Abstract
Magnesium and zinc are known to exert multiple beneficial effects including anti-inflammatory and antioxidant actions. To our knowledge, data on the effects of magnesium and zinc co-supplementation on biomarkers of inflammation and oxidative stress and gene expression related to inflammation in subjects of polycystic ovary syndrome (PCOS) are scarce. This study was conducted to evaluate the effects of magnesium and zinc co-supplementation on biomarkers of inflammation and oxidative stress and gene expression related to inflammation in subjects with PCOS. This randomized double-blind, placebo-controlled trial was conducted among 60 subjects with PCOS diagnosed according to the Rotterdam criteria, aged 18–40 years old. Participants were randomly assigned into two groups to take either 250 mg of magnesium oxide plus 220 mg of zinc sulfate (containing 50 mg zinc) supplements (n = 30) or placebo (n = 30) twice a day for 12 weeks. Biomarkers of inflammation and oxidative stress were assessed at baseline and at end of treatment. Gene expression related to inflammatory cytokines was assessed in peripheral blood mononuclear cells (PBMCs) of PCOS women with RT-PCR method. After the 12-week intervention, compared with the placebo, magnesium and zinc co-supplementation significantly decreased serum high-sensitivity C-reactive protein (hs-CRP) (− 1.6 ± 2.4 vs. + 0.1 ± 0.7 mg/L, P = 0.001) and protein carbonyl (PCO) (− 0.14 ± 0.28 vs. + 0.02 ± 0.07 mmol/mg protein, P = 0.002) and significantly increased plasma total antioxidant capacity (TAC) levels (+ 60.7 ± 69.4 vs. − 1.5 ± 141.5 mmol/L, P = 0.03). Results of RT-PCR demonstrated that compared with the placebo, magnesium and zinc co-supplementation downregulated gene expression of interleukin-1 (IL-1) (P = 0.007) and tumor necrosis factor alpha (TNF-α) (P = 0.03) in PBMCs of subjects with PCOS. Overall, magnesium and zinc co-supplementation, compared with the placebo, for 12 weeks among PCOS women had beneficial effects on serum hs-CRP, plasma PCO, TAC, and gene expression of IL-1 and TNF-α. Clinical trial registration number: http://www.irct.ir: IRCT201706075623N121.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrinopathies in subjects of reproductive age that involves clinical and metabolic disorders, including oligo menorrhoea or amenorrhoea, insulin resistance, cardiovascular abnormities, hyperandrogenemia, hirsutism, and androgen alopecia [1]. It affects 6–15% of reproductive-aged women among different geographic regions according to the Rotterdam criteria [2]. Previous studies in PCOS women have showed that hyperglycemia and insulin resistance induce an increase in reactive oxygen species (ROS) production by peripheral blood leukocytes [3, 4] and the pro-inflammatory transcription factor nuclear κB (NF-κB) [5] and an increase of pro-inflammatory markers [6].
Calcium-vitamin D, magnesium-zinc-calcium-vitamin D co-supplementation has earlier been used by other researchers. Co-supplementation may be efficient more than single supplementation. In addition, previous studies have documented that calcium and vitamin D co-supplementation might have a strong synergistic effect on metabolic profiles. For instance, we have previously indicated that calcium and vitamin D co-supplementation compared with calcium or vitamin D only for 8 weeks among PCOS women had beneficial effects on biomarkers of inflammation and oxidative stress [7]. In addition, magnesium-zinc-calcium-vitamin D co-supplementation resulted in significant reductions in hirsutism, high-sensitivity C-reactive protein (hs-CRP), and plasma malondialdehyde (MDA) and a significant increase in plasma total antioxidant capacity (TAC) levels, but did not affect other biomarkers of inflammation and oxidative stress [8]. Calcium (1000 mg/day) and vitamin D (50,000 IU/week) co-supplementation might significantly improve systemic inflammation through decreasing interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) levels in vitamin D-insufficient subjects with type 2 diabetes mellitus (T2DM) [9]. However, magnesium supplementation as magnesium oxide at dosage of 250 mg/day for 8 weeks in overweight women did not influence inflammatory markers [10]. In addition, no significant effect in hs-CRP concentrations was seen after the intake of 10 mg rosuvastatin for 4 months with or without zinc supplements (30 mg/day) in subjects with atherosclerosis [11].
