Abstract
This article explores the application of hyperthermia mediated by alpha human folate receptor (αHFR) targeted gold nanoparticles (GNPs) for potentiating the cytotoxicity of cyclophosphamide (CPA) in αHFR positive breast cancer cells. Folate functionalized GNPs were delivered to highly αHFR positive breast cancer cells MDA-MB-231 and to MCF-7 breast cancer cells that does not express detectable levels of αHFR followed by hyperthermia. We have shown that hyperthermia induced by folate functionalized GNPs sensitized MDA-MB-231 cells by ten-fold to CPA treatment, whereas MCF-7 cells exhibited only onefold chemosensitization. Collectively, the study suggests the feasibility of using αHFR targeted GNPs for facilitating increased cellula r uptake of CPA in cancer cells expressing elevated αHFR, allowing reduction in drug dosage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy refers to the use of one or more cytotoxic drugs that prevents the growth of cancerous cells and triggers cancer cell death. However, such drugs may also cause damage to other dividing cells which are normal and healthy, leading to treatment-related serious adverse toxicities such as cerebral dysfunction, liver failure, pulmonary fibrosis, cardiomyopathy, and gonadal dysfunction due to the administration of large doses of the chemotherapeutic agent [1]. Hence, there is a necessity of an effective therapeutic approach that involves relatively low doses of the chemotherapeutic agent, which selectively targets and kills only the cancer cells thereby minimizing or eliminating the adverse side effects of chemotherapy.
Cyclophosphamide (CPA), among several others, is a commonly used drug for the treatment of several types of cancers including lymphomas, leukemia and cancers of ovaries, breasts, and lung. The main concern regarding heavy doses of long-term CPA administration is the adverse and chronic side effects such as bone marrow toxicity, hemorrhagic cystitis, infertility, nausea, vomiting, and hair loss [2].
Nanotechnology based cancer therapy has emerged as one of the promising fields of biomedicine that has been extensively studied over the last few decades to provide effective and targeted delivery of the chemotherapeutic agents rendering reduction in drug dosage and minimizing nonspecific adverse effects. Cancer nanomedicine overcomes the drawbacks of the conventional drug delivery systems such as nonspecific biodistribution and targeting, lack of water solubility, poor oral bioavailability, and low therapeutic indices [3, 4].
Recently, gold nanomaterials are of immense attention in potential cancer therapy and diagnostics. The plasmonic gold nanoparticles are stable, inert, well-tolerated clinically as they do not result in any serious side effects of treatment, have longer circulation half-life and can absorb low energy light source very effectively in the near-infrared wavelength to convert the absorbed light to heat, resulting in the death of the cancer cells. Moreover, gold nanoparticles exhibit versatile physical and chemical properties such as high surface-to-volume ratio, extensive thermal stability, multiple receptor targeting, well controlled size distribution, multifunctionality, ease of synthesis and highly tunable optical properties [3, 5, 6]. Gold nanostructures such as nanoparticles, nanorods, nanoshells, nanocages, and surface enhanced Raman scattering (SERS) particles have shown much promise in fighting cancer and are emerging as drug carriers, photothermal agents, optical detection agents, and radiosensitizers in cancer treatment [3, 7–18]. However, numerous types of other nanoparticles such as super paramagnetic iron oxide nanoparticles (SPIONs), cobalt-ferrite nanoparticles, and carbon nanotubes are under investigation to identify the ideal nanoparticles for tumor hyperthermia [19, 20].
Gold nanomaterial mediated hyperthermic chemosensitization is one of the most exciting and novel approaches to selectively kill the cancerous cells and is being studied extensively for the treatment of various cancers [21, 22]. In this approach, the gold nanostructures are specifically targeted to the cancerous cells followed by the exposure to an energy source such as an intense light source, which enables the optical absorbing gold nanostructures to transform the incident energy into heat to induce photothermal ablation of the cancerous cells, killing them selectively by disrupting the cell membrane [18, 23–25].
A major advance over the approach of gold nanostructure mediated hyperthermia for cancer therapy is the ligand-targeted therapeutics that provides selective delivery of large quantities of the nanostructures to only the cancer cells. This is achieved by conjugating the gold nanostructures to targeting ligands that can selectively bind to the surface of the cancerous cells and penetrate through receptor-mediated endocytosis. Folic acid is a water soluble B-complex vitamin that is widely used a targeting agent for selectively delivering the anticancer drugs and diagnostic aids to αHFR positive cancer cells. The αHFRs are highly expressed in a variety of cancers like kidney, endometrium, lung, breast, bladder, pancreas, and ovarian carcinoma [26, 27]. Thus, folic acid is preferred over the other targeting ligands in drug delivery.
