBS wash and storage at 4 °C for 12 h. The stained cells were observed under Nikon Eclipse 80i fluorescence microscope to observe the significant cellular morphological changes, including signs of apoptosis and necrosis, in the MCF-7 cells after the nanoparticle treatment.
Estimation of Levels of p53 and Bcl-2 Using Immunoassays
The modulation of expression of the pro-apoptotic gene p53 and anti-apoptotic gene Bcl-2 can be measured using the immunoassays. After exposure of the MCF-7 breast cancer cells to the metal nanoparticles for 24 h, the cell suspension was collected and the cell culture supernatants after centrifugation were transferred to microwell plates coated with p53 monoclonal antibody. Then, biotin-conjugated anti-human p53 monoclonal antibody, streptavidin-horseradish peroxidase (HRP), and the chromogenic substrate tetramethylbenzidine (TMB) were added to the microwells. The absorbance of the colored solution was measured at 450 nm to quantitate the p53 protein expression. In the same manner, the expression of Bcl-2 protein was screened by adding the MCF-7 cell lysates, after treatment with nanoparticles, to microwell plates coated with Bcl-2 monoclonal antibody, and then, adding the biotin-conjugate anti-human Bcl-2 monoclonal antibody, streptavidin-HRP, and TMB followed by measuring the absorbance of the colored solution at 450 nm. A standard curve was made using the known concentrations of the p53 and Bcl-2 proteins; and the quantitation of the p53 and Bcl-2 proteins after the nanoparticle treatment was determined by interpolating from the standard curve in comparison with the control.
Summary and Conclusions
Ionic gold and silver were reduced using the biological reducing agents present in the extract of date palm pollen to form the biogenic gold and silver nanoparticles surface capped with biomolecules from the extract that were characterized using techniques such as UV-visible spectroscopy, SEM, and EDX. Furthermore, the cytotoxicity of the metal nanoparticles were tested on MCF-7 breast cancer cells and the results showed that the metal nanoparticles had a significant dose-dependent cytotoxicity, whereas the date palm pollen extract showed no significant cytotoxicity on the breast cancer cells as we believe that the anti-cancerous phytochemicals were not delivered into the cancer cells. Researches show that the metal nanoparticles when synthesized using non-toxic chemicals are almost entirely harmless, especially when used at low dosages. So, the cancer cell destruction could be attributed to the anti-cancerous bioflavanoids available on the surface of metal nanoparticles as stabilizing and capping agents, which are delivered to the cancerous cell using GNP as a drug delivery vehicle. Also, the mechanism of cancer cell death is by apoptotic pathway via the modulation of gene expression of the pro-apoptotic protein p53 and the anti-apoptotic protein Bcl-2.
References
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (Accessed august 2014), GLOBOCAN 2012 v1.0, http://globocan.iarc.fr/Default.aspx
Ansari SH, Islam F, Sameem M (2012) Influence of nanotechnology on herbal drugs: a review. J Adv Pharm Technol Res 3:142–146
Scheepens A, Tan K, Paxton JW (2010) Improving the oral bioavailability of beneficial polyphenols through designed synergies. Genes Nutr 5:75–87
Kesarwani K, Gupta R (2013) Bioavailability enhancers of herbal origin: an overview. Asian Pac J Trop Biomed 3:253–256
Bhadoriya SS, Mangal A, Madoriya N, Dixit P (2011) Bioavailability and bioactivity enhancement of herbal drugs by “nanotechnology”: a review. J Curr Pharm Res 8:1–7
