Abstract
Synergistic approach of magnesium and vitamin E may benefit clinical symptoms of patients with polycystic ovary syndrome (PCOS) through improving their metabolic profiles and reducing oxidative stress and inflammation. This study was designed to determine the effects of magnesium and vitamin E co-supplementation on hormonal status and biomarkers of inflammation and oxidative stress in women with PCOS. This randomized, double-blind, placebo-controlled trial was conducted among 60 women with PCOS, aged 18–40 years old. Participants were randomly divided into two groups to take 250 mg/day magnesium plus 400 mg/day vitamin E supplements or placebo (n = 30 each group) for 12 weeks. Fasting blood samples were taken at baseline and after the 12-week intervention to quantify related variables. Magnesium and vitamin E co-supplementation resulted in a significant reduction in hirsutism (β − 0.37; 95% CI, − 0.70, − 0.05; P = 0.02) and serum high-sensitivity C-reactive protein (hs-CRP) (β − 0.67 mg/L; 95% CI, − 1.20, − 0.14; P = 0.01), and a significant increase in plasma nitric oxide (NO) (β 3.40 μmol/L; 95% CI, 1.46, 5.35; P = 0.001) and total antioxidant capacity (TAC) levels (β 66.32 mmol/L; 95% CI, 43.80, 88.84; P < 0.001). Overall, magnesium and vitamin E co-supplementation for 12 weeks may benefit women with PCOS on hirsutism, serum hs-CRP, plasma NO, and TAC levels. Clinical trial registration number http://www.irct.ir: IRCT2017082733941N8
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovary syndrome (PCOS) is the most common metabolic disorder presented with hyperinsulinemic- and hyperandrogenic-related disorders. This complex metabolic disorder affects nearly 6% of women in reproductive age [1]. Earlier, investigators have documented a systemic low-grade inflammation in women diagnosed with PCOS, which subsequently leads to long-term consequences of PCOS, including enhanced risk of type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD) [2, 3]. On the other hand, oxidative damage is closely linked with inflammation and further clinical and metabolic disorders in women diagnosed with PCOS [4].
The pathophysiology of PCOS might be associated with the deficiency of essential micronutrients such as magnesium and vitamin E in patients with insulin resistance and oxidative stress. Current evidence has shown that women suffering from PCOS have lower values of magnesium and vitamin E than normal individuals [5, 6]. In addition, few studies conducted in individuals with no PCOS have suggested that combined magnesium and vitamin E intake might be useful to improve metabolic profiles. A meta-analysis study conducted by Simental-Mendia et al. [7] showed that magnesium intake could reduce C-reactive protein (CRP) levels. Furthermore, magnesium supplementation for 12 weeks resulted in a considerable reduction of high-sensitivity C-reactive protein (hs-CRP) and an elevation of total antioxidant capacity (TAC) among diabetic patients with foot ulcer [8]. In another meta-analysis, vitamin E supplementation decreased CRP levels [9]. Recently, there is a growing interest to use the combination approach including combined magnesium and vitamin E, which might improve metabolic profiles in a number of diseases with metabolic abnormalities. Due to the synergistic impact of vitamin E and magnesium [10, 11], a further important question will be whether combined vitamin and micronutrient would provide a better protection against disease. The basis of this approach relies on a clinical correlation between vitamin E and cellular magnesium as well as tissue glucose metabolism [12].
Considering the antioxidant and anti-inflammatory effects of magnesium with vitamin E, we hypothesized that magnesium plus vitamin E might be useful on metabolic profiles in women with PCOS. However, data on studies investigating the impact of magnesium plus vitamin E on hormonal profiles, and biomarkers of inflammation and oxidative stress among women with PCOS, are scarce. Therefore, this study evaluates the effects of magnesium with vitamin E co-supplementation on metabolic profiles in women with PCOS.