According to our knowledge, data on the effects of magnesium and zinc co-supplementation on biomarkers of inflammation and oxidative stress and gene expression related to inflammation in subjects with PCOS are scarce. Therefore, we hypothesized that taking magnesium plus zinc might affect metabolic status of PCOS population. We investigated this aim by conducting the effects of magnesium and zinc co-supplementation on biomarkers of inflammation and oxidative stress, and gene expression related to inflammation in women with PCOS.
Patients and Methods
The current randomized double-blind, placebo-controlled trial, registered in the Iranian website for registration of clinical trials as http://www.irct.ir: IRCT201706075623N121, was performed among 60 subjects with PCOS diagnosed according to the Rotterdam criteria [12], aged 18–40 years old who were referred to the Naghavi Clinic in Kashan, Iran, from June 2017 to August 2017. The present study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines, and written informed consent was taken from all participants. We excluded women who were pregnant during the intervention, and metabolic disorders, including androgen-secreting tumors, thyroid dysfunction, diabetes or impaired glucose tolerance at enrollment, insulin injection, and taking anti-inflammatory drugs.
Study Design
At first, participants were matched according to BMI (< 25 and ≥ 25 kg/m2), age (< 30 and ≥ 30 years), and phenotypes A (14 subjects in each group) and D (16 subjects in each group) of PCOS. Then, PCOS women were randomized into two groups to take either 250 mg of magnesium oxide plus 220 mg of zinc sulfate (containing 50 mg zinc) supplements (n = 30) or placebo (n = 30) twice a day for 12 weeks. Shape and size of magnesium and zinc supplements and placebos were similar and manufactured by 21st Century Pharmaceutical Company (AZ, USA), Alhavi Pharmaceutical Company (Tehran, Iran), and Barij Essence Pharmaceuticals (Kashan, Iran), respectively. Randomization assignment was performed using computer-generated random numbers. Randomization and allocation concealment were done from the researchers and participants and were carried out by a trained staff member at the gynecology clinic. Participants were asked not to alter their routine physical activity or usual dietary intakes during the study and were asked not to consume any supplements that might influence related markers during the intervention. All participants provided 3-day dietary records and three physical activity records to verify that they maintained their usual diet and physical activity during the intervention. Both dietary records and physical activity were taken at the baseline and weeks 3, 6, 9, and 12 during the intervention. To obtain information on participant nutrient intake based on these 3-day food diaries, we used Nutritionist IV software (First Databank, San Bruno, CA) modified for Iranian foods.
Assessment of Anthropometric Measures
A trained midwife at the clinic took anthropometric measurements at baseline and 12 weeks following the intervention. Height and weight (Seca, Hamburg, Germany) were measured while the participants wore light clothing and no shoes. Body mass index (BMI) was calculated as weight (in kilograms) divided by height (in meters) squared.
Assessment of Outcomes
Inflammatory markers were considered as the primary outcomes, and biomarkers of oxidative stress were considered as the secondary outcomes.
Biochemical Assessment
Twenty-milliliter fasting blood samples were collected at the baseline and after the 12-week intervention at Kashan Reference Laboratory, Kashan, Iran. Serum hs-CRP values were quantified using an ELISA kit (LDN, Nordhorn, Germany) with inter- and intra-assay coefficient variances (CVs) of 4.8 to 6.5%, respectively. The plasma nitric oxide (NO) using Griess method [13] was determined. Plasma TAC concentrations using the method of ferric-reducing antioxidant power developed by Benzie and Strain [14], total glutathione (GSH) using the method of Beutler et al. [15], and MDA concentrations by the thiobarbituric acid reactive substance spectrophotometric test [16] were determined. CVs for plasma TAC, GSH, and MDA were lower than 5%, respectively. Plasma protein carbonyl (PCO) levels were quantified using a spectrophotometric method [17] with inter- and intra-assay CVs of lower than 5%.