With this prospect, herein, we report the use of αHFR targeted GNPs for potentiating the cytotoxicity of the drug CPA in highly folate positive cancers. For demonstration, we have used highly αHFR positive MDA-MB-231 breast cancer cell lines and MCF-7 cell line that does not express detectable levels of αHFR as models [28–34]. Our results illustrated that the combined approach of hyperthermia of αHFR targeted GNPs followed by CPA treatment tremendously increased the sensitivity of highly αHFR positive MDA-MB-231 cells to chemotherapy relative to the MCF-7 cells that does not express detectable levels of αHFR.
Experimental Methods
Materials
Positively charged GNPs (uniformly spherical in shape; 1.4-nm diameter) were purchased from Nanoprobes Inc., USA (Positively Charged Nanogold®; catalog number 2022). Anhydrous dimethyl sulfoxide (DMSO), folic acid, triethylamine, N,N’-dicyclohexylcarbodiimide (DCC), CPA, dichloromethane (DCM), Dulbecco’s modified Eagle’s media (DMEM), fetal bovine serum, l-glutamine, penicillin, streptomycin, trypsin-EDTA, trypan blue, phosphate buffered saline, and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich Corporation, USA. All other chemicals and reagents used in the experiments were of analytical reagent grade and used without further purification.
Cell Lines and Cell Culture
The human breast cancer cell lines MCF-7 and MDA-MB-231 were used in this study. Both the cell lines were grown in DMEM supplemented with 10 % fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37 °C in a humidified atmosphere containing 5 % CO2. The cell lines were subcultured twice a week upon reaching approximately 80 % confluency. About 70–80 % confluent cells were used for the experiments.
Synthesis and Characterization of Folate Functionalized GNPs
The positively charged GNPs (30 nmol) were reconstituted in anhydrous DMSO. Triethylamine (360 nmol) in DMSO was added to the GNP solution and stirred for 5–10 min. Folic acid (1 mg) in DMSO was then added to the GNP-triethylamine solution and stirred for 20 min. To this mixture, N,N’-dicyclohexylcarbodiimide solution was added and stirred overnight at room temperature to give a brown colored aggregate of folic acid conjugated GNPs, which were then purified and collected by repeated centrifugation and stored at −20 °C. The folate functionalized GNP was characterized using UV-visible spectrophotometry and Fourier transform Infrared spectrometry. The conjugation of the folic acid to the positively charged GNPs through the formation of amide bond was examined using UV-visible absorption spectroscopy (Shimadzu, PharmaSpec UV-1700) by comparing the absorption spectra of folate functionalized GNPs with folic acid and positively charged GNPs. Also, the functional group responsible for folate conjugation was analyzed using a Bruker Fourier transform infrared (FTIR) IFS 66 V spectrometer.
Photoexcitation of the GNPs with Intense Pulse Light
The intense pulse light (IPL) source used for the experimentation is Philips Lumea®. IPL is a continuous noncoherent broad spectrum light emitted from a flash lamp with a wavelength range of 575–1200 nm. The instrument was operated with a light fluence of 6.5 J/cm2 for a pulse duration of 3–100 ms at a wavelength of about 570–600 nm.
Screening Cytotoxicity of IPL on Cancer Cell Lines
The cytotoxic effect of IPL on MCF-7 and MDA-MB-231 cell lines were studied to determine the optimum number of pulses for use in the experiment by subjecting the cell lines to increasing number of IPL pulses, before the nanoparticle treatment. About 80 % confluent monolayer cell lines of both MCF-7 and MDA-MB-231 were harvested and plated at densities of 300,000 cells per ml and cultured for 24 h. The attached cell lines, after decanting the media, were exposed to 0, 10, 20, 50, 75, and 100 single IPL pulses with a pulse duration of 3 ms and then cultured with fresh media. After 48 h of incubation, the cell survival was assessed by MTT assay by measuring the absorbance of formazan product formed by the reduction of MTT by the mitochondrial dehydrogenase of viable cells at 570 nm using a Microplate reader.
Drug Sensitivity Assay
Drug sensitivity assay was performed to determine the median effective concentration (EC50) of the drug CPA from a dose response curve. The breast cancer cell lines were plated at densities of 300,000 cells per ml and were grown for 24 h. Both the MCF-7 and MDA-MB-231 cell culture plates were then incubated with a fresh medium containing CPA at an increasing concentration of 0, 0.1, 10, 20, 50, and 100 μM. After 48 h of incubation, the cell viability was determined by MTT assay.