Methods
Trial Design and Participants
This randomized, double-blinded, placebo-controlled clinical trial is registered in the Iranian clinical trials website (http://www.irct.ir: IRCT2017082733941N8), has followed the Declaration of Helsinki guideline, and informed consent was given by all participants. The study protocol was approved by the Ethics Committee of Arak University of Medical Sciences (AUMS). This study was conducted among 60 women with PCOS, diagnosed based on the Rotterdam Criteria [13], aged 18–40 years, who referred to the Kosar Clinic in Arak, Iran, between October 2017 and January 2018. The main exclusion criteria were as follows: pregnancy, adrenal hyperplasia, androgen-secreting tumors, hyperprolactinemia, thyroid dysfunction, diabetes and other metabolic disorders at enrollment, and antioxidant and/or anti-inflammatory supplements within 3 months prior to the enrollment in the study.
Study Design
All patients were matched for BMI and age. They were then randomly allocated into two groups to receive either 250 mg/day magnesium oxide (Twenty First Century Pharmaceutical Company, AZ, USA) plus 400 IU/day vitamin E (Zahravi Pharmaceutical Company, Tabriz, Iran) (n = 30) or placebo (Barij Essence Pharmaceuticals, Kashan, Iran) (n = 30) for 12 weeks. The placebos were matched in color, shape, size, packaging, smell, and taste with the vitamin E and magnesium capsules. The compliance rate was assessed by measuring serum magnesium levels. Intake of the magnesium, vitamin E, and placebo capsules was monitored through asking participants to return the medication containers. To increase the compliance rate, all patients received brief daily cell phone reminders to take the supplements. Patients were requested to have their regular physical activity and not to take any extra nutritional supplements during the 12-week trial. All patients completed a 3-day food record and three physical activity records at the baseline of the study, weeks 3, 6, and 9, and the end of the intervention. Daily macro- and micronutrient intakes were calculated by analyzing food records using the Nutritionist IV software (First Databank, San Bruno, CA).
Anthropometric Measures
Patient’s weight and height were measured after an overnight fasting, using a standard scale (Seca, Hamburg, Germany), at both the onset of the study and after the 12-week intervention. BMI was calculated as weight in kilogram divided by height in meters squared.
Clinical Assessments
Hirsutism was determined using a modified Ferriman–Gallwey scoring system, as the clinical outcome [14].
Biochemical Assessment
Fasting blood samples were collected at the beginning and end of the trial, at the Arak reference laboratory. Serum total testosterone and sex hormone-binding globulin (SHBG) were measured using Elisa kits (DiaMetra, Milano, Italy) with inter- and intra-assay coefficient of variations (CVs) less than 7%. Serum hs-CRP concentrations were measured using an ELISA kit (LDN, Nordhorn, Germany) with the intra- and inter-assay CVs less than 7%. Other biomarkers were assessed as follows: plasma nitric oxide (NO) using the Griess method [15], TAC using ferric reduction antioxidant power method developed by Benzie and Strain [16], glutathione (GSH) applying Beutler et al.’s method [17], and malondialdehyde (MDA) concentrations using the thiobarbituric acid reactive substance method [18] with the inter- and intra-assay CVs less than 5%.
Sample Size
Sample size was calculated using the standard formula for clinical trials, considering type one error (α) of 0.05 and type two error (β) of 0.20 (power = 80%). According to a previously published study [19], we used 1.93 mg/L as the effect size (d) of hs-CRP and 2.4 mg/L as standard deviation (SD). Using this information, 25 individuals were required to be included in each treatment group. Considering 5 probable dropouts in each group, the final sample size was determined as 30 patients in each group.
Randomization
Randomization was conducted using computer-generated random numbers. Randomization and allocation were concealed from the researchers and patients until the final analyses were completed. The randomized allocation sequence, enrolling patients, and allocating them into intervention groups were performed by a trained staff at the clinic.