Isolation of Lymphocyte, RNA Extraction, and cDNA Synthesis
Lymphocytes were isolated using 50% Percoll solution (Sigma-Aldrich, Dorset, UK) gradient by centrifugation for 20 min and 3000 rpm at 4 °C [18]. Total RNA was extracted based on acid guanidinium-phenol-chloroform procedure using RNX™-plus reagent (Cinnacolon, Tehran, Iran) according to the manufacturer’s instructions. RNAs were treated with DNase I (Fermentas, Lithuania) for the elimination of any genomic DNA contamination. Concentration, integration, and purity of RNA samples were determined by spectrometry and gel electrophoresis. Three micrograms of total RNA was used for cDNA synthesis with random hexamer and oligo (dT) 18 primers through RevertAid™ Reverse Transcriptase (Fermantase, Canada) in total 20 μL reaction mixture [18].
Real-Time PCR Analysis
Appropriate primers for interleukin-1 (IL-1), IL-8, TNF-α, transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), and glyceraldehyde-3-phosphate dehydrogenase—as an internal control—were designed (Table 1). Quantitative real-time PCR was performed by the LightCycler® 96 sequence detection systems (Roche Diagnostics, Rotkreuz, Switzerland) using 4 μL of 5× EvaGreen I master mix (Salise Biodyne, Japan), 10 ng cDNA, and 200 nM of each forward and reverse primers in final volume of 20 μL. The PCR was performed through the following instruction: an initial denaturation at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 10 s, annealing at 54–62.1 °C for 15 s, and extension at 72 °C for 30 s. The specificity of PCR products was evaluated by 1.5% agarose gel electrophoresis and melting curve analysis. All experiments were performed at least in triplicate.
Statistical Methods
To calculate the sample size, we used the standard formula suggested for clinical trials by considering type 1 error (α) of 0.05 and type 2 error (β) of 0.20 (power = 80%). Based on a previous study [19], we used 1391.56 ng/mL as SD and 1120.0 ng/mL as the difference in mean (d) of hs-CRP as primary variable. Based on this, we needed 25 subjects in each group. Considering a dropout of 5 subjects per group, we calculated to have 30 subjects per group.
The Shapiro-Wilk test was applied to control the normal distribution of variables. The analyses were carried out based on intention-to-treat (ITT) principle. To detect differences in anthropometric measures, macro- and micro-nutrient intakes, and gene expression related to inflammation between the two groups, we used independent t test. To determine the effects of magnesium and zinc co-supplementation on biomarkers of inflammation and oxidative stress, we used one-way repeated measures analysis of variance. Adjustment for changes in baseline values of biochemical parameters was performed by analysis of covariance (ANCOVA) using general linear models. The P value of < 0.05 was considered statistically significant. All statistical analyses used the Statistical Package for Social Science version 18 (SPSS Inc., Chicago, IL, USA).
Results
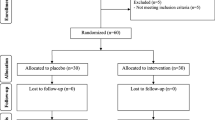
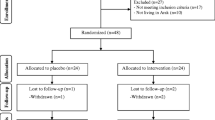
Firstly, we invited 75 subjects with PCOS; however, 15 subjects were excluded from the study because of not meeting inclusion criteria [(not meeting inclusion criteria (n = 10) and not living in Kashan (n = 5)] (Fig. 1). As demonstrated in the study flow diagram, during the intervention phase of the study, three participants in the placebo group and four participants in the intervention group due to personal reasons were excluded. Finally, 60 participants [placebo (n = 27) and intervention (n = 26)] completed the trial. However, as the analysis was based on ITT principle, all 60 participants (30 in each group) were included in the final analysis.
Participants’ mean age, height, and weight and BMI at the baseline and end of trial were not statistically different between the two groups (data not shown).
Based on the 3-day dietary records obtained at the baseline, end of treatment, and throughout the study, we found no significant effect in mean dietary macro- and micro-nutrient intakes between the two groups (data not shown).