Jeyaraj M, Rajesh M, Arun R, Mubarak Ali DB, Sathishkumar G, Sivanandhan G, Deva GK, Manickavasagam M, Premkumar K, Thajuddin N, Ganapathi A (2013) An investigation on the cytotoxicity and caspase-mediated apoptotic effect of biologically synthesized silver nanoparticles using Podophyllum hexandrum on human cervical carcinoma cells. Colloids Surf B Biointerfaces 102:708–717
Govender R, Phulukdaree A, Gengan RM, Anand K, Chuturgoon AA (2013) Silver nanoparticles of Albizia adianthifolia: the induction of apoptosis in human lung carcinoma cell line. J Nanobiotechnol 11:5. https://doi.org/10.1186/1477-3155-11-5
Rosarin FS, Arulmozhi V, Nagarajan S, Mirunalini S (2012) Antiproliferative effect of silver nanoparticles synthesized using amla on Hep2 cell line. Asian Pac J Trop Biomed 6:1–10
Nune SK, Chanda N, Shukla R, Kavita K, Kulkarni RR, Thilakavathi S, Mekapothula S, Kannan R (2009) Katti KV (2009) green nanotechnology from tea: phytochemicals in tea as building blocks for production of biocompatible gold nanoparticles. J Mater Chem 19:2912–2920
Mukundan D, Mohankumar R, Vasanthakumari R, ICAN (2014) Green synthesis of gold nanoparticles using leaves extract of Bauhinia tomentosa Linn and in vitro anticancer activity. Int Conf Adv New Mater 2:375–380
Zohary D, Hopf M (2000) Domestication of plants in the old world: the origin and spread of cultivated plants in West Asia, Europe and the Nile Valley. Third edition. Oxford University Press, Oxford
Nixon RW (1951) The date palm: “tree of life” in subtropical deserts. Econ Bot 5:274–301
Duke JA, Wain KK (1981) Medicinal plants of the world. In: Computer index with more than 85,000 entries 3 volumes Plant Genetics and Germplasm Institute. Agricultural Research Service, Beltsville, Maryland
Hartwell JL (1967–1971) Plants used against cancer a survey. Lloydia, p 30.
El-Sohaimy SA, Hafez EE (2010) Biochemical and nutritional characterizations of date palm fruits (Phoenix dactylifera L). J App Sci Res 6:1060–1067
Al-Farsi MA, Lee CY (2008) Nutritional and functional properties of dates: a review. Crit. Rev Food Sci Nutr 48:877–887
Mohamed SM, Bosila HA, El-Sharabsy SF, Ibrahim IA, Refay KA (2001) Phytochemical screening of some in vivo and in vitro date palm tissues. The Second International Conference on Date Palms, Al-Ain, UAE, March 25-27, p 87.
Abdul A, Allaith A (2008) Antioxidant activity of Bahraini date palm (Phoenix dactylifera L) fruit of various cultivars. Int J Food Sci Tech 43:1033–1040
Ahmed IA, Ahmed A, Robinson RK (1995) Chemical composition of date varieties as influenced by the stage of ripening. Food Chem 54:305–309
Mansouri A, Embarek G, Kokkalou E, Kefalas P (2005) Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera). Food Chem 89:411–420
Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, Prior RL (2003) Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J Agric Food Chem 51:7513–7521
Hong YJ, Tomas-Barberan FA, Kader AA, Mitchel AE (2006) The flavonoid glycosides and procyanidin composition of Deglet Noor dates (Phoenix dactylifera). J Agric Food Chem 54:2405–2411
Biglari F, Alkarkhi AFM, Easa AM (2008) Antioxidant activity and phenolic content of various date palm (Phoenix dactylifera) fruits from Iran. Food Chem 107:1636–1641
Boukouada M, Yousfi M (2009) Phytochemical study of date seeds lipids of three fruits (Phoenix dactylifera L) produced in Ouargla region. Annales de la Faculté des Sciences et Sciemces de I’Ingénieur 1:66–74
Mahran GH, Abdel-Wahab SM, Athan AM (1976) A phytochemical study of date palm pollen. Planta Med 29:171–175
Bosila HA, Mohamed SM, Ibrahim SIA, Refay KA (2001) Phytochemical screening of some in vivo and in vitro date palm tissue. The Second International Conference on Data Palms, Al-Ain, United Arab Emirates, March 25–27.
Al-Samarai AH, Al-Salihi FG, Al-Samarai RR (2016) Phytochemical constituents and nutrient evaluation of date palm (Phoenix dactylifera, L) pollen grains. Tikrit Journal of Pure Science 21(1):2016
Hassan HM (2011) Chemical composition and nutritional value of palm pollen grains. Global journal of biotechnology and Biochemistry 6(1):1–7
Mahran GH, Abdul-Wahab SM, Attia AM (1985) Constituents of the Egyptian date palm pollen: Saponin and lipid constituents of pollen grains. First International Conference, Vol I, Zag University.
Bosila HA, El-Sharabasy SF, Mohamed SM, Ibrahim SIA, Refay KA (2001) Production of some secondary products from date palm tissue cultures (Sewi cultivar) using some precursors I. Callus stage. Second International Conference on Date Palms, Al Ain, United Arab Emirates March 25–27.
Bacha MA, Ali MA, Farahat FA (1997) Chemical composition of pollen grains of some date palm males grown in Riyadh, Saudi Arabia. Arab Gulf J Sci Res 15(3):783–803
Abed AM (2005) Determination of carbohydrates, protein, phenolic compounds content in pollen grains of three date palm (Phoenix dactylifera) male cultivars. Basrah, J Date Palm Res 4(2):141–151
Campos MG, Bogdanov S, Almeida LB, Szczesna T, Mancebo Y, Frigerio C, Ferreira F (2008) Pollen composition and standardization of analytical methods. J Agri Res bee worlds 47(2):156–163
Abbas FA, Ateya AM (2011) Estradiol, esteriol, estrone and novel flavonoids from date palm pollen Aust. J App Sci Res 7:606–614
Dash SS, Sikder AK, Bag BG, Bandyopadhyay S (2013) Phoenix dactylifera (Date Palm) seed extract mediated green synthesis of gold nanoparticles and its application as a catalyst for the reduction of 4-nitrophenol to 4-aminophenol Int. J Nanomat Biostr 3:42–46
Zayed MF, Eisa WH (2014) Phoenix dactylifera L leaf extract phytosynthesized gold nanoparticles; controlled synthesis and catalytic activity. Spectrochim Acta A Mol Biomol Spectrosc 121:238–244
Khatami M, Pourseyedi S (2015) Phoenix dactylifera (date palm) pit aqueous extract mediated novel route for synthesis of high stable AgNPs with high antifungal and antibacterial activity. IET Nanobiotechnol 9:1–7
Aitenneite H, Abboud Y, Tanane O, Solhy A, Sebti S, El Bouari A (2016) Rapid and green microwave-assisted synthesis of silver nanoparticles using aqueous phoenix Dactylifera L (date palm) leaf extract and their catalytic activity for 4-Nitrophenol reduction. J Mater Environ Sci 7:2335–2339
Azizi S, Namvar F, Mohamad R, Mahdavi M (2015) Facile biosynthesis and characterization of palm pollen stabilized ZnO nanoparticles. Mater Lett 148:106–109
Hou CT, Shaw JF (2010) Biocatalysis and biomolecules engineering. John Willey & sons, Hoboken, New Jersey, p 452
Singh PP, Bhakat C (2012) Green synthesis of gold nanoparticles and silver nanoparticles from leaves and bark of Ficus carica for nanotechnological applications. Int J Sci Res Pub 2:1
Ahmad T, Wani IA, Ahmed J, Al-Hartomy OA (2014) Effect of gold ion concentration on size and properties of gold nanoparticles in TritonX-100 based inverse microemulsions. Appl Nanosci 4:491–498
Ray D, Aswal VK (2010) SANS studies insight into improving of yield of block copolymer-stabilized gold nanoparticles. AIP conference proceedings 19:219