Statistical Methods
Anthropometric measures and dietary intakes were compared between intervention groups, using independent samples t test. Multiple linear regression models were used to assess treatment effects on the study outcomes after adjusting for confounding variables including the baseline values, age, and BMI. The effect sizes were presented as the mean differences with 95% confidence intervals. Paired-samples t test was used to detect within-group differences. The normality of model residual was tested using the Kolmogorov-Smirnov test. The P value of < 0.05 was considered as statistically significant. All statistical analyses were conducted using the Statistical Package for Social Science version 18 (SPSS Inc., Chicago, IL, USA).
Results
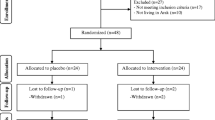
In the current study, all 60 subjects (intervention (n = 30) and placebo (n = 30)) completed the trial (Fig. 1). Overall, the compliance rate in this study was high, such that more than 90% of the capsules were consumed throughout the study in both groups. No side effects were reported following co-administration of magnesium and vitamin E capsules in patients with PCOS throughout the study.
Mean age, height, weight, and BMI at baseline and end-of-trial were not statistically different between intervention groups (Table 1).
Mean dietary macro- and micronutrient intakes were also not statistically different between the two groups throughout the trial (data not shown).
After the 12-week intervention, magnesium and vitamin E co-supplementation significantly reduced hirsutism (β − 0.37; 95% CI, − 0.70, − 0.05; P = 0.02) and serum hs-CRP (β − 0.67 mg/L; 95% CI, − 1.20, − 0.14; P = 0.01), and significantly increased serum magnesium (β 0.17 mg/dL; 95% CI, 0.12, 0.21; P < 0.001), plasma NO (β 3.40 μmol/L; 95% CI, 1.46, 5.35; P = 0.001), and TAC levels (β 66.32 mmol/L; 95% CI, 43.80, 88.84; P < 0.001) compared with the placebo (Table 2). Serum total testosterone, SHBG, plasma GSH, and MDA levels did not significantly change with magnesium and vitamin E co-supplementation. Within-group changes demonstrated a significant reduction in hirsutism (P = 0.02), and a significant increase in serum magnesium (P < 0.001), SHBG (P = 0.004), plasma NO (P < 0.001), TAC (P < 0.001), and GSH (P = 0.03) in the magnesium and vitamin E group. In addition, within-group change showed a significant increase in serum hs-CRP (P = 0.03) in the placebo group.
Discussion
We evaluated the effect of co-administration of magnesium and vitamin E, to our best knowledge for the first time, on metabolic status in patients with PCOS. The results showed that taking magnesium and vitamin E supplements together for 12 weeks had beneficial effects on hirsutism, hs-CRP, NO, and TAC levels.
Effects on Hormonal Profiles
Women with PCOS are susceptible to multiple metabolic disorders [20, 21]. The current study demonstrated that magnesium and vitamin E co-supplementation for 12 weeks was associated with a significant reduction in hirsutism, but did not affect serum total testosterone and SHBG levels. Our previous study demonstrated that magnesium-zinc-calcium-vitamin D co-supplementation after 12 weeks to women with PCOS significantly decreased hirsutism, but did not affect other hormonal profiles [22]. In addition, vitamin E (400 IU/day) and omega-3 (1000 mg/day) co-supplementation for 12 weeks to women with PCOS significantly improved total and free testosterone levels, but did not affect other endocrine parameters [23]. In another study, coenzyme Q10 (200 mg/day) with or without vitamin E (400 IU/day) supplementation for 8 weeks to women with PCOS had beneficial effects on serum total testosterone levels, but did not influence SHBG concentrations [24]. A cross-sectional study demonstrated an inverse association between serum α-tocopherol and circulating testosterone, estradiol, and SHBG levels in smoker men [25]. Elevated androgen level which is a hallmark of PCOS in a self-perpetuating cycle promotes hyperandrogenism and results in neuroendocrine abnormality [26]. Previous evidence showed that reducing androgens is attributed to the restoration of ovulatory functions, decreasing hirsutism, and improving health-related quality of life [27, 28]. The absence of beneficial effects of magnesium and vitamin E co-supplementation on total testosterone and SHBG may be due to low-dose magnesium and vitamin E used as well as short-duration intervention. A number of potential mechanisms through which magnesium and vitamin E may improve ovarian functions are proposed including the antioxidant and anti-inflammatory activities of magnesium and vitamin E, as well as their effects on improved insulin sensitivity [24, 29, 30].
Effects on Biomarkers of Inflammation and Oxidative Stress
The co-supplementation of magnesium and vitamin E for 12 weeks was found to significantly decrease serum hs-CRP and increase plasma NO and TAC levels in patients with PCOS, but did not affect plasma GSH and MDA levels. We have previously showed that taking magnesium plus zinc supplements for 12 weeks by women with PCOS had beneficial effects on hs-CRP and TAC levels [19]. In addition, a meta-analysis conducted by Simental-Mendia et al. [7] showed that magnesium supplementation could decrease CRP levels in people with high inflammation (CRP levels > 3 mg/dL). A 4-week magnesium supplementation to rugby players and people with sedentary lifestyle significantly decreased oxidative damage [31]. However, magnesium supplementation at a dosage of 250 mg/day as magnesium oxide after 8 weeks to middle-aged overweight women did not influence inflammatory markers [32]. Moreover, the administration of 440 mg magnesium supplements as magnesium oxide 3 times per week for 6 months to hemodialysis patients did not influence CRP concentrations [33]. Anti-inflammatory and antioxidative effects of vitamin E have been previously reported. In a meta-analysis conducted by Saboori et al. [9], vitamin E supplementation significantly reduced CRP levels. Furthermore, vitamin E supplementation at a dosage of 200 mg/day for 12 weeks to workers significantly reduced biomarkers of oxidative stress [34]. However, no significant changes in biomarkers of oxidative damage were observed following the administration of 400 IU/day vitamin E for 8 weeks to patients with chronic obstructive pulmonary disease [35]. Synergistic anti-inflammatory effects of vitamin E plus magnesium might boost their impact on clinical and biochemical symptoms. It is now obvious that PCOS is a pro-inflammatory condition linking with oxidative stress [36]. Impaired antioxidative defense and increased inflammatory markers such as CRP contribute to insulin resistance and the promotion of atherosclerosis which increase the risk of CVD [37]. Anti-inflammatory effects of magnesium supplements may be due to its antagonism to calcium [38] and blocking nuclear factor-kappa B (NF-κB) [39]. In addition, anti-inflammatory and antioxidative effects of vitamin E may be explained by suppressing NF-κB and JAK-signal transducer and activator of transcription 6 (STAT6) or JAK-STAT3 signaling pathways in various types of cells [40].
This study had a few limitations. In the present study, we did not evaluate circulating vitamin E before and after supplementation. Further, this study did not assess gene expression related to inflammatory cytokines and biomarkers of oxidative stress.
Conclusions
In summary, the current study demonstrated that taking magnesium and vitamin E supplements together for 12 weeks by patients with PCOS has beneficial effects on hirsutism, hs-CRP, NO, and TAC, but did not affect serum total testosterone, SHBG, plasma GSH, and MDA levels.