After the 12-week intervention, compared with the placebo, magnesium and zinc co-supplementation significantly increased serum magnesium (+ 0.21 ± 0.24 vs. − 0.05 ± 0.17 mg/dL, P < 0.001) and zinc (+ 6.6 ± 3.9 vs. − 0.6 ± 3.9 mg/dL, P < 0.001). In addition, compared with the placebo, magnesium and zinc co-supplementation significantly decreased serum hs-CRP (− 1.6 ± 2.4 vs. + 0.1 ± 0.7 mg/L, P = 0.001) and PCO (− 0.14 ± 0.28 vs. + 0.02 ± 0.07 mmol/mg protein, P = 0.002) and significantly increased plasma TAC levels (+ 60.7 ± 69.4 vs. − 1.5 ± 141.5 mmol/L, P = 0.03) (Table 2). We did not observe any significant effect of magnesium and zinc co-supplementation on plasma NO, GSH, and MDA compared with the placebo.
Baseline values of plasma NO (P = 0.01), TAC (P = 0.01), and GSH (P = 0.01) were significantly different between the two groups. Therefore, we controlled the analyses for baseline values. When we adjusted the analysis for baseline values of biochemical parameters, plasma TAC (P = 0.14) became non-significant, while plasma NO (P = 0.03) became statistically significant, and other findings did not alter (Table 3).
Results of RT-PCR demonstrated that compared with the placebo, magnesium and zinc co-supplementation downregulated gene expression of IL-1 (P = 0.007) and TNF-α (P = 0.03) in PBMCs of subjects with PCOS (Fig. 2).
We did not observe any significant effect of magnesium and zinc co-supplementation on gene expression of IL-8, TGF-β, and VEGF compared with the placebo (Fig. 3).
Effect of 12-week supplementation with magnesium plus zinc or placebo on expression ratio of IL-8, TGF-β, and VEGF gene in blood mononuclear cells of PCOS women. IL-1 interleukin-1, IL-8 interleukin-8, PCOS polycystic ovary syndrome, TNF-α tumor necrosis factor alpha, TGF-β transforming growth factor beta, VEGF vascular endothelial growth factor
Discussion
To our knowledge, data on magnesium and zinc co-supplementation on biomarkers of inflammation and oxidative stress and gene expression related to inflammation among PCOS women are limited. We found that magnesium and zinc co-supplementation, compared with the placebo, for 12 weeks among PCOS women had beneficial effects on serum hs-CRP, plasma PCO, TAC, and gene expression of IL-1 and TNF-α, but did not affect NO, GSH, MDA, and gene expression of IL-8, TGF-β, and VEGF. It must be considered that there was a significant difference in baseline levels of plasma NO, TAC, and GSH between the magnesium-zinc and the placebo groups at study baseline. This difference might have been occurred due to several reasons. The diagnosis of PCOS in our study was done based on the Rotterdam criteria. Therefore, different patients might had different plasma NO, TAC, and GSH levels, which could in turn lead to a different mean of NO, TAC, and GSH at study baseline. Furthermore, we did not randomize participants based on their NO, TAC, and GSH levels because all participants had PCOS. Random assignment to two groups was done after stratification for BMI (< 25 and ≥ 25 kg/m2), age (< 30 and ≥ 30 years), and phenotypes A and D of PCOS, and random assignment was done by the use of computer-generated random numbers. Therefore, the difference in NO, TAC, and GSH levels between the two groups was occurred by random. In addition, when we adjusted the analyses for baseline values, no significant changes in our findings were observed except for TAC and No levels.