Treatment of Cell Lines with Folate Functionalized GNPs Followed by Hyperthermia and Chemotherapy
The cell lines MCF-7 and MDA-MB-231 were plated at densities of 300,000 cells per ml and cultured for 24 h. Photoexcitation of the nanoparticle can be accomplished by the exposure of cancer cells incubated with the folate functionalized GNPs to IPL. This is achieved by incubation of the cell lines with culture media containing folate functionalized GNPs at a concentration of 5 μg/ml for 4 h, irradiation of the wells with the predetermined number of IPL pulses after the decantation of the culture media, incubation with a fresh medium containing CPA at increasing concentrations of 0, 0.1, 1, 10, 20, 50, and 100 μM for 48 h followed by analysis of the cell survival by performing MTT assay.
Data Analysis
The EC50 was determined from a sigmoidal dose response curve with a variable slope, where the curve is fitted by nonlinear regression analysis using GraphPad Prism version 6.0 (GraphPad Software Inc., San Diego, CA, USA). Other data analysis and graphs were made using the graphing software Origin version 8.0 and the commercial spreadsheet Microsoft Excel. All the experiments were repeated at least three times with similar results.
Results and Discussion
Development of Folate Functionalized GNPs Targeted to αHFR
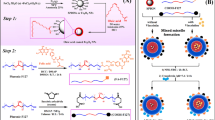
In an attempt to formulate the αHFR targeted GNPs, the positively charged GNPs were conjugated to the folic acid through a carbodiimide coupling reaction, in which an amine-reactive ester is formed on the folate that facilitates the coupling of the positively charged primary amine groups of GNPs to the activated folate (Fig. 1). Each molecule of GNP used for the experiment bears approximately six primary amine groups that are ionized to assume positive charge and are therefore suitable to tag functional groups like the carboxyl group of folic acid that has a net negative charge. The primary amine groups of GNPs were covalently conjugated to the carboxylate group of folic acid through amide bond formation in the presence of triethyl amine and N,N’-dicyclohexylcarbodiimide. In this coupling reaction, the amine-reactive ester group formed on the folate favors the binding of the positively charged primary amine groups of the GNPs with the activated folate [35].
Biofunctionalization of gold nanoparticle with folic acid. Each of the positively charged gold nanoparticle has six reactive primary amine groups. The primary amine groups of GNPs were conjugated to the carboxylate group of folic acid via a CO-NH bond in the presence of triethyl amine (TEA) and N,N’-dicyclohexylcarbodiimide (DCC)
Characterization by UV-Visible Spectrophotometry
The absorbance peak of the gold nanoparticle around 260 nm can be attributable to the fact that each of the positively charged gold nanoparticle is surrounded with almost six primary amine groups [36, 37], and the shift in absorbance peak to around 230 nm after folate conjugation of the gold nanoparticle is due to the amide carbonyl [CO-NH] group formed between the carboxylic group of the folate and the amine groups of the gold nanoparticle [38]. The UV absorption spectrum of folic acid functionalized GNPs when compared with pure folic acid and positively charged gold nanoparticle showed a characteristic absorption peak at around 233 nm indicating the formed amide bond between the positively charged GNPs and folic acid (Fig. 2).
The UV-visible absorption spectra of positively charged gold nanoparticles, pure folic acid, and the folate functionalized gold nanoparticles were compared. The absorbance peak of the gold nanoparticle around 260 nm is attributable to the fact that each of the positively charged gold nanoparticle is surrounded with almost six primary amine groups [36, 37], and the shift in absorbance peak to 233 nm after folate conjugation of the gold nanoparticle is due to the amide carbonyl group formed between the carboxylic group of the folate and the amine groups of the gold nanoparticle [38]
Characterization by Fourier Transform Infrared Spectroscopy
FTIR spectroscopic analysis of folate functionalized GNPs were performed to verify the conjugation of folic acid to the surface of positively charged GNPs via a CO-NH bond. The approximate frequency of an amide group, in an infrared spectral data, is expected to be at around 1600 to 1690 cm−1 [39]. The FTIR spectra detected over a frequency range of 4000–350 cm−1 resulted in a peak at wave number 1666.67 and 1500 cm−1 as represented in Fig. 3, due to C = O stretching and N-H bending vibrations of the CO-NH bond formed between the amino group of the positively charged GNP and the carboxyl group of the folic acid [38, 40].