References
Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO (2016) The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 31:2841–2855
Boots CE, Jungheim ES (2015) Inflammation and human ovarian follicular dynamics. Semin Reprod Med 33:270–275
Shorakae S, Teede H, de Courten B, Lambert G, Boyle J, Moran LJ (2015) The emerging role of chronic low-grade inflammation in the pathophysiology of polycystic ovary syndrome. Semin Reprod Med 33: 257–269
Artimani T, Karimi J, Mehdizadeh M et al (2018) Evaluation of pro-oxidant-antioxidant balance (PAB) and its association with inflammatory cytokines in polycystic ovary syndrome (PCOS). Gynecol Endocrinol 34:148–152
Chakraborty P, Ghosh S, Goswami SK, Kabir SN, Chakravarty B, Jana K (2013) Altered trace mineral milieu might play an aetiological role in the pathogenesis of polycystic ovary syndrome. Biol Trace Elem Res 152:9–15
Zhang D, Luo WY, Liao H, Wang CF, Sun Y (2008) The effects of oxidative stress to PCOS. Sichuan Da Xue Xue Bao Yi Xue Ban 39:421–423
Simental-Mendia LE, Sahebkar A, Rodriguez-Moran M, Zambrano-Galvan G, Guerrero-Romero F (2017) Effect of magnesium supplementation on plasma c-reactive protein concentrations: a systematic review and meta-analysis of randomized controlled trials. Curr Pharm Des 23:4678–4686
Razzaghi R, Pidar F, Momen-Heravi M, Bahmani F, Akbari H, Asemi Z (2018) Magnesium supplementation and the effects on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res 181:207–215
Saboori S, Shab-Bidar S, Speakman JR, Yousefi Rad E, Djafarian K (2015) Effect of vitamin E supplementation on serum C-reactive protein level: a meta-analysis of randomized controlled trials. Eur J Clin Nutr 69:867–873
Dou M, Ma AG, Wang QZ et al (2009) Supplementation with magnesium and vitamin E were more effective than magnesium alone to decrease plasma lipids and blood viscosity in diabetic rats. Nutr Res 29:519–524
Chang W, Ma A, Wang Q, Mao R, Li C (2014) Effects of vitamin E and magnesium on glucolipid metabolism in obese rats. Wei Sheng Yan Jiu 43:713–718
Barbagallo M, Dominguez LJ, Tagliamonte MR, Resnick LM, Paolisso G (1999) Effects of vitamin E and glutathione on glucose metabolism: role of magnesium. Hypertension 34:1002–1006
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81:19–25
Hatch R, Rosenfield RL, Kim MH, Tredway D (1981) Hirsutism: implications, etiology, and management. Am J Obstet Gynecol 140:815–830
Tatsch E, Bochi GV, Pereira Rda S et al (2011) A simple and inexpensive automated technique for measurement of serum nitrite/nitrate. Clin Biochem 44:348–350
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76
Beutler E, Gelbart T (1985) Plasma glutathione in health and in patients with malignant disease. J Lab Clin Med 105:581–584
Janero DR (1990) Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med 9:515–540
Afshar Ebrahimi F, Foroozanfard F, Aghadavod E, Bahmani F, Asemi Z (2018) The effects of magnesium and zinc co-supplementation on biomarkers of inflammation and oxidative stress, and gene expression related to inflammation in polycystic ovary syndrome: a randomized controlled clinical trial. Biol Trace Elem Res 184:300–307
Asemi Z, Foroozanfard F, Hashemi T, Bahmani F, Jamilian M, Esmaillzadeh A (2015) Calcium plus vitamin D supplementation affects glucose metabolism and lipid concentrations in overweight and obese vitamin D deficient women with polycystic ovary syndrome. Clin Nutr 34:586–592
Foroozanfard F, Jamilian M, Bahmani F et al (2015) Calcium plus vitamin D supplementation influences biomarkers of inflammation and oxidative stress in overweight and vitamin D-deficient women with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Clin Endocrinol 83:888–894
Maktabi M, Jamilian M, Asemi Z (2018) Magnesium-zinc-calcium-vitamin D co-supplementation improves hormonal profiles, biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res 182:21–28
Ebrahimi FA, Samimi M, Foroozanfard F et al (2017) The effects of omega-3 fatty acids and vitamin E co-supplementation on indices of insulin resistance and hormonal parameters in patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Exp Clin Endocrinol Diabetes 125:353–359