Vigneshwaran N, Kathe AA, Varadarajan PV, Nachane RP, Balasubramaniya R (2006) Biomimetics of silver nanoparticles by white rot fungus, Phaenerochaete chrysosporium. Colloids Surf B Biointerfaces 53:55–59
Boken J, Dalela S, Sharma CK, Kumar D (2013) Detection of Pathogenic Escherichia coli (E.coli) Using Robust Silver and Gold Nanoparticles. J Chem Eng Process Technol 4:1. https://doi.org/10.4172/2157-7048.1000175
Raliya R, Tarafdar JC (2013) Biosynthesis of gold nanoparticles using rhizoctonia bataticola TFR-6. Adv Sci Eng Med 5:1073–1076
Khan MAM, Kumar S, Ahamed M, Alrokayan SA, Al-Salhi MS (2011) Structural and thermal studies of silver nanoparticles and electrical transport study of their thin films. Nanoscale Res Lett 6:1–8
Al-Farsi M, Alasalvar C, Morris A, Baron M, Shahidi F (2005) Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J Agric Food Chem 53:7592–7599
Guo C, Yang J, Wei J, Li Y, Xu J, Jiang Y (2003) Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr Res 23:1719–1726
Vayalil PK (2002) Antioxidant and antimutagenic properties of aqueous extract of date fruit (Phoenix dactylifera L. Arecaceae). J Agric Food Chem 50:610–617
Vinson JA, Zubic L, Bose P, Samman N, Proch J (2005) Dried fruits: excellent in vitro and in vivo antioxidants. J Am Coll Nutr 24:44–50
Al-Shoaibi Z, Al-Mamary MA, Al-Habori MA, Al-Zubairi AS, Abdelwahab SI (2012) In vitro antioxidative and hepatoprotective effects of palm date fruits (Phoenix dactylifera). Int J Pharmacol 8:185–191
Dammak I, Abdallah FB, Boudaya S, Besbes S, Keskes L, El Gaied A, Turki H, Attia H, Hentati B (2007) Date seed oil limit oxidative injuries induced by hydrogen peroxide in human skin organ culture. Biofactors 29:137–145
Elberry AA, Mufti ST, Al-Maghrabi JA, Abdel-Sattar EA, Ashour OM, Ghareib SA, Mosli HA (2011) Anti-inflammatory and antiproliferative activities of date palm pollen (Phoenix dactylifera) on experimentally-induced atypical prostatic hyperplasia in rats. J Inflamm (Lond) 8:40–53
Mie G (1908) Beiträge zur Optik trüber Medien speziell kolloidaler Goldlö sungen contributions to the optics of diffuse media, especially colloid metal solutions. Ann Phys 25:377–445
Gnanajobitha G, Paulkumar K, Vanaja M, Rajeshkumar S, Malarkodi C, Annadurai G, Kannan C (2013) Fruit-mediated synthesis of silver nanoparticles using Vitis vinifera and evaluation of their antimicrobial efficacy. J Nanostr Chem 3:1–6
Tadros T (2012) In: Ohsima H (ed) Electrical phenomena at interfaces and biointerfaces. John Wiley & Sons, Hoboken, p 153
Monteiro-Riviere NA, Tran CL (2014) Nanotoxicology: progress toward nanomedicine, Second Edition. CRC Press, Taylor & Francis Group, Boca Raton, p 8
Akhavan O, Ghaderi E, Akhavan A (2012) Size dependent genotoxicity of graphene nanoplatelts in human stem cells. Biomaterials 33:8017–8025
Hashemi E, Akhavan O, Shamsara M, Daliri M, Dashtizad M, Farmany A (2016) Synthesis and cyto-genotoxicity evalulation of graphene on mice spermatogonial stem cells. Colloids Surf B Biointerfaces 146:770–776
Barillet S, Jugan ML, Lave M, Leconte Y, Herlin-Boime N, Reynaud C, Carrière M (2010) In vitro evaluation of SiC nanoparticles impact on A549 pulmonary cells: cyto-, genotoxicity and oxidative stress. Toxicol Lett 198:324–330
Sankar R, Karthik A, Prabu A, Karthik S, Shivashangari KS, Ravikumar V (2013) Origanum vulagare mediated biosyntheis of silver nanoparticles for its antibacterial and anticancer activity. Colloids Surf B Biointerfaces 108:80–84
Nayak D, Ashe S, Rauta PR, Kumari M, Nayak B (2016) Bark extract mediated green synthesis of silver nanoparticles: evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater Sci Eng C 58:44–52
Periyasami VS, Athinarayanan J, Alfawaz MA, Alshatwy AA (2016) Carbon nanoparticle induced cytotoxicity in human mesenchymal stem cells through upregulation of TNF3, NFKBIA, BCL2L1 genes. Chemosphere 144:275–284
Banu H, Sethi DK, Edgar A, Sheriff A, Rayees N, Renuka N, Faheem SM, Premkumar K, Vasanthakumar G (2015) Doxorubicin loaded polymeric gold nanoparticles targeted to human folate receptor upon hyperthermia potentiates chemotherapy in breast cancers. J Photochem Photobiol B 149:116–128
Banu H, Stanley B, Faheem SM, Renuka N, Premkumar K, Vasanthakumar G (2014) Thermal chemosensitization of breast cancer cells to cyclophosphamide treatment using folate receptor targeted gold nanoparticles. Plasmonics 9:1341–1349
Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD (2005) Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 1:325–327
Shukla R, Bansal V, Chaudhary M, Basu A, Bhonde R, Sastry M (2005) Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview. Langmuir 21:10644–10654
Faedmaleki F, Shirazi FH, Salarian AA, Ashtiani HA, Rastegara H (2014) Toxicity effect of silver nanoparticles on mice liver primary cell culture and HepG2 cell line. Iran J Pharm Res 13:235–242
Speshock JL, Braydich-Stolle LK, Szymanski ER, Hussain SM (2011) Silver and gold nanoparticles alter cathepsin activity in vitro. Nanoscale Res Lett 6:17. https://doi.org/10.1007/s11671-010-9746-3
Evan GI, Vousden KH (2001) Proliferation, cell cycle and apoptosis in cancer. Nature 411:342–348
Makin G, Hickman JA (2000) Apoptosis and cancer chemotherapy. Cell Tissue Res 301:143–152
May P, May E (1999) Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene 18:7621–7636
Chan SL, Yu VC (2004) Proteins of the Bcl2 family in apoptosis signalling: from mechanistic insights into therapeutic oppurtunities. Clin Exp Pharmacol Physiol 31:119–128
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Acknowledgements
We would like to acknowledge Dr. Firdos Alam Khan, Chairperson of School of Life Sciences, Manipal University, Dubai for his cooperation and encouragement; Dr. Taher Rizvi, Professor, College of Medicine and Health Sciences, UAE University; Al-Ain for providing us the cell lines; Dr. Elango, Researcher at Masdar Institute, Abu Dhabi for helping us with some of the characterization studies; Dr. N.K. Rao, Plant Genetic Resources Scientist at the International Center for Biosaline Agriculture (ICBA) for arranging the plant source; Dr. Zakiya Ansari, Assistant Professor – English Language Teaching, Manipal University, Dubai for proof-reading the manuscript; and the undergraduate students Ms. Sajidah Hashim and Ms. Sahadiya Zubair for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Banu, H., Renuka, N., Faheem, S. et al. Gold and Silver Nanoparticles Biomimetically Synthesized Using Date Palm Pollen Extract-Induce Apoptosis and Regulate p53 and Bcl-2 Expression in Human Breast Adenocarcinoma Cells. Biol Trace Elem Res 186, 122–134 (2018). https://doi.org/10.1007/s12011-018-1287-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1287-0