Cytotoxicity of IPL on Cancer Cell Lines
The cytotoxicity of IPL was tested on both the MCF-7 and MDA-MB-231 breast cancer cell lines. The percentage of cell survival at 10, 20, 50, 75, and 100 IPL pulses was 100, 98, 90, 80, and 60 for MDA-MB-231 cell lines and 100, 90, 88, 76, and 50 for MCF-7 cell lines, respectively (Fig. 4). The exposure of MCF-7 as well as MDA-MB-231 cell lines up to about 20 single IPL pulses, for a duration of 3 ms, did not show any significant reduction in the cell survival. However, beyond 20 pulses, the percentage cell survival of both the cell lines was found to decrease with the increase in the number of IPL pulses, which can be attributed to localized hyperthermia resulting in membrane alterations, followed by necrosis and programmed cell death [41]. Thus, 20 IPL pulses with a duration of 3 ms were chosen to be almost harmless for both the cell lines and were used for the experiments to mediate hyperthermia.
Cytotoxicity of CPA on Breast Cancer Cell Lines
The half maximal effective concentration (EC50) of the drug CPA, calculated using sigmoidal dose response nonlinear regression curve fit with variable slope, was 9.752 μM and 10.79 μM for MCF-7 and MDA-MB-231 cell lines, respectively (Figs. 5 and 6).
Thermal Chemosensitization of Highly αHFR Positive Cancer Cells Compared to Cancer Cells Having Low αHFR
The combined treatment of hyperthermia by folate functionalized GNPs followed by chemotherapy had a significant cytotoxic effect on highly folate positive MDA-MB-231 cell line, whereas this approach did not have much influence on MCF-7 cell line that does not express detectable levels of αHFR. The EC50 of CPA with and without folate GNP mediated hyperthermia were 9.219 and 9.752 μM, respectively, in the MCF-7 breast cancer cells that does not express detectable levels of αHFRs, whereas the EC50 of CPA with and without folate GNP-mediated hyperthermia was 1.074 and 10.79 μM, respectively, in the highly αHFR positive MDA-MB-231 breast cancer cells (Figs. 7, 8, and 9).
Combination therapy is a potential breakthrough in cancer treatment, which involves the concurrent use of two or more cancer fighting strategies, which targets diverse cellular pathways, to provide safe and effective treatment of cancers. The effective combination of the standard chemotherapy with nanoparticle mediated hyperthermia can bring about improved clearance of the cancers by simultaneous apoptosis and necrosis of the cancer cells, thereby improving the overall efficacy of the anticancer drugs that can be used at an optimal dose avoiding the adverse side effects [41–46]. The combination therapy also reduces the probability of developing drug resistant cancer cells as the cancer fighting strategies mediate cell death in cancer cells by different mechanisms and also minimizes individual drug-related toxicity [42].
Targeted photothermal therapy mediated by plasmon resonant gold nanoparticle and chemotherapy is becoming increasingly accepted for the treatment of cancers due to the selective destruction of tumor cells with minimum side effects on normal cells. Combination therapy of cisplatin and hyperthermia produced by the optically excited gold nanorods kills 78 % more cells than cisplatin alone [47]. Doxorubicin loaded dual functional gold nanospheres were reported to be capable of mediating photothermal ablation of MDA-MB-231 cancer cells and drug release upon excitation by near-infrared light radiation [48]. Folate conjugated gold nanoparticles stabilized with a monolayer of doxorubicin conjugated amphiphilic copolymer exhibited higher cytotoxicity against the mouse mammary carcinoma cell line [49].
We have shown in our study that folate targeted gold nanoparticles after heat treatment had resulted in improved chemosensitization of cyclophosphamide in folate positive breast cancer cells when compared to breast cancer cells that does not express detectable levels of αHFRs. Previous researches have shown that the MDA-MB-231 breast cancer cell line is highly αHFR positive and MCF-7 cell line does not express detectable levels of αHFR [30–34], and therefore, we have chosen MDA-MB-231 and MCF-7 breast cancer cell lines as cancer cell models that expresses high and low αHFRs on their surfaces. Folic acid is essential for the cellular survival and proliferation of cancerous cells in which it serves as cofactor of enzymes associated with the biosynthesis of nucleic acids and amino acids [50, 51]. The agents conjugated to the folic acid is internalized and absorbed by the cancer cells through αHFR-mediated endocytosis [52, 53].
The half maximal effective concentration of CPA that caused fifty percentage of cell death in MDA-MB-231 cell line was only 1.074 μM after the hyperthermia, whereas only CPA could produce the same 50 % cytotoxicity at a concentration of 10.79 μM, which leads to greater than ten-fold reduction in the EC50 of the drug. Under similar circumstances, the EC50 of CPA with and without the folate GNP-mediated hyperthermia was 9.219 and 9.752 μM, respectively on MCF-7 cell line, which is about only about onefold reduction in the median effective concentration. It can be clearly seen that, the αHFR targeted GNPs sensitized highly folate positive MDA-MB-231 cell line by 10-fold to treatment with cyclophosphamide.