Izadi A, Ebrahimi S, Shirzai S et al (2018) Hormonal and metabolic effects of coenzyme q10 and/or vitamin e in patients with polycystic ovary syndrome. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2018-01221
Mondul AM, Rohrmann S, Menke A et al (2011) Association of serum alpha-tocopherol with sex steroid hormones and interactions with smoking: implications for prostate cancer risk. Cancer Causes Control 22:827–836
Blank SK, McCartney CR, Helm KD, Marshall JC (2007) Neuroendocrine effects of androgens in adult polycystic ovary syndrome and female puberty. Semin Reprod Med 25:352–359
Dokras A, Sarwer DB, Allison KC et al (2016) Weight loss and lowering androgens predict improvements in health-related quality of life in women with PCOS. J Clin Endocrinol Metab 101:2966–2974
Boztosun A, Acmaz G, Ozturk A, Muderris II (2013) Clinical efficacy of low dose flutamide plus Diane-35 in the treatment of idiopathic hirsutism and polycystic ovary syndrome. Ginekol Pol 84:258–262
Barbagallo M, Dominguez LJ, Galioto A et al (2003) Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol Asp Med 24:39–52
Rizzo MR, Abbatecola AM, Barbieri M et al (2008) Evidence for anti-inflammatory effects of combined administration of vitamin E and C in older persons with impaired fasting glucose: impact on insulin action. J Am Coll Nutr 27:505–511
Petrovic J, Stanic D, Dmitrasinovic G et al (2016) Magnesium supplementation diminishes peripheral blood lymphocyte DNA oxidative damage in athletes and sedentary young man. Oxidative Med Cell Longev 2016(2019643)
Moslehi N, Vafa M, Rahimi-Foroushani A, Golestan B (2012) Effects of oral magnesium supplementation on inflammatory markers in middle-aged overweight women. J Res Med Sci 17:607–614
Mortazavi M, Moeinzadeh F, Saadatnia M, Shahidi S, McGee JC, Minagar A (2013) Effect of magnesium supplementation on carotid intima-media thickness and flow-mediated dilatation among hemodialysis patients: a double-blind, randomized, placebo-controlled trial. Eur Neurol 69:309–316
Kasperczyk S, Dobrakowski M, Kasperczyk A et al (2017) alpha-Tocopherol supplementation and the oxidative stress, homocysteine, and antioxidants in lead exposure. Arch Environ Occup Health 72:153–158
Nadeem A, Raj HG, Chhabra SK (2008) Effect of vitamin E supplementation with standard treatment on oxidant-antioxidant status in chronic obstructive pulmonary disease. Indian J Med Res 128:705–711
Rajendran S, Willoughby SR, Chan WP et al (2009) Polycystic ovary syndrome is associated with severe platelet and endothelial dysfunction in both obese and lean subjects. Atherosclerosis 204:509–514
Hyderali BN, Mala K (2015) Oxidative stress and cardiovascular complications in polycystic ovarian syndrome. Eur J Obstet Gynecol Reprod Biol 191:15–22
Aneiros E, Philipp S, Lis A, Freichel M, Cavalie A (2005) Modulation of Ca2+ signaling by Na+/Ca2+ exchangers in mast cells. J Immunol 174:119–130
Mazur A, Maier JA, Rock E, Gueux E, Nowacki W, Rayssiguier Y (2007) Magnesium and the inflammatory response: potential physiopathological implications. Arch Biochem Biophys 458:48–56
Jiang Q (2014) Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med 72:76–90
Funding
The present study was supported by a grant from the Vice-chancellor for Research, AUMS, Arak, and Iran.
Author information
Authors and Affiliations
Contributions
ZA and MS contributed in conception, design, statistical analysis, and drafting of the manuscript. ZA supervised the study.
Corresponding author
Ethics declarations
This randomized, double-blinded, placebo-controlled clinical trial is registered in the Iranian clinical trials website (http://www.irct.ir: IRCT2017082733941N8), has followed the Declaration of Helsinki guideline, and informed consent was given by all participants. The study protocol was approved by the Ethics Committee of Arak University of Medical Sciences (AUMS).
Competing Interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shokrpour, M., Asemi, Z. The Effects of Magnesium and Vitamin E Co-Supplementation on Hormonal Status and Biomarkers of Inflammation and Oxidative Stress in Women with Polycystic Ovary Syndrome. Biol Trace Elem Res 191, 54–60 (2019). https://doi.org/10.1007/s12011-018-1602-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1602-9