Subjects with PCOS are susceptible to some metabolic aberrations, including hormonal disturbances and increased biomarkers of inflammation and oxidative stress [7, 20]. The current study demonstrated that magnesium and zinc co-supplementation for 12 weeks to PCOS subjects led to significant reductions in serum hs-CRP levels and gene expression of IL-1 and TNF-α, but could not influence plasma NO levels and gene expression of IL-8, TGF-β, and VEGF. Supporting our study, results of a meta-analysis study indicated that magnesium supplementation decreased CRP concentrations among individuals with inflammation [21]. Some cross-sectional studies have documented inverse relationships between magnesium intake and some inflammatory markers, such as hs-CRP and IL-6 [22, 23]. In addition, a significant reduction in serum CRP levels was observed following the supplementation of magnesium as oral magnesium citrate at a dosage of 300 mg/day for 5 weeks among patients with heart failure [24]. Maternal magnesium supplementation as magnesium chloride suppressed cytokine/chemokine levels in the amniotic fluid and placentas in a rat model [25]. Moreover, neonates with sepsis who received zinc supplements in addition to antibiotics demonstrated a significant reduction in inflammatory cytokines, including IL-6 and TNF-α [26]. Supplementation with 30 mg/day of zinc supplements as zinc gluconate for 8 weeks to obese women resulted in a significant reduction in hs-CRP concentrations [27]. However, supplementation with 250 mg of magnesium as magnesium oxide for 8 weeks did not affect inflammatory markers in middle-aged overweight women [10]. In addition, no significant difference in hs-CRP values was seen following the intake of 10 mg rosuvastatin for 4 months with or without zinc supplements (30 mg/day) in subjects with atherosclerosis [11]. Biochemical levels and gene expression of some inflammatory cytokines and mediators have been shown to be elevated in PCOS women [28]. Prior studies have documented that increased inflammatory factors play a main role in hyperinsulinemia and the vascular inflammation process through multiple actions [29, 30]. Magnesium intake may decrease inflammatory factors due to its antagonism to calcium, the ion playing an important role in inflammation [31]. In addition, zinc intake may be associated with the regulation of NF-κB activation via anti-inflammatory protein A20 and peroxisome proliferator-activated receptor-α signaling pathway [27]. NF-κB as a component of the adhesion molecule upregulation process increases CRP concentrations and inflammatory markers, including IL-1β and TNF-α [32].
Our study demonstrated that magnesium and zinc co-supplementation for 12 weeks to women with PCOS resulted in a significant rise in plasma TAC and a significant reduction in plasma PCO levels, but did not influence plasma GSH levels. In line with our study, potassium magnesium citrate supplementation for 4 weeks significantly decreased biomarkers of oxidative stress in prehypertensive and hypertensive subjects [33]. Furthermore, high magnesium diet has been shown to attenuate aldosterone-induced rise in NADPH oxidase activity in the kidneys of mice with genetically low intracellular magnesium levels [34]. Zinc supplementation could also attenuate diabetes-induced oxidative stress in circulation as well as in cardiac and hepatic tissues in diabetic rats [35]. Unlike our study, the kidney and serum levels of MDA were increased significantly in non-diabetic rats treated with magnesium sulfate for 10 days [36]. Furthermore, Tang et al. [37] demonstrated that zinc supplementation did not influence MDA concentrations in rats with severe acute pancreatitis. Increased oxidative stress has been implicated in the pathogenesis of insulin resistance, dyslipidemia, and diabetes mellitus [38]. In addition, increased biomarkers of oxidative stress associated with increased risk of atherosclerosis [39]. Magnesium intake might decrease oxidative stress via reducing ROS production [40] and increasing glutathione-peroxidase activity [41]. Furthermore, zinc may contribute to the antioxidant role through its ability to compete with transition metals iron and copper for the binding sites on the cell membrane [35]. Iron and copper ions catalyze the production of lipid peroxides, and thereby, replacement of these metals by zinc in the plasma membrane could inhibit lipid peroxides in insulin resistance condition.
The current study had a number of limitations. Due to limited funding, we could not assess the effects of magnesium and zinc co-supplementation on gene expression related to insulin resistance and lipid in the current study. In addition, further studies are needed with single supplementation of each compared with co-supplementation to evaluate the beneficial effects on biomarkers of inflammation and oxidative stress.
Overall, our study demonstrated that magnesium and zinc co-supplementation, compared with the placebo, for 12 weeks among PCOS women had beneficial effects on serum hs-CRP, plasma PCO, TAC, and gene expression of IL-1 and TNF-α, but did not affect NO, GSH, MDA, and gene expression of IL-8, TGF-β, and VEGF. This suggests that magnesium and zinc co-supplementation may confer advantageous therapeutic potential for subjects with PCOS management. Further research is needed in other patients with longer periods to determine the safety of this supplemental approach.
Change history
28 February 2020
The Editors-in-Chief are currently investigating this article [Afshar Ebrahimi, F., Foroozanfard, F., Aghadavod, E. et al. The Effects of Magnesium and Zinc Co-Supplementation on Biomarkers of Inflammation and Oxidative Stress, and Gene Expression Related to Inflammation in Polycystic Ovary Syndrome: a Randomized Controlled Clinical Trial. Biol Trace Elem Res 184, 300–307 (2018). https://doi.org/10.1007/s12011-017-1198-5] as concerns have been raised about integrity of the clinical trial reported here. There is also an ongoing investigation by the Iranian National Committee for Ethics in Biomedical Researches. Further editorial action will be taken as appropriate once the investigation into the concerns is complete and all parties have been given an opportunity to respond in full.
References
Ullah A, Jahan S, Razak S, Pirzada M, Ullah H, Almajwal A, Rauf N, Afsar T (2017) Protective effects of GABA against metabolic and reproductive disturbances in letrozole induced polycystic ovarian syndrome in rats. J Ovarian Res 10(1):62. https://doi.org/10.1186/s13048-017-0359-7
Churchill SJ, Wang ET, Pisarska MD (2015) Metabolic consequences of polycystic ovary syndrome. Minerva Ginecol 67(6):545–555
Gonzalez F, Rote NS, Minium J, Kirwan JP (2006) Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab 91(1):336–340. https://doi.org/10.1210/jc.2005-1696
Victor VM, Rocha M, Banuls C, Sanchez-Serrano M, Sola E, Gomez M, Hernandez-Mijares A (2009) Mitochondrial complex I impairment in leukocytes from polycystic ovary syndrome patients with insulin resistance. J Clin Endocrinol Metab 94(9):3505–3512. https://doi.org/10.1210/jc.2009-0466
Gonzalez F, Rote NS, Minium J, Kirwan JP (2006) Increased activation of nuclear factor kappaB triggers inflammation and insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab 91(4):1508–1512. https://doi.org/10.1210/jc.2005-2327
Gonzalez F, Rote NS, Minium J, Kirwan JP (2006) In vitro evidence that hyperglycemia stimulates tumor necrosis factor-alpha release in obese women with polycystic ovary syndrome. J Endocrinol 188(3):521–529. https://doi.org/10.1677/joe.1.06579
Foroozanfard F, Jamilian M, Bahmani F, Talaee R, Talaee N, Hashemi T, Nasri K, Asemi Z, Esmaillzadeh A (2015) Calcium plus vitamin D supplementation influences biomarkers of inflammation and oxidative stress in overweight and vitamin D-deficient women with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Clin Endocrinol 83(6):888–894. https://doi.org/10.1111/cen.12840
Maktabi M, Jamilian M, Asemi Z (2017) Magnesium-zinc-calcium-vitamin D co-supplementation improves hormonal profiles, biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res. https://doi.org/10.1007/s12011-017-1085-0
Tabesh M, Azadbakht L, Faghihimani E, Esmaillzadeh A (2014) Calcium-vitamin D cosupplementation influences circulating inflammatory biomarkers and adipocytokines in vitamin D-insufficient diabetics: a randomized controlled clinical trial. J Clin Endocrinol Metab 99(12):E2485–E2493. https://doi.org/10.1210/jc.2014-1977
Moslehi N, Vafa M, Rahimi-Foroushani A, Golestan B (2012) Effects of oral magnesium supplementation on inflammatory markers in middle-aged overweight women. J Res Med Sci 17(7):607–614
Dias PC, Sena-Evangelista KC, Paiva MS, Ferreira DQ, Ururahy MA, Rezende AA, Abdalla DS, Pedrosa LF (2014) The beneficial effects of rosuvastatin are independent of zinc supplementation in patients with atherosclerosis. J Trace Elem Med Biol 28(2):194–199. https://doi.org/10.1016/j.jtemb.2014.01.003
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81(1):19–25. https://doi.org/10.1016/j.fertnstert.2003.10.004