This difference in response, between the cell lines, can be attributed to the fact that the elevated levels of αHFR expression in MDA-MB-231 had facilitated the cellular uptake of increased quantities of folate functionalized GNPs via receptor-mediated endocytosis thereby resulting in the intracellular accumulation of GNPs. Subsequently, these accumulated GNPs when subjected to photoexcitation by IPL had resulted in localized heat (hyperthermia) through absorption of light that had produced heat-induced alterations in the tumor microenvironment leading to changes in the cytoskeletal organization [41, 43]. The hyperthermia treatment had increased the fluidity of cell membrane that in turn had been the reason for increased sensitization of cancer cells to the drug CPA. Thus, the folate functionalized GNPs after heat treatment had enabled CPA to enter the highly folate cancer cells at a higher concentration in comparison with the MCF-7 cell line that does not express detectable levels of αHFR.
Moreover, this approach of conjugation of nanoparticles to folic acid as the targeting ligand will also help the noncancerous cells to escape the hyperthermia treatment, by preventing entry of the gold nanoparticle into the normal healthy tissue. The increased intracellular accumulation of GNPs through folate receptor-mediated endocytosis and subsequent hyperthermia is expected to occur only in the αHFR overexpressing cancerous cells when compared to normal healthy cells because the αHFRs are overexpressed on the surface of cancerous cells because they grow and divide uncontrollably, whereas the αHFR distribution in the normal healthy cells is restricted [26, 27]. On account of the poor blood supply of the cancerous tissue when compared to the healthy tissue, the gold nanostructure-mediated hyperthermia produces overheating of the cancerous cell that causes fatal damage to the cancerous cell, without causing much damage to the normal healthy cells [23, 28, 29]. Also, the low pH and hypoxic state of the cancer cells makes them more vulnerable to hyperthermia when compared to the normal healthy cells [54]. Therefore, we speculate that approach of using αHFR targeted GNPs and subsequent photothermal therapy, specifically targets and selectively kills only the cancer cells, as the folate GNP-mediated hyperthermia provides increased localized heating in the cancer cells when compared to the surrounding healthy tissue.
Based on the data presented, it is understood that the cytotoxic effects obtained with higher doses of CPA could be achieved when much lower doses of CPA is administered after the thermal chemosensitization of highly folate positive cancer cells using folate conjugated GNPs. Our results are in well agreement with the previous researches based on the approach of thermal chemosensitization of highly folate positive cancer cells using folate receptor targeted gold nanoconjugates. The folate conjugated gold nanorods when delivered to malignant human oral cancer KB cells that overexpresses high affinity folate receptors and NIH/3T3 mouse embryonic fibroblast cell line that has low folate receptor expression followed by photothermal lysis using near infrared radiation produced severe membrane damage in KB cells when compared to NIH/3T3 cells [25]. The MCF-7 breast cancer and human adenocarcinoma HeLa cell lines, when subjected to photothermal treatment using folate conjugated gold nanoparticles had resulted in approximately 9 % cytotoxicity in MCF-7 cell line that has low levels of folate receptor expression, and approximately 98 % cell lethality in HeLa cell lines that has overexpressed folate receptors [28, 29]. Folate receptor overexpressing human ovarian carcinoma cell line (SKOV3) and human lung adenocarcinoma epithelial cell line (A549) when treated with folic acid conjugated and radioactive-iodine-labeled gold nanorods resulted in an increased cellular uptake of about 2.7-fold in SKOV3 cells when compared to the A549 cells and upon consequent photothermal treatment had better thermal ablation in the folate overexpressing ovarian cancer cell line SKOV3 [55].
Future work may focus on the standardization of the combination therapy to make it a more effective treatment, further enhancement of the folate conjugated GNPs by co-functionalization with polymers like polyethylene glycol to make it more suitable for in vivo studies so that the nanocomposite escapes the nonspecific phagocytosis of the reticulo-endothelial system of liver and spleen, and investigation of the molecular mechanisms behind the in vitro cellular response after hyperthermia, particularly study of genes associated with apoptosis including the expression of p53 and Bcl-2 genes. This approach also could also be extended to the treatment of multidrug resistant cancers as the nanoparticles may be able to surpass the drug efflux transporters like P-glycoprotein, which are overexpressed in the multidrug resistant cancers.
