Abstract
This study aimed to investigate the effects of oral administration of trivalent chromium on the contents of Ca, Mg, Mn, Fe, Cu, and Zn in the heart, liver, and kidney. Different levels of 1/8, 1/4, and 1/2 LD50 (LD50 = 5000 mg/kg body mass) CrCl3 milligrams per kilogram body mass daily were added into the water to establish the chronic poisoning model. Ca, Mg, Mn, Fe, Cu, and Zn were detected with the flame atomic absorption spectrometry in the organs exposed 14, 28, and 42 days to CrCl3, respectively. Results showed that Cr was accumulated in the heart, liver, and kidney significantly (P < 0.05) with extended time and dose. The contents of Ca and Fe increased, whereas those of Mg, Mn, Cu, and Zn decreased in the heart, liver, and kidney of each treated group, which had a dose- and time-dependent relationship, but the contents of Mg and Zn in the heart took on a fluctuated change. These particular observations were different from those in the control group. In conclusion, the oral administration of CrCl3 could change the contents of Ca, Mg, Mn, Fe, Cu, and Zn in the heart, liver, and kidney, which may cause disorders in the absorption and metabolism of the metal elements of chickens.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium (Cr) at high exposure belongs to the toxic metals. It primarily exists in two valence states, namely trivalent and hexavalent Cr [1]. With the massive use of Cr mainly in pharmaceutical industries and the continuous attention devoted to chromium poisoning for the past few years, people are more prone to exposure to Cr. Hence, Cr exposure is strictly monitored. However, Cr can also be deemed as an important trace element in human and animal bodies; Cr is actively involved in the metabolism of carbohydrates, lipids, and proteins [2]. In particular, Cr potentiates insulin action, resulting in the increased cellular uptake of glucose and improved intracellular carbohydrate and lipid metabolism [3]. Studies have reported that both trivalent and hexavalent chromium incur toxic effects on human beings and experimental animals; in fact, oral administration of Cr(VI) has induced oxidative stress, DNA damage, and certain tumors in rats [4, 5], and Cr(III) can cause oxidative damage in goldfish and mice [6, 7]. Studies also showed that Cr(III) can induce histopathological changes and oxidative stress in the liver and kidney in chicken [8, 9]. In low content, trivalent chromium can promote the growth and development of chickens, thereby improving the quality of their meat [10]. This is the potential reason why Cr is added into animal feed. Nonetheless, in the actual process of adding Cr into the fodder, people may put in excess trivalent chromium to gain more benefits, adversely causing such element to be poisonous and dangerous for chicken.
Trace elements are a commonly explored research topic and have drawn significantly more attention in recent years. Certain kinds of essential trace elements play an important role in the everyday life of both human beings and animals although the content of each trace element is less than 0.01 % [11]. Trace elements are directly involved in metabolism and maintain the dynamic balance in the body with a variety of forms [12]. The lack or excess of these trace elements can induce changes in the normal physiological activity and biochemical process of a body, thereby incurring abnormal cell metabolism, growth and reproductive disorders, and severe oxidative damage [13]. Therefore, humans and animals must significantly maintain the relative balance of the trace elements that exist in their different organs. Accordingly, trace elements increasingly arouse scholarly attention for explaining the relationship between the change of metal trace elements on physiological and pathological effects. Many animal experiments have been conducted under a condition of either overload or lack of a specific element, after which the effects on the content and distribution of other elements in various organs were investigated [14, 15]; however, few studies had reported the Cr contents in chickens after adding excess Cr. Several reports have also been made to detect the effects on trace element contents in the organs of mammals and investigate the accumulation of toxic trace elements after exposure to a certain element [16–18]. Nevertheless, few studies have examined the effects of Cr3+ exposure in drinking water on Ca, Mg, Mn, Fe, Cu, and Zn contents in the heart, liver, and kidney of chickens. Such changes in contents may play an important role in metal metabolism. Therefore, a substantial research is needed to provide a more detailed insight into chromium-induced changes in Ca, Mg, Mn, Fe, Cu, and Zn content in chickens.
The current study aimed to investigate the effect on the contents of Ca, Mg, Mn, Fe, Cu, and Zn in the heart, liver, and kidney of Hyland chickens when oral administration of chromium (Cr3+) is excessive and to identify the relationship between time and dose with regard to Cr exposure.
Materials and Methods
Reagents
Chromium chloride (CrCl3·6H2O) (AR, purity ≥99 %) was acquired from Putian Tedia Company Inc. in Tai’an, China. Meanwhile, nitric (HNO3, GR) and perchloric acids (HClO4, GR) were provided by the Chemical Institute of Shandong Agricultural University. All plastic and glassware materials used for the experiment were rinsed by soaking them in dilute HNO3 (1 %) and were washed with distilled water prior to their use. All other reagents were of analytical grade.