Tatsch E, Bochi GV, Pereira Rda S, Kober H, Agertt VA, de Campos MM, Gomes P, Duarte MM, Moresco RN (2011) A simple and inexpensive automated technique for measurement of serum nitrite/nitrate. Clin Biochem 44(4):348–350. https://doi.org/10.1016/j.clinbiochem.2010.12.011
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239(1):70–76. https://doi.org/10.1006/abio.1996.0292
Beutler E, Gelbart T (1985) Plasma glutathione in health and in patients with malignant disease. J Lab Clin Med 105(5):581–584
Janero DR (1990) Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med 9(6):515–540. https://doi.org/10.1016/0891-5849(90)90131-2
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478. https://doi.org/10.1016/0076-6879(90)86141-H
Gmelig-Meyling F, Waldmann TA (1980) Separation of human blood monocytes and lymphocytes on a continuous Percoll gradient. J Immunol Methods 33(1):1–9. https://doi.org/10.1016/0022-1759(80)90077-0
Jamilian M, Foroozanfard F, Bahmani F, Talaee R, Monavari M, Asemi Z (2016) Effects of zinc supplementation on endocrine outcomes in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res 170(2):271–278. https://doi.org/10.1007/s12011-015-0480-7
Asemi Z, Foroozanfard F, Hashemi T, Bahmani F, Jamilian M, Esmaillzadeh A (2015) Calcium plus vitamin D supplementation affects glucose metabolism and lipid concentrations in overweight and obese vitamin D deficient women with polycystic ovary syndrome. Clin Nutr 34(4):586–592. https://doi.org/10.1016/j.clnu.2014.09.015
Simental-Mendia LE, Sahebkar A, Rodriguez-Moran M, Zambrano-Galvan G, Guerrero-Romero F (2017) Effect of magnesium supplementation on plasma C-reactive protein concentrations: a systematic review and meta-analysis of randomized controlled trials. Curr Pharm Des 23(999):1. https://doi.org/10.2174/1381612823666170525153605
Bo S, Durazzo M, Guidi S, Carello M, Sacerdote C, Silli B, Rosato R, Cassader M, Gentile L, Pagano G (2006) Dietary magnesium and fiber intakes and inflammatory and metabolic indicators in middle-aged subjects from a population-based cohort. Am J Clin Nutr 84(5):1062–1069
Song Y, Li TY, van Dam RM, Manson JE, FB H (2007) Magnesium intake and plasma concentrations of markers of systemic inflammation and endothelial dysfunction in women. Am J Clin Nutr 85(4):1068–1074
Almoznino-Sarafian D, Berman S, Mor A, Shteinshnaider M, Gorelik O, Tzur I, Alon I, Modai D, Cohen N (2007) Magnesium and C-reactive protein in heart failure: an anti-inflammatory effect of magnesium administration? Eur J Nutr 46(4):230–237. https://doi.org/10.1007/s00394-007-0655-x
Roman A, Desai N, Rochelson B, Gupta M, Solanki M, Xue X, Chatterjee PK, Metz CN (2013) Maternal magnesium supplementation reduces intrauterine growth restriction and suppresses inflammation in a rat model. Am J Obstet Gynecol 208(383):e381–e387
Banupriya N, Vishnu Bhat B, Benet BD, Sridhar MG, Parija SC (2017) Efficacy of zinc supplementation on serum calprotectin, inflammatory cytokines and outcome in neonatal sepsis—a randomized controlled trial. J Matern Fetal Neonatal Med 30(13):1627–1631. https://doi.org/10.1080/14767058.2016.1220524
Kim J, Ahn J (2014) Effect of zinc supplementation on inflammatory markers and adipokines in young obese women. Biol Trace Elem Res 157(2):101–106. https://doi.org/10.1007/s12011-013-9885-3
Duleba AJ, Dokras A (2012) Is PCOS an inflammatory process? Fertil Steril 97(1):7–12. https://doi.org/10.1016/j.fertnstert.2011.11.023
Jialal I, Devaraj S, Venugopal SK (2004) C-reactive protein: risk marker or mediator in atherothrombosis? Hypertension 44(1):6–11. https://doi.org/10.1161/01.HYP.0000130484.20501.df
Wellen KE, Hotamisligil GS (2005) Inflammation, stress, and diabetes. J Clin Invest 115(5):1111–1119. https://doi.org/10.1172/JCI25102