In summary, the current study demonstrates that cancer cells expressing high levels of αHFRs can be sensitized to the chemotherapy when used in combination with hyperthermia using αHFR targeted GNPs. Under similar circumstances, folate-conjugated surface plasmon resonant GNPs were delivered to MDA-MB-231 and MCF-7 breast cancer cell lines that expresses high and low αHFRs on their surfaces, respectively, followed by exposure to intense pulse light for hyperthermia. Hyperthermia mediated by folate functionalized GNPs sensitized MDA-MB-231 cells by ten-fold to cyclophosphamide treatment, whereas MCF-7 cells exhibited only one-fold chemosensitization. We speculate that the elevated levels of αHFR expression in MDA-MB-231 had facilitated the accumulation of GNPs in cancer cells, and the subsequent application of localized heat apparently had resulted in increase in the fluidity of cell membrane enabling sensitization of cancer cells to CPA treatment. Thus, sensitization of cancer cells to cyclophosphamide is directly proportional to the levels of expression of the folate receptor on the breast cancer cell surface, which had directly influenced the internalization of folate-conjugated gold nanoparticles through folate receptor-mediated endocytosis. These accumulated large population of gold nanoparticles in highly folate positive cancer cells, due to their surface plasmon resonance, had exhibited intense hyperthermia due to absorption of light, which had resulted in increased fluidity of cell membrane enabling sensitization of cancer cells to cyclophosphamide treatment.
With this approach, cytotoxicity levels achieved by using higher doses of cyclophosphamide can be replaced with hyperthermia mediated by folate functionalized GNPs followed by the administration of much lower doses of cyclophosphamide, allowing reduction in drug dosage and overcoming the adverse side effects of the drug; and GNPs can be selectively delivered to cancer cells by conjugation with folic acid that enables selective binding to the surface of the cancerous cells and penetrate through folate receptor mediated endocytosis. Photothermal ablation of cancer cells resulting in sensitization to cyclophosphamide occurs selectively in cancer cells due to the αHFR receptor targeting, minimizing the effects on the surrounding healthy tissue. In vivo studies using folate-conjugated GNPs coated with a biocompatible polymer that helps the nanocomposite to escape immune reactions, investigation of the expression of apoptotic genes after hyperthermia, and extension of this approach to the multidrug resistant cancers are the future prospects of this research.
Conclusions
GNPs are considered as efficient and unique agents in terms of their toxicity and biocompatibility in an array of applications. They have already made their way effectively into cancer diagnosis and treatment. The ease of production of GNPs and increased drug efficacy in combination treatment makes them attractive drug delivery vehicles in chemotherapy. We summarize that the folate conjugated gold nanoparticles were synthesized, characterized by UV-visible spectrophotometry and FTIR techniques and studied on two different breast cancer cell lines, MDA-MB-231 that is rich in αHFRs on its surface and the MCF-7 cells that doesn’t express detectable levels of αHFRs. The folate targeted GNPs, which had accumulated in highly αHFR positive MDA-MB-231 cell line via folate receptor-mediated endocytosis, had resulted in the hyperthermia-mediated cell membrane permeability to CPA due to cellular membrane damage upon photoexcitation of gold nanoparticles, whereas no such accumulation of folate-targeted GNP occurred in MCF-7 cell line that doesn’t express detectable levels of αHFRs. The αHFR-targeted GNPs upon photothermal therapy were able to sensitize the highly αHFR positive breast cancer cells to cyclophosphamide treatment making this a promising approach for the treatment of folate positive cancers.
References
Khoo KS, Ang PT, Lim AG (1993) Common toxicities of cancer chemotherapy. Singap Med J 34(4):418–420
Haubitz M (2007) Acute and long-term toxicity of cyclophosphamide. Transplantationsmedizin 19(2):26–31
Cai W, Gao T, Hong H, Sun J (2008) Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol Sci Appl 1(1):17–32
Sultana N, Shenoy SB, Sham ME, Keshav S, Kaul R (2012) Nanogoldtechnology-imaging, sensing and target therapy in head and neck cancer. Clin Cancer Investig J 1(1):6–12
Tiwari PM, Vig K, Dennis VA, Singh SR (2011) Functionalized gold nanoparticles and their biomedical applications. Nanomaterials 1(1):31–63
Kennedy LC, Bickford LR, Lewinski NA et al (2011) A new era for cancer treatment: gold-nanoparticle-mediated thermal therapies. Small 7(2):169–183
Turkevich J, Stevenson PC, Hillier J (1951) The nucleation and growth process in the synthesis of colloidal gold. Discuss Faraday Soc 11:55–75
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature 241:20–22
Martin CR (1994) Nanomaterials: a membrane-based synthetic approach. Science 266(5193):1961–1966
Van der Zande BMI, Bӧehmer MR, Fokkink LGJ, Schӧnenberger C (1997) Aqueous gold sols and rod-shaped particles. J Phys Chem B 101(6):852–854
Oldenburg SJ, Jackson JB, Westcott SL, Halas NJ (1999) Infrared extinction properties of gold nanoshells. Appl Phys Lett 75(19):2897–2899
Caruso F, Spasova M, Salgueirino-Maceira V, Liz-Marzan LM (2001) Multilayer assemblies of silica-encapsulated gold nanoparticles on decomposable colloid templates. Adv Mater 13(14):1090–1094
Chen J, McLellan JM, Siekkinen A, Xiong Y, Li ZY, Xia Y (2006) Facile synthesis of gold-silver nanocages with controllable pores on the surface. J Am Chem Soc 128:14776–14777
Chen J, Saeki F, Wiley BJ et al (2005) Gold nanocages: bioconjugation and their potential use as optical imaging contrast agents. Nano Lett 5(3):473–477
Chen JY, Wang DL, Xi JF et al (2007) Immuno gold nanocages with tailored optical properties for targeted photothermal destruction of cancer cells. Nano Lett 7(5):1318–1322
Sha MY, Xu H, Penn SG, Cromer R (2007) SERS nanoparticles: a new optical detection modality for cancer diagnosis. Nanomed 2(5):725–734
Hering K, Cialla D, Ackermann K et al (2008) SERS: a versatile tool in chemical and biochemical diagnostics. Anal Bioanal Chem 390:113–124
Jain S, Hirst DG, O’Sullivan JM (2012) Gold nanoparticles as novel agents for cancer therapy. Br J Radiol 85(1010):101–113
Kumar CSSR, Mohammad F (2011) Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv Drug Deliv Rev 63(9):789–808
Burlaka A, Lukin S, Prylutska S et al (2010) Hyperthermic effect of multi-walled carbon nanotubes stimulated with near infrared irradiation for anticancer therapy: in vitro studies. Exp Oncol 32(1):48–50
Issels RD (2008) Hyperthermia adds to chemotherapy. Eur J Cancer 44(17):2546–2554
Krishnan S, Diagaradjane P, Cho SH (2010) Nanoparticle-mediated thermal therapy: evolving strategies for prostate cancer therapy. Int J Hyperthermia 26(8):775–789
Conde J, Doria G, Baptista P (2012) Noble metal nanoparticles applications in cancer. J Drug Deliv 2012:751075
Jain PK, Huang XH, El-Sayed IH, El-Sayed MA (2008) Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology and medicine. Acc Chem Res 41(12):1578–1586
Tong L, Zhao Y, Huff TB, Hansen MN, Wei A, Cheng JX (2007) Gold nanorods mediate tumor cell death by compromising membrane integrity. Adv Mater 19(20):3136–3141
Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP (2005) Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem 338:284–293
Garin-Chesa P, Campbell I, Saigo PE, Lewis JL, Old LJ, Rettig WJ (1993) Trophoblast and ovarian cancer antigen LK26: sensitivity and specificity in immunopathology and molecular identification as a folate-binding protein. Am J Pathol 142(2):557–567
Shakeri-Zadeh A, Mansoori GA, Hashemian AR, Eshghi H, Sazgarnia A, Montazerabadi AR (2010) Cancerous cells targeting and destruction using folate conjugated gold nanoparticles. Dyn Biochem Process Biotechnol Mol Biol 4(1):06–12
Mansoori GA, Brandenburg KS, Shakeri-Zadeh A (2010) A comparative study of two folate-conjugated gold nanoparticles for cancer nanotechnology applications. Cancers 2(4):1911–1928
Chen H, Ahn R, Van den Bossche J, Thompson DH, O'Halloran TV (2009) Folate-mediated intracellular drug delivery increases the anticancer efficacy of nanoparticulate formulation of arsenic trioxide. Mol Cancer Ther 8(7):1955–1963