Animal Treatment and Experimental Design
All procedures related to this experiment were conducted under the protocols approved by the Institutional Animal Care and Use Committee of Shandong Agricultural University (SDAUA-2014-013).
Prior to performing this study, the median lethal dose (LD50) of CrCl3 for chicken was confirmed according to Karber methods [19], and the LD50 was 5000 mg/kg body mass for male Hyland [20]. A total of 72 Hyland male chickens (1 day old) were obtained from the Animal Center of Shandong Agriculture University (Tai’an, China). The chickens were randomly divided into four groups, each consisting of 18 chickens. The animals were housed in cages and allowed to acclimate 14 days prior to experimental use. CrCl3 was orally administered to the first three groups by allowing them to drink water for 42 days with the following doses: 1/2 LD50 (high-dose group), 1/4 LD50 (middle-dose group), and 1/8 LD50 (low-dose group) mg/kg body mass/day. The fourth group (control) was treated with only water. The animals were maintained at an appropriate room temperature and within a relative humidity with a 12-h light/dark cycle. All animals were granted free access to local feed throughout the experiment period. At the end of 14, 28, and 42 days, 6 chickens of each group per time point were euthanized by intraperitoneal injection of thiopental (40 mg/kg body mass) and were dissected to collect their heart, liver, and kidney for element assay.
Assay of Metal Concentrations
The heart, liver, and kidney of the chickens were dealt with to remove the blood, vessels, and fat around them. Each sample accurately weighed 1.0 g (wet mass) and was placed in an oven at 80 °C, allowing them to dry for a whole night. Subsequently, the samples were placed into a triangle flask containing a 25-mL mixture of nitric and perchloric acids (4:1), digesting for 24 h. The digested mixture was slowly heated the next day in the ventilation with electric furnace until the remaining liquid measured to approximately 2 to 3 mL. The system was then slightly cooled. A constant 10-mL volume with 0.5 % of nitric acid was preserved at 4 °C and was prevented from being exposed to light. The contents of the elements (Ca, Mg, Mn, Fe, Cu, and Zn) were determined via flame atomic absorption spectrophotometry method F-AAS with Zeeman background correction (Hitachi Model Z-8000, Hitachi-naka City, Japan). Concentration of Cr was determined by an inductively coupled plasma mass spectrometer (ICP-MS; Hewlett-Packard, HP-4500, Avondale, PA, USA) according to the manufacturer’s recommendation. The National Institute of Standards and Technology (NIST) Standard Reference Materials® 1577c Bovine Liver was used as the quality controls. The detection limits (LOD) of Cr was 0.00004 μg/mL. The measured value multiplied by the dilution ratio was the actual content of each element in the heart, liver, and kidney. The operating conditions of F-AAS for detecting each element are specified in Table 1. The achieved results were in good agreement with certified values are given in Table 2. All experiments were conducted three times to guarantee their reproducibility.
Statistical Analysis
All statistical analyses were performed with Statistical Package for Social Sciences (SPSS) program version 18.0 (SPSS Inc., Chicago, IL, USA). Meanwhile, one-way ANOVA was used to identify the significant values (P < 0.05). Data are expressed as mean ± S.D.
Results
Contents of Cr in the Heart, Liver, and Kidney
The Cr contents in the liver, kidney, and heart tissues at different times are given in Table 3. In particular, the findings revealed that the content of Cr distinctly accumulated (P < 0.05) in these organs. With extended time and dose of CrCl3 exposure, the contents of Cr in the high, middle, and low groups significantly increased (P < 0.05) compared with those of the control group, which incurred the same content in each organ, particularly the liver and kidney. At 42 days, the content of Cr in the kidney was nearly the same among the three Cr-treated groups (P > 0.05).
Element Contents in the Heart
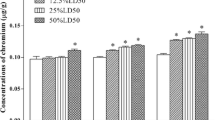
In the heart, the contents of Ca and Fe were higher in the three Cr-treated groups than those in the control groups all the time. Contrarily, the contents of Mn and Cu were lower in the three Cr-treated groups than those in the control groups all the time. However, with the increased time and dose of CrCl3, the contents of Ca, Mn, and Cu were significantly decreased (P < 0.05) compared with those of the control group. The content of Mg was increased at 28 days, but was significantly reduced (P < 0.05) at 42 days, whereas the contents of Zn did not significantly change (P > 0.05). Only the content of high-dose group was significantly decreased (P < 0.05) at 42 days (Fig. 1).