Aneiros E, Philipp S, Lis A, Freichel M, Cavalie A (2005) Modulation of Ca2+ signaling by Na+/Ca2+ exchangers in mast cells. J Immunol 174(1):119–130. https://doi.org/10.4049/jimmunol.174.1.119
De Martin R, Hoeth M, Hofer-Warbinek R, Schmid JA (2000) The transcription factor NF-kappa B and the regulation of vascular cell function. Arterioscler Thromb Vasc Biol 20(11):E83–E88. https://doi.org/10.1161/01.ATV.20.11.e83
Vongpatanasin W, Peri-Okonny P, Velasco A, Arbique D, Wang Z, Ravikumar P, Adams-Huet B, Moe OW, Pak CYC (2016) Effects of potassium magnesium citrate supplementation on 24-hour ambulatory blood pressure and oxidative stress marker in prehypertensive and hypertensive subjects. Am J Cardiol 118(6):849–853. https://doi.org/10.1016/j.amjcard.2016.06.041
Yogi A, Callera GE, O’Connor SE, He Y, Correa JW, Tostes RC, Mazur A, Touyz RM (2011) Dysregulation of renal transient receptor potential melastatin 6/7 but not paracellin-1 in aldosterone-induced hypertension and kidney damage in a model of hereditary hypomagnesemia. J Hypertens 29(7):1400–1410. https://doi.org/10.1097/HJH.0b013e32834786d6
Barman S, Srinivasan K (2017) Attenuation of oxidative stress and cardioprotective effects of zinc supplementation in experimental diabetic rats. Br J Nutr 117(03):335–350. https://doi.org/10.1017/S0007114517000174
Soltani N, Nematbakhsh M, Eshraghi-Jazi F, Talebi A, Ashrafi F (2013) Effect of oral administration of magnesium on cisplatin-induced nephrotoxicity in normal and streptozocin-induced diabetic rats. Nephrourol Mon 5(4):884–890. https://doi.org/10.5812/numonthly.11624
Tang QQ, SY S, Fang MY (2014) Zinc supplement modulates oxidative stress and antioxidant values in rats with severe acute pancreatitis. Biol Trace Elem Res 159(1-3):320–324. https://doi.org/10.1007/s12011-014-9971-1
Evans JL, Goldfine ID, Maddux BA, Grodsky GM (2003) Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes 52(1):1–8. https://doi.org/10.2337/diabetes.52.1.1
Gross M, Steffes M, Jacobs DR Jr, Yu X, Lewis L, Lewis CE, Loria CM (2005) Plasma F2-isoprostanes and coronary artery calcification: the CARDIA study. Clin Chem 51(1):125–131. https://doi.org/10.1373/clinchem.2004.037630
Liu YX, Guo YM, Wang Z (2007) Effect of magnesium on reactive oxygen species production in the thigh muscles of broiler chickens. Br Poult Sci 48(1):84–89. https://doi.org/10.1080/00071660601148187
Boujelben M, Ghorbel F, Vincent C, Makni-Ayadi F, Guermazi F, Croute F, El-Feki A (2006) Lipid peroxidation and HSP72/73 expression in rat following cadmium chloride administration: interactions of magnesium supplementation. Exp Toxicol Pathol 57(5-6):437–443. https://doi.org/10.1016/j.etp.2006.02.012
Acknowledgments
Research reported in this publication was supported by Elite Researcher Grant Committee under award number (958632) from the National Institutes for Medical Research Development (NIMAD), Tehran, Iran.
Author information
Authors and Affiliations
Contributions
ZA contributed in conception, design, statistical analysis, and drafting of the manuscript. FA-E, FF, EA, and FB contributed in data collection and manuscript drafting. BB and HJ contributed in the revised version. All authors approved the final version for submission. ZA supervised the study.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Afshar Ebrahimi, F., Foroozanfard, F., Aghadavod, E. et al. The Effects of Magnesium and Zinc Co-Supplementation on Biomarkers of Inflammation and Oxidative Stress, and Gene Expression Related to Inflammation in Polycystic Ovary Syndrome: a Randomized Controlled Clinical Trial. Biol Trace Elem Res 184, 300–307 (2018). https://doi.org/10.1007/s12011-017-1198-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1198-5