Chung KN, Saikawa Y, Paik TH et al (1993) Stable transfectants of human MCF-7 breast cancer cells with increased levels of the human folate receptor exhibit an increased sensitivity to antifolates. J Clin Invest 91:1289–1294
Feng D, Song Y, Shi W, Li X, Ma H (2013) Distinguishing folate-receptor-positive cells from folate-receptor-negative cells using a fluorescence off–on nanoprobe. Anal Chem 85(13):6530–6535
Mahalwar A, Sharma A, Sahu R, Rathore DS (2012) Evaluation of receptor mediated endocytosis on cellular internalization: a comparative study of PEGylated nanoparticles and folate anchored PEGylated nanoparticles on MDA-MB-231 cells. Int J Biol Pharm Res 3(3):431–443
Meier R, Henning TD, Boddington S et al (2010) Breast cancers: MR imaging of folate-receptor expression with the folate-specific nanoparticle P1133. Radiol 255(2):527–535
Hermanson GT (2008) Bioconjugate techniques, 2nd edn. Academic Press Publishers, US, pp 224–226
Park IS, Heo E, Nam YS (2012) Colorimetric detection of aliphatic primary amines and a molecular logic gate based on a photochromic phenoxyquinone derivative. J Photochem Photobiol A Chem 238(1):1–6
Sorensen H, Sorensen S, Bjergegaard C et al. (1999) Chromatography and capillary electrophoresis in food analysis, First Edition, Royal Society of Chemistry, 102–104
Pompeo F, Resasco DE (2002) Water solubilization of single-walled carbon nanotubes by functionalization with glucosamine. Nanolett 2(4):369–373
Mejri M, Rogé B, BenSouissi A, Michels F, Mathhlouthi M (2005) Effects of some additives on wheat gluten solubility: a structural approach. Food Chem 92(1):7–15
Kong J, Yu S (2007) Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin 39(8):549–559
Hildebrandt B, Wust P, Ahlers O et al (2002) The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol 43(1):33–56
Parhi P, Mohanty C, Sahoo SK (2012) Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Discov Today 17(17–18):1044–1052
Yamamoto D, Inui T, Tsubota Y et al. (2012) The utility of hyperthermia for local recurrence of breast cancer. World J Surg Oncol 10 (201), doi: 10.1186/1477-7819-10-201
Bull JM (1984) An update on the anticancer effects of a combination of chemotherapy and hyperthermia. Cancer Res 44(10):4853–4856
Dahl O (1988) Interaction of hyperthermia and chemotherapy. Rec Res Cancer Res 107:157–169
Engelhardt R (1987) Hyperthermia and drugs. Rec Res Cancer Res 104:136–203
Tanya SH, Travis LJ, Tetyana Y, Kumaradas JC, Warren CWC (2008) Enhancing the toxicity of cancer chemotherapeutics with gold nanorod hyperthermia. Adv Mater 20(20):3832–3838
You J, Zhang G, Li C (2010) Exceptionally high payload of doxorubicin in hollow gold nanospheres for near-infrared light-triggered drug release. ACS Nano 4(2):1033–1041
Prabaharan M, Grailer JJ, Pilla S, Steeber DA, Gong S (2009) Gold nanoparticles with a monolayer of doxorubicin-conjugated amphiphilic block copolymer for tumor-targeted drug delivery. Biomaterials 30(30):6065–6075
Muller C, Schibli R (2011) Folic acid conjugates for nuclear imaging of folate receptor positive cancer. J Nucl Med 52(1):1–4
Lu Y, Sega E, Leamon CP, Low PS (2004) Folate receptor targeted immunotherapy of cancer: mechanism and therapeutic potential. Adv Drug Deliv Rev 56(8):1161–1176
Kularatne SA, Low PS (2010) Targeting of nanoparticles: folate receptor. Methods Mol Biol 624:249–265
Bhattacharya R, Patra CR, Earl A et al (2007) Attaching folic acid on gold nanoparticles using noncovalent interaction via different polyethylene glycol backbones and targeting of cancer cells. Nanomed Nanotechnol Biol Med 3(3):224–238
Van Der Zee J (2002) Heating the patient: a promising approach? Ann Oncol 13(8):1173–1184
Jang B, Park S, Kang SH et al (2012) Gold nanorods for target selective SPECT/CT imaging and photothermal therapy in vivo. Quant Imaging Med Surg 2(1):1–11
Acknowledgments
We would like to thank Dr. K. Satyamoorthy, the Director of Manipal Life Sciences Centre, Manipal University, India for providing us the MCF-7 and MDA-MB-231 cell lines. We would also like to thankfully acknowledge Dr. Firdos Khan, the Chairperson of School of Life Sciences, Manipal University—Dubai Campus, for his cooperation and encouragement, and the students Ms. Deepika Bhagavatula, Ms. Jia Anne Jacob, and Mr. Sanju Simon for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banu, H., Stanley, B., Faheem, S.M. et al. Thermal Chemosensitization of Breast Cancer Cells to Cyclophosphamide Treatment Using Folate Receptor Targeted Gold Nanoparticles. Plasmonics 9, 1341–1349 (2014). https://doi.org/10.1007/s11468-014-9747-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11468-014-9747-7