Element contents in the heart of chickens at different chromium exposition for increasing time. The bars represent arithmetic means, and the upper tiny strokes represent SD from n = 6. *P < 0.05 is significantly different compared to the control group at the same exposure time for the same one element. a Ca. b Mg. c Mn. d Fe. e Cu. f Zn. SD standard deviations
Element Contents in the Liver
In the liver, the content of Ca in the three Cr-treated groups was higher (P < 0.05) than in the control group during the experiment. With increased time and dose of the CrCl3 the Ca content slightly increased. The content of Mg in the three Cr-treated groups was higher than in the control group at 14 and 28 days, but was lower at 42 days (Fig. 2b). The content of Mn showed no significant differences (P > 0.05) in the three Cr-treated groups compared with that of the control group at 14 and 28 days, but was lower at 42 days (Fig. 2c). The content of Fe increased and was higher than the control group at 42 days (Fig. 2d). With increased time and dose of CrCl3, the contents in the high groups at 28 and 42 days of Cu significantly decreased (P < 0.05) compared with those in the control group (Fig. 2e). The content of Zn in the three Cr-treated groups was significantly lower (P < 0.05) than those in the control group at 28 and 42 days (Fig. 2f).
Element contents in the liver of chickens at different chromium exposition for increasing time. The bars represent arithmetic means, and the upper tiny strokes represent SD from n = 6. *P < 0.05 is significantly different compared to the control group at the same exposure time for the same one element. a Ca. b Mg. c Mn. d Fe. e Cu. f Zn. SD standard deviations
Element Contents in the Kidney
In the kidney, the content of Ca in the three Cr-treated groups was higher (P < 0.05) than those in the control group at different time (Fig. 3a). These contents, however, were decreased with the increased time and dose of CrCl3. The contents of Mg, Mn, Fe, Cu, and Zn were below the control group level at all times and were significantly decreased (P < 0.05). These contents were therefore determined time- and dose-dependent (Fig. 3).
Element contents in the kidney of chickens at different chromium exposition for increasing time. The bars represent arithmetic means, and the upper tiny strokes represent SD from n = 6. *P < 0.05 is significantly different compared to the control group at the same exposure time for the same one element. a Ca. b Mg. c Mn. d Fe. e Cu. f Zn. SD standard deviations
Discussion
In general circumstances, the proportion and concentration of trace elements in the body maintain a normal level. All kinds of trace element induce synergy or antagonism effects on one another, directly affecting their metabolism and utilization [21]. Any kind of trace elements whose content is extremely high or low could affect the other and cause damage to the body [22]. In this study, the excessive amounts of Cr can promote or inhibit the absorption and metabolism of certain elements to break the balance among the other elements in the body [23, 24]. This certain condition subsequently induces physiological injury. Such antagonistic relationships among the elements are common. In fact, these links were observed in the present experiment.
Trace elements, which are a key to many metabolic pathways, play an important physiological role; their content and distribution in the body are often associated with various diseases [25]. This study discovered that excess Cr in drinking water significantly increased the content of such element in the heart, liver, and kidney of chickens more than the control level. With the increased dose of Cr and the extension of poisoning time, the contents of Cr and Fe presented an increasing trend, but the contents of Cu and Zn decreased. In the blood, Cr and Fe used the same transfer protein called β-globulin to transport; however, they combined with the protein in different positions and transported to various organs to use in the body [26]. Accordingly, the protein delivered more Cr to the organism, thereby increasing the content of Fe. Zheng [27] and Vlad et al. [28] proved that Cr incurs antagonistic effects on Cu by identifying that the hearing impairment of rats was poisoned by Cr; the result was basically identical with ours. When the content of Cr in the body significantly (P < 0.05) increased, the content of Cu was significantly reduced, and these contents subsequently presented a negative correlation. Cr was mainly absorbed in the form of small molecule organic compounds through intestinal mucosa into the body [29]. In the intestines, Cr and Zn were collectively used as same metabolic pathway [30, 31]. Based on this analysis, giving animals a considerable amount of Cr correspondingly decreased the content of Zn in organs.
Numerous studies have revealed that damages in the tissues could increase the content of Ca in the organs [12, 14, 32]. The current study determined that, with different chromium exposure for increasing dose, the content of Ca was significantly increased (P < 0.05), and its content in the high-dose group was obviously higher than that of the middle- and low-dose groups. However, when the exposure time was increased, the content of Ca exhibited a declining trend. Meanwhile, the content of Mn was also significantly decreased (P < 0.05). In particular, when the exposure doses were higher and the exposure times were longer, the content of Mn in the organisms was lower. In the body, Mn combines with the binding protein, but could be replaced by Cr; this instance speeds up the metabolism of Mn [33]. Consequently, a large part of Mn was exported out of the body, greatly decreasing its content in the tissue. Liu et al. [34] verified that Mn and Fe have antagonistic effects; this certain observation is consistent with the experimental results of the present study. Meanwhile, the contents of Mg in the liