Abstract
An 11-week feeding trial was carried out to investigate the effects of supplemented chromium picolinate (Cr-Pic) on the growth, whole-body composition, and relative mRNA expression related to lipogenesis and glucose metabolism in juvenile blunt snout bream. Seven isonitrogenous and isoenergetic diets with graded Cr supplementation levels were fed to triplicate groups. The final weight (FW), feed conversion ratio (FCR), and specific growth rate (SGR) were improved with increasing dietary Cr supplementation levels up to 0.4 mg/kg, and thereafter showed relatively constant. However, 12.0 mg/kg dietary Cr supplementation decreased growth and feed utilization. Based on SGR and FCR, the optimal dietary Cr supplementation level for the juvenile was estimated to be 0.28 mg/kg. Significantly higher plasma insulin levels were found in juvenile fed diets with 0.4 and 0.8 mg/kg Cr supplementation compared to those fed diet sans supplemented Cr. Plasma glucose levels decreased with increasing dietary Cr supplementation, and the lowest value was remarked in the group added 3.2 mg/kg of Cr. Adding 0.4–0.8 mg/kg Cr enhanced insulin receptor substrate 1 (IRS-1), phosphoinositide-3-kinase (PI3K), and pyruvate kinase (PK) and inhibited expression of phosphoenolpyruvate carboxykinase (PEPCK), glucose-6-phosphatase (G6Pase), and glycogen synthase (GS) mRNA levels. High dietary Cr (12.0 mg/kg) supplementation resulted in high G6Pase and PEPCK expression. The highest content of whole-body lipid was remarked in fish fed with 0.4 mg/kg dietary Cr, which related to the enhanced gene expression related to lipogenesis; thereafter, mRNA levels showed a diminishing trend. These findings indicate that optimum dietary Cr-Pic supplementation has a positive effect on growth and blood glucose homeostasis by modifying the mRNA levels related to glucose metabolism and lipogenesis in juvenile blunt snout bream.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chromium III (Cr) has been accepted as an essential nutrient for animals for decades [1]. Although some studies show that Cr has not been an indispensable trace element for healthy mammals recently [2], supplemental Cr given to diabetics has increased the insulin sensitivity and interfered with general metabolism [3, 4]. In skeletal muscle cells of rat, chromium increased insulin and significantly enhanced the mRNA levels of the insulin receptor, glycogen synthase, and glucose transporter 4 [5]. Sundaram et al. [6] reported that Cr supplementation is beneficial for hyperglycemia correction by glucose metabolism modification in a diabetic rat. The Cr supplementation also can decrease proinflammatory cytokine blood levels, oxidative stress, and lipid levels, and then diminishes the vascular inflammation risk in diabetes [7]. In different farmed livestock, the Cr also has been confirmed as a vital element involved in lipid and glucose metabolism and subsequently growth improvement [8]. The previous studies suggest the supplemental Cr form greatly influences Cr absorption and its effectiveness in animals [6]. Generally, the Cr bioavailability is higher in the organic forms compared with the inorganic forms. It is accepted that chromium picolinate (Cr-Pic) is a convenient form of Cr that is used more efficiently, slightly affected by nutritional and environmental conditions compared with other forms of Cr [9, 10].
The dietary carbohydrate utilization in fish is limited [11]. Precedent studies have provided evidence of the positive effect of chromium as a growth inducer in several fish species [12], such as in tilapia Oreochromis niloticus x O. aureus [13], grass carp Ctenopharyngodon idellus [14], common carp Cyprinus carpio L. [15], mirror carp Cyprinus carpio L [16], and yellow croaker Larmichthys crocea [17]. The supplementation of Cr in the diets significantly improved growth performance, immunity, and carbohydrate utilization. In contrast, no growth benefits have been remarked in channel catfish Ictalurus punctatus [18], gilthead seabream Sparus aurata L. [19], and rainbow trout Oncorhynchus mykiss [20] fed supplemented Cr in the diets. Although Liu et al. [14] stated that Cr supplementation could significantly improve the growth, plasma lipid, and carbohydrate profile at a level 0.8 mg/kg in grass carp fingerlings, the mechanism by which Cr effects growth, lipids, and glucose metabolism in fish is still unclear.
Blunt snout bream, Megalobrama amblycephala, is a mean reared freshwater fish species in China, and its production reached approximately 0.8 million tons in 2015 [21]. In our previous studies, we have investigated the effects of differences at dietary carbohydrate level [22] and kinds (wheat starch, corn starch, dextrin, maltose, glucose, and cellulose) [23] on growth performance, carbohydrate utilization, and immunity in this fish species, which indicated that high dietary carbohydrate level impaired growth performance and resulted in glucose metabolism disorder. This study was aimed to investigate the potential nutritional benefits of Cr-Pic supplementation in practical diets for juvenile blunt snout bream, and to reveal the mechanism by which Cr affects gene expression related to glucose metabolism and lipogenesis involving the insulin signaling pathway.
Materials and Methods
Diet Preparation
The experimental diets are shown in Table 1. Fish meal, canola meal, soybean meal, and cotton seed meal were used as protein sources. Soybean oil and lecithin were used as lipid sources. The basal diet was supplemented with 0, 0.2, 0.4, 0.8; 1.6, 3.2, and 12.0 mg/kg Cr in the form of Cr-Pic (Sigma-Aldrich (Shanghai) Trading Co., Ltd., Shanghai, China). The analyzed Cr concentrations of dry diet were 0.84, 1.13, 1.42, 1.94, 2.46, 4.24, and 12.6 mg/kg, respectively. Ingredients were crushed and crumbled into powder and passed via mesh sieve, afterwards entirely mixed with soybean oil, lecithin, and water for stiff dough production; the pelletizer (F-26 (II), South China University of Technology, China) was used to transfer this dough and produce pellets; the latter was dried in an aerated furnace overnight. After drying, the diets were broken up and sieved into 1.5 × 2.0 mm pellet size. All diets were sealed in bags and kept at − 10 °C until used.
Experimental Procedure
The present study was approved by the Animal Care and Use Committee of Nanjing Agricultural University (Nanjing, China). Juvenile blunt snout breams were caught from the Freshwater Fisheries Research Center (Wuxi, China). Fish were reared and acclimatized to the experimental conditions in floating cages (located in a 2000 m3 culture pond) for 2 weeks and fed a commercial diet containing 6% lipid and 30% protein (Wuxi Tongwei feedstuffs Co., Ltd., Wuxi, China). Prior to the feeding trial, the fish were starved for 24 h, caught and weighed, and then randomly placed into floating cages (1 m × 1 m × 1 m) with a group of 20 fish in each cage. Each group was haphazardly allotted to triplicate cages. Fish were hand-fed three times a day at 8:00, 12:00, and 16:00 until apparent satisfaction based on the visual observation. During the 11 weeks of the feeding trial, the feed consumption and dead fish were weighed and recorded daily. The water temperature varied from 24 to 30 °C and dissolved oxygen was about 6 mg L−1 throughout the feeding trial, while the Cr level in the water was less than 6 μg/L.
Sample Collection and Chemical Analysis
At the experiment termination, the fish were harvested from the cages, anesthetized with 100 mg/L MS-222, and steadily weighed and counted after the last feeding time. Five fish were randomly taken up from each cage and separately weighed, and then blood sampling operation has been carried out from the caudal vein followed by the centrifugation process to separate the plasma. Liver and plasma samples were stored at − 80 °C for subsequent measurements of glycogen contents and relative genes mRNA levels. Meanwhile, five others from each cage were sampled and kept at similar temperature degree for body composition analysis.
Crude protein, lipid, dry matter, and ash contents in the diets and whole body were found out in accordance with the Association of Official Analytical Chemists (AOAC) methods [24]. The diets and whole body of dry matter was obtained after drying in a furnace at 105 °C until constant weight. While crude protein was determined after acid digestion by the Kjeldahl method, crude percent lipid was determined by the Soxhlet method and ash was obtained after combustion during 5 h at 550 °C. The diet Cr content was analyzed in a professional laboratory (Jiangnan University, Wuxi, China) using the method of graphite furnace atomic absorption spectrometric. Glucose analyses, protein and triglyceride in the plasma were measured by an automatic biochemical analyzer (Mindray BS-400, Mindray International Ltd., Shenzhen, China) with these three methods, respectively; glucose oxidase, biuret and glycerine phosphate oxidase-peroxidase coupling (GPO-POD). Plasma insulin levels were determined using an automatic chemiluminescence analyzer (MAGLUMI 1000, Snibe Diagnostic Ltd., Shenzhen, China) according to the chemiluminescence method. The hepatic glycogen content was determined by a glycogen assay kit (JianCheng Bioengineering Institute, Nanjing, China).
A real-time PCR was used to determine levels of mRNA using PrimerScript TM RT reagent kit and analyze the relative gene expressions as mentioned in our prior study [25]. Concisely, an RNAiso plus kit (Takara, Dalian, China) was used for a total RNA extraction from the fish liver. After purification, quality and quantity were assessed and a PrimeScript TM RT reagent kit (Takara, Dalian, China) was used to synthesize a complementary DNA. Specific primers (Table 2) were designed by the M. amblycephala transcriptome analysis [26] according to the partial cDNA sequences of the related genes. The levels of relative gene expression were normalized to the β-actin expression level. The method of 2-∆∆CT has been used to analyze the expression findings after confirmation that the amplification efficiency primers is nearly 100% [27].
Statistical Analysis
After assumptions’ evaluation of normality, outliers, and variances equality, the software SPSS 13.0 for Windows was used to subject them to one-way ANOVA, where the significant difference between treatments was evaluated by Turkey’s multiple comparison tests. Meanwhile, the estimation of the ideal dietary Cr supplementation level in the practical diet has been carried out by the linear-broken-line regression analysis [28, 29] according to FCR and SGR after the comparison of the estimated coefficient (R2) using the second-order polynomial regression model.
Results
Growth Performance
The growth performance results for the juvenile blunt snout bream are shown in Table 3. The survival rate was not significantly affected by dietary Cr (Cr as a form of Cr-Pic) supplementation (P > 0.05), but dietary Cr supplementation significantly affected the growth performance of juvenile blunt snout bream (P < 0.05). The highest values of the final weight (FW) and specific growth rate (SGR) were remarked in fish fed with 0.4 mg/kg of supplemented Cr in the diet, thereafter showed a relatively constant trend. In the same diet (0.4 mg/kg of Cr supplementation), the lowest value of feed conversion ratio (FCR) has been recorded. However, high dietary Cr (12.0 mg/kg) supplementation level has decreased the growth and feed utilization of juvenile fish (P < 0.05). Based on SGR (Fig. 1) and FCR (Fig. 2) using the broken-line regression model, the optimum level of Cr supplementation in the practical diet was estimated at 2.8 mg/kg.
Table 4 presented whole-body compositions. The contents of whole-body moisture, protein, and ash significantly were not affected by dietary treatments. In fish fed diet supplemented with 0.4 mg/kg of Cr, the highest content of whole-body lipid was found compared to other fed diets supplemented with 0, 0.2, 0.8, and 12.0 mg/kg Cr (P < 0.05). As shown in Fig. 3, the addition of 0.2–1.6 mg/kg of Cr in the diets significantly reduced the content of hepatic glycogen in juvenile blunt snout bream in comparison to the fish fed without Cr supplementation in the diets.
Plasma Parameters
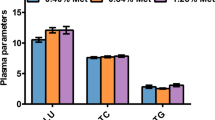
The results are shown in Table 5, where total protein concentration in the plasma was not significantly affected by Cr supplementation (P > 0.05). Likewise, the high levels of triglyceride were remarked in fish fed with supplements of 0.4 and 0.8 mg/kg Cr in the diets and were significantly higher than those fed other diets. Glucose concentrations decreased with rising dietary Cr supplementation and reached the lowest level when the juveniles were fed with 3.2 mg/kg of supplemented Cr in the diets (P < 0.05). Significantly higher plasma insulin levels were found in juvenile fed diets with 0.4 and 0.8 mg/kg Cr supplementation compared to those fed diet sans supplemented Cr.
Relative Gene Expression
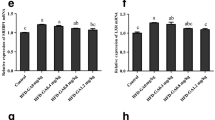
Relative mRNA levels of IRS-1, PI3K (insulin signaling pathway-related genes), and plasma insulin levels were significantly affected by supplemented Cr in the diets. The highest IRS-1 expression was observed in juvenile fed with 0.8 mg/kg Cr supplementation in the diet, and thereafter showed a decreased trend (P < 0.05). Significantly higher PI3K expressions were found in juvenile fed diets with 0.4, 0.8, and 1.6 mg/kg Cr supplementation compared to those fed diets with 3.2 and 12.0 mg/kg Cr supplementation (P < 0.05) (Fig. 4).
Relative expression of insulin signaling pathway-related genes including insulin receptor substrate 1 (IRS-1) (a) and phosphoinositide-3-kinase (PI3K) (b) genes in the liver of juvenile blunt snout bream fed experimental diets for 11 weeks. Values are means for nine fish per treatment, with standard errors represented by vertical bars. Relative mRNA levels of IRS-1 and PI3K were normalized, and significant differences within the diets are indicated by different superscripts (P < 0.05)
The levels of relative mRNA expression are presented in Fig. 5. The highest pyruvate kinase (PK) was remarked in the fish fed with 0.4 of supplemented Cr in the diets (P < 0.05). Phosphoenolpyruvate carboxykinase (PEPCK), glycogen synthase (GS), and glucose-6-phosphatase (G6pase) mRNA expressions showed a downward trend with increasing of the Cr supplementation level in the diets, and the lowest value was recorded in the dietary treatments supplemented with 0.4 or 0.8 mg/kg of Cr, respectively. Subsequently, these three gene expressions showed an increased trend. The mRNA levels of lipogenesis-related genes are shown in Fig. 6. The sterol regulatory element binding protein-1 (SREBP1) and fatty synthase (FAS) augmented with the supplementation of dietary Cr up to 0.8 mg/kg, and thereafter showed a decreased trend (P < 0.05).
Relative expression of glucose metabolism-related genes including pyruvate kinase (PK) (a), phosphoenolpyruvate carboxykinase (PEPCK) (b), glucose-6-phosphatase (G6Pase) (c), and glycogen synthase (GS) (d) in the liver of juvenile blunt snout bream fed experimental diets for 11 weeks. Values are means for nine fish per treatment, with standard errors represented by vertical bars. Relative mRNA levels of PK, PEPCK, G6Pase, and GS were normalized, and significant differences within the diets are indicated by different superscripts (P < 0.05)
Relative expression of lipogenesis-related genes including sterol regulatory element binding protein-1 (SREBP1) (a) and fatty acid synthase (FAS) (b) in the liver of juvenile blunt snout bream fed experimental diets for 11 weeks. Values are means for nine fish per treatment, with standard errors represented by vertical bars. Relative mRNA levels of SREBP1 and FAS were normalized and significant differences within the diets are indicated by different superscripts (P < 0.05)
Discussion
In the current study, the survival rate of the fish was high and independent of dietary Cr supplementation, which is in agreement with previous reports in the mirror and common carp [15, 16], while adding 5.0 mg/kg of chromium polynicotinate in the yellow croaker diet has significantly improved its survival rate [17]. Dietary 0.2–3.2 mg/kg Cr supplementation has improved growth performance of the juvenile, and optimal Cr supplementation level in the practical diet for the fish was assessed to be 0.28 mg/kg. Similar benefits on other farmed animals were detailed and explained in the scientific literature [12]. Many studies have reported that the Cr supplementation in fish diets improves the growth performance. Supplemental 0.5 mg/kg organic Cr in a starch-based diet revealed positive effects on growth performance for the common carp [15]. Adding 0.8 mg/kg of Cr in the diet could increase growth and change serum lipid and carbohydrate profiles in the grass carp [14]. However, trivalent chromium supplementation has not improved growth performance for the channel catfish and rainbow trout [18, 20, 30]. Based on the previous studies, the beneficial advantage of supplemented Cr in fish diets might be species-specific and dependent on the dose and form of the used Cr.
In this study, 0.4 mg/kg of supplemented Cr has improved feed utilization of juvenile blunt snout bream versus to those fed diet sans supplemented Cr, which might be due to the increase of dietary nutrient utilization [17]. It has been confirmed that in mammals, the IRS-PI3k-Akt insulin pathway plays a key role in glucose and lipid utilization and metabolism [31].The findings of this study indicated that highest mRNA expression values of IRS-1 and PI3K were observed in fish diet supplemented with 0.4–0.8 mg/kg of Cr and were significantly higher than those sans Cr supplementation. Furthermore, in our study, we found that plasma insulin content shows a positive relationship with IRS-1 and PI3K. Identical findings have been found in higher animals, where the chromium has been proved as motivator for the insulin binding to cells, enhancer for insulin receptor number, and stimulator for IRS-1 phosphorylation and PI3K activity in mice [32,33,34], the latter associated with the blood glucose homeostasis and used by the cells [35]. In agreement with aforementioned findings in mammals, our results indicated that Cr supplementation in the diets has decreased plasma glucose concentrations in juvenile blunt snout bream, and we suggest that the optimal Cr supplementation has a beneficial effect on blood glucose homeostasis which might be involved in insulin signaling molecules and insulin production. Similarly, Hertz et al. [36] reported that chromium chloride supplementation decreased serum glucose level in the common carp.
Likewise, the glycolysis is the route of glucose catabolism in the animals including fish, and the regulation of gluconeogenesis is depends on key enzymes’ activities such as PK [37]. In our research, the highest level of relative mRNA expression of PK was detected in the diet supplemented with 0.8 mg/kg of Cr. Similar results have been obtained in dietary treatments for the common carp where the high activities of hexokinase (HK) have been recorded in the fish fed starch-based supplemented with 0.5–1.0 mg/kg of Cr in the diet [15]. The present study showed that 0.4 mg/kg dietary Cr supplementation significantly lowered the relative mRNA expression of PEPCK and G6Pase. Similarly, Hertz et al. [36] reported that chromium chloride supplementation inhibited gluconeogenesis from amino acids in the common carp. In this study, we suggested that optimal Cr supplementation in juvenile blunt snout bream commercial diet could regulate mRNA expression related to glycolysis and gluconeogenesis. Similarly, in streptozotocin-induced experimental diabetes, there is an ameliorating effect of chromium administration on hepatic glucose metabolism by improving glycolysis and suppressing gluconeogenesis [6]. In fish, glycogen plays a key role in the metabolism and homeostasis of glucose, and a synthesis of the glycogen is catalyzed by GS as the same procedure in mammals [37]. In the current study, the content of hepatic glycogen and GS relative mRNA level was regulated by dietary Cr supplementation and presented alike trend. In a like manner, a positive relationship between total GS activity and hepatocyte glycogen content was reported in rainbow trout [38]. Liu et al. [14] reported although the higher hepatic glycogen level was indicated in grass carp fed a diet with low chromium level, glycogen content showed a downward trend with the augmentation of dietary chromium supplementation (above than 0.4 mg/kg), which may be considered as partially in agreement with our results. The findings from this research about GS hepatic mRNA level and content of glycogen showed negative relation with IRS-1 and PI3K expression, which suggested glycogen synthesis might be sensitive to insulin signaling regulated by supplemented Cr in the diets. In agreement with the current trial, after insulin injection in the teleost Pimelodus maculatus, some abnormities have been recorded such as the depletion of hepatic glycogen content and has led to the diminution of glucose level in the plasma [39]. In contrast, Cr has proven as an insulin activator and stimulator for glycogen synthesis by increasing or stabilizing GS mRNA in higher animals [40]. The mechanism that dietary Cr regulates hepatic glycogen synthesis in juvenile blunt snout bream is still unclear and needs to be further investigated.
In the current study, the supplementation of Cr in the diets has not changed the moisture, protein, and ash content of whole-body composition, which is in agreement with the reports in other carp species [16]. Though the fish fed diet with milligram/kilogram Cr supplementation showed highest whole-body lipid content among the dietary treatments. Similar results have been recorded in other fish species such as in grass carp [14] and mirror carp nourished with maize starch [16], also in higher animals such as in beef cows [41]. The same trends were shown in plasma triacylglyceride level and relative expression of lipogenesis-related genes such as SREBP1 and FAS. It is worth noting that whole-body lipid, plasma triacylglyceride level, and the relative mRNA expression levels of lipogenesis-related genes declined with the increasing dietary Cr supplementation, when the adding levels of Cr in the diets were higher than 0.4 mg/kg, suggesting that high supplemented level in the diet might inhibit the synthesis of fatty acids at least in the level of mRNA of juvenile blunt snout bream. In pigs, dietary Cr supplementation might also inhibit the synthesis of fatty acids, which supports our result [42]. Recently, a new understanding into the insulin signaling pathway that controls SREBP1 has been revealed in mammals [43]. SREBP1 can be responsible for the biosynthesis of fatty acids by regulating the FAS gene; the latter plays a fault-finding role in lipogenesis metabolic actions [44,45,46]. Regardless of mammals, SREBP1 and FAS mRNA have indicated a positive relation to the expression of the insulin signaling pathway-related gene (IRS-1 and PI3K) in this research, suggesting that the lipogenesis gene expression upregulation by dietary Cr could be related to the improved insulin signaling molecules. However, this study reports the Cr effect on the gene expression related to lipogenesis for the first time in fish. Whether Cr supplementation affects the aforementioned enzymes’ activities or genes in fish needs more investigation and clarification.
In this study, significant depressions of feed utilization and growth were observed in the fish fed diet with 12.0 mg/kg (extremely high) Cr supplementation, whereas reflect the same results reported in fish and other animals [12]. In the previous studies, the decreases in feed utilization and growth were attributed to Cr toxic effect with a high supplemented level. This extreme level as a form of chromium chloride in the common carp diet had an adverse impact on the hepatic structural level and midsection of the intestine which negatively affected the growth performance and fish health [15]. The exposure of goldfish to the chromium also results in an oxidative stress in the liver and kidney [47]. Several experiments in cell culture have been undertaken to grasp the effect of high level of Cr and led to suggest that the possibility of increasing DNA damage and caused toxicity when the chromium taken in excess [48]. However, Komorowski et al. [49] reported that Cr-Pic did not provoke damage of chromosomes in the cell of the bone marrow even with a dose of 2000 mg/kg body weight and consequently any Cr-Pic toxicity has been recorded. In the current study, the poor growth performance and feed utilization seem to be due to depressed utilization and metabolism of glucose by the fish fed diet with high Cr level. We clearly observed a high level of blood glucose and relative expression of gluconeogenesis-related gene (PEPCK and G6Pase) which might be affected by depressed gene expression related to the insulin signaling pathway (IRS-1 and PI3K) in the fish given diet supplemented with 12.0 mg/kg of Cr compared to those fed other diets.
Conclusions
The findings of this study suggested that Cr (as the form of Cr-Pic) supplementation in the practical diet of juvenile blunt snout bream has positive effects on growth and blood glucose homeostasis by modifying glucose metabolism, and the optimal dietary Cr supplementation level is estimated to be 0.28 mg/kg. However, excess dietary Cr (12.0 mg/kg) supplementation results in poor growth performance and enhancement of gene expression related to gluconeogenesis.
References
Vincent JB (2000) The biochemistry of chromium. J Nutr 130(4):715–718
Bona KRD, Love S, Rhodes NR, McAdory D, Sinha SH, Kern N, Kent J, Strickland J, Wilson A, Beaird J, Ramage J, Rasco JF, Vincent JB (2011) Chromium is not an essential trace element for mammals: effects of a “low- chromium” diet. J Biol Inorg Chem 16(3):381–390. https://doi.org/10.1007/s00775-010-0734-y
Anderson RA (1997) Chromium as an essential nutrient for humans. Regul Toxicol Pharmacol 26(1):35–41. https://doi.org/10.1006/rtph.1997.1136
Dallago BS, Braz S, Marçola TG, McManus C, Caldeira DF, Campeche A, Gomes EF, Paim TP, Borges BO, Louvandini H (2015) Blood parameters and toxicity of chromium picolinate oral supplementation in lambs. Biol Trace Elem Res 168(1):91–102. https://doi.org/10.1007/s12011-015-0347-y
Qiao W, Peng ZL, Wang ZS, Wei J, Zhou AG (2009) Chromium improves glucose uptake and metabolism through upregulating the mRNA levels of IR, GLUT4, GS, and UCP3 in skeletal muscle cells. Biol Trace Elem Res 131(2):133–142. https://doi.org/10.1007/s12011-009-8357-2
Sundaram B, Singhal K, Sandhir R (2012) Ameliorating effect of chromium administration on hepatic glucose metabolism in streptozotocin-induced experimental diabetes. Biofactors 38(1):59–68. https://doi.org/10.1002/biof.194
Jain SK, Rains JL, Croad JL (2007) Effect of chromium niacinate and chromium picolinate supplementation on lipid peroxidation, TNF-α, IL-6, CRP, glycated hemoglobin, triglycerides, and cholesterol levels in blood of streptozotocin-treated diabetic rats. Free Radic Biol Med 43(8):1124–1131. https://doi.org/10.1016/j.freeradbiomed.2007.05.019
NRC (National Research Council) (1997) The role of chromium in animal nutrition. National Academy, Washington, DC
Evans GW, Pouchnik DJ (1993) Composition and biological activity of chromium-pyridine carboxylate complexes. J Inorg Biochem 49(3):177–187. https://doi.org/10.1016/0162-0134(93)80003-R
Preuss HG, Echard B, Perricone NV, Bagchi D, Yasmin T, Stohs SJ (2008) Comparing metabolic effects of six different commercial trivalent chromium compounds. J Inorg Biochem 102(11):1986–1990. https://doi.org/10.1016/j.jinorgbio.2008.07.012
Wilson RP (1994) Utilization of dietary carbohydrate by fish. Aquaculture 124(1-4):67–80. https://doi.org/10.1016/0044-8486(94)90363-8
NRC (National Research Council) (2011) Nutrient requirements of fish and shrimp. National Academy, Washington, DC
Shiau SY, Chen MJ (1993) Carbohydrate utilization by tilapia Oreochromis niloticus × O.aureus was influenced by different chromium sources. J Nutr 123(10):1747–1753
Liu T, Wen H, Jiang M, Yuan DN, Gao P, Zhao YJ, Wu F, Liu W (2010) Effect of dietary chromium picolinate on growth performance and blood parameters in grass carp fingerling, Ctenopharyngodon idellus. Fish Physiol Biochem 36(3):565–572. https://doi.org/10.1007/s10695-009-9327-5
Ahmed AR, Jha AN, Davies SJ (2012) The effect of dietary organic chromium on specific growth rate, tissue chromium concentrations, enzyme activities and histology in common carp, Cyprinus carpio L. Biol Trace Elem Res 149(3):362–370. https://doi.org/10.1007/s12011-012-9436-3
Ahmed AR, Jha AN, Davies SJ (2012) The efficacy of chromium as a growth enhancer for mirror carp (Cyprinus carpio L): an integrated study using biochemical, genetic, and histological responses. Biol Trace Elem Res 148(2):187–197. https://doi.org/10.1007/s12011-012-9354-4
Wang J, Ai QH, Mai KS, Xu HG, Zuo RT (2014) Dietary chromium polynicotinate enhanced growth performance, feed utilization, and resistance to Cryptocaryon irritans in juvenile large yellow croaker (Larmichthys crocea). Aquaculture 432:321–326. https://doi.org/10.1016/j.aquaculture.2014.05.027
Ng WK, Wilson RP (1997) Chromium oxide inclusion in the diets does not affect glucose utilization and chromium retention by channel catfish, Ictalurus punctatus. J Nutr 12:2357–2362
Gatta PP, Piva A, Paolini M, Testi S, Bonaldo A, Antelli A, Mordenti A (2001) Effects of dietary organic chromium on gilthead seabream (Sparus aurata L.) performances and liver microsomal metabolism. Aquac Res 32(Suppl. 1):60–69. https://doi.org/10.1046/j.1355-557x.2001.00005.x
Selcuk Z, Tiril SU, Alagil F, Belen V, Salman M, Cenesiz S, Muglali OH, Yagci FB (2010) Effects of dietary L-carnitine and chromium picolinate supplementations on performance and some serum parameters in rainbow trout (Oncorhynchus mykiss). Aquacult Int 18(2):213–221. https://doi.org/10.1007/s10499-008-9237-z
Ministry of Agriculture of the People’s Republic of China (2016) China fishery statistical yearbook. Chinese Agricultural Press, Beijing (in Chinese)
Zhou CP, Liu B, Ge XP, Xie J, Xu P (2013) Effect of dietary carbohydrate on the growth performance, immune response, hepatic antioxidant abilities and heat shock protein 70 expression of Wuchang bream, Megalobrama amblycephala. J Appl Ichthyol 29(6):1348–1356. https://doi.org/10.1111/jai.12264
Ren MC, Habte-Tsion HM, Xie J, Liu B, Zhou QL, Ge XP, Pan LK, Chen RL (2015) Effects of dietary carbohydrate source on growth performance, diet digestibility and liver glucose enzyme activity in blunt snout bream, Megalobrama amblycephala. Aquaculture 438:75–81. https://doi.org/10.1016/j.aquaculture.2015.01.008
AOAC (Association of Official Analytical Chemists) (2003) Official methods of analysis of the association of official analytical chemists, 15th edn. Association of Official Analytical Chemists Inc, Arlington
Habte-Tsion HM, Liu B, Ren MC, Ge XP, Xie J, Zhou QL, Miao LH, Pan LK, Chen RL (2015) Dietary threonine requirement of juvenile blunt snout bream (Megalobrama amblycephala). Aquaculture 437:304–311. https://doi.org/10.1016/j.aquaculture.2014.12.018
Gao ZX, Luo W, Liu H, Zeng C, Liu XL, Yi SK, Wang WM (2012) Transcriptome analysis and SSR/SNP markers information of the blunt snout bream (Megalobrama amblycephala). PLoS One 7(8):e42637. https://doi.org/10.1371/journal.pone.0042637
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2- ∆∆CT method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Robbins KR, Norton HW, Baker DH (1979) Estimation of nutrient requirement from growth data. J Nutr 109(10):1710–1714
Mukhtar AK, Shabi FA (2013) Dietary methionine requirement of Indian major carp fry, Cirrhinus mrigala (Hamilton) based on growth, feed conversion and nitrogen retention efficiency. Aquac Res 44(2):268–281. https://doi.org/10.1111/j.1365-2109.2011.03030.x
Tacon AGJ, Beveridge MM (1982) Effects of dietary trivalent chromium on rainbow trout. Nutr Rep Int 25:49–56
Kahn BB, Flier JS (2000) Obesity and insulin resistance. J Clin Invest 106(4):473–481. https://doi.org/10.1172/JCI10842
Yang X, Palanichamy K, Ontko AC, Rao MN, Fang CX, Ren J, Sreejayan N (2005) A newly synthetic chromium complex chromium (phenylalanine) 3 improves insulin responsiveness and reduces whole body glucose tolerance. FEBS Lett 579(6):1458–1464. https://doi.org/10.1016/j.febslet.2005.01.049
Chen WY, Chen CJ, Liu CH, Mao FC (2009) Chromium supplementation enhances insulin signalling in skeletal muscle of obese KK/HlJ diabetic mice. Diabetes Obes Metab 11(4):293–303. https://doi.org/10.1111/j.1463-1326.2008.00936.x
Wang ZQ, Zhang XH, Russell JC, Hulver M, Cefalu WT (2006) Chromium picolinate enhances skeletal muscle cellular insulin signaling in vivo in obese, insulin-resistant JCR: LA-cp rats. J Nutr 136(2):415–420
Anderson RA, Polansky MM, Bryden NA, Canary JJ (1991) Supplemental chromium effects on glucose, insulin, glucagon and urinary loss in subjects consuming low chromium diets. Am J Cardiovasc Drugs 54:909–916
Hertz Y, Mader Z, Hepher B, Gertler A (1989) Glucose metabolism in the common carp (Cyprinus carpio L.): the effect of cobalt and chromium. Aquaculture 76(3-4):255–267. https://doi.org/10.1016/0044-8486(89)90079-3
Enes P, Panserate S, Kaushik S, Oliva-Teles A (2009) Nutritional regulation of hepatic glucose metabolism in fish. Fish Physiol Biochem 35(3):519–539. https://doi.org/10.1007/s10695-008-9259-5
Pereira C, Vijayan MM, Storey KB, Jones RA, Moon TW (1995) Role of glucose and insulin in regulating glycogen synthase and phosphorylase activities in rainbow trout hepatocytes. J Comp Physiol 165B:62–70. https://doi.org/10.1007/BF0026468
Carneiro NM, Amaral AD (1983) Effects of insulin and glucagon on plasma glucose levels and glycogen content in organs of the freshwater teleost Pimelodus maculatus. Gen Comp Endocr 49(1):115–121. https://doi.org/10.1016/0016-6480(83)90014-X
Wang ZQ, Zhang XH, Cefalu WT (2000) Chromium picolinate and biotin enhance glycogen synthesis and glycogen synthase gene expression in human skeletal muscle culture. Diabetes Res Clin Pract 50:395–395. https://doi.org/10.1016/S0168-8227(00)81348-0
Stahlhut HS, Whisnant CS, Lloyd KE, Baird EJ, Legleiter LR, Hansen SL, Spears JW (2006) Effect of chromium supplementation and copper status on glucose and lipid metabolism in Angus and Simmental beef cows. Anim Feed Sci Technol 128(3-4):253–265. https://doi.org/10.1016/j.anifeedsci.2005.11.002
Xi G, Xu Z, Wu SH, Chen SJ (2000) Effect of chromium picolinate on growth performance, carcass characteristics, serum metabolites and metabolism of lipid in pigs. Asian-Aust J Anim Sci 14:258–262
Wong RHF, Sul HS (2000) Insulin signaling in fatty acid and fat synthesis: a transcriptional perspective. Curr Opin Pharmacol 10(6):684–691. https://doi.org/10.1016/j.coph.2010.08.004
Shimano H, Horton JD, Shimomura I, Hammer RE, Brown MS, Goldstein JL (1997) Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J Clin Invest 99(3):846–854. https://doi.org/10.1111/acel.12576
Sul HS, Wang D (1998) Nutritional and hormonal regulation of enzymes in fat synthesis: studies of fatty acid synthase and mitochondrial glycerol-3-phosphate acyltransferase gene transcription. Annu Rev Nutr 18(1):331–351. https://doi.org/10.1146/annurev.nutr.18.1.331
Kim KH, Shin HJ, Kim K, Choi HM, Rhee SH (2007) Hepatitis B virus X protein induces hepatic steatosis via transcriptional activation of SREBP1 and PPARgamma. Gastroenterology 132(5):1955–1967. https://doi.org/10.1053/j.gastro.2007.03.039
Lushchak OV, Kubrak OI, Lozinsky OV, Storey JM, Storey KB, Lushchak VI (2009) Chromium (III) induces oxidative stress in goldfish liver and kidney. Aquat Toxicol 93(1):45–52. https://doi.org/10.1016/j.aquatox.2009.03.007
Landman GWD, Bilo HJD, Houweling ST, Kleefstra N (2014) Chromium does not belong in the diabetes treatment arsenal: current evidence and future perspectives. World J Diabetes 5(2):160–164. https://doi.org/10.4239/wjd.v5.i2.160
Komorowski JR, Greenberg D, Juturu V (2008) Chromium picolinate does not produce chromosome damage. Toxicol in Vitro 22(3):819–826. https://doi.org/10.1016/j.tiv.2007.12.007
Acknowledgements
The funding of the current research was supported by the National Science Foundation of China (31772820), the National Science Foundation of Jiangsu Province (BK20161143), and the Modern Agriculture Industrial Technology System special project-the national technology system for Conventional Freshwater Fish Industries (CARS-46).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The present study was approved by the Animal Care and Use Committee of Nanjing Agricultural University (Nanjing, China).
Rights and permissions
About this article
Cite this article
Ren, M., Mokrani, A., Liang, H. et al. Dietary Chromium Picolinate Supplementation Affects Growth, Whole-Body Composition, and Gene Expression Related to Glucose Metabolism and Lipogenesis in Juvenile Blunt Snout Bream, Megalobrama amblycephala. Biol Trace Elem Res 185, 205–215 (2018). https://doi.org/10.1007/s12011-018-1242-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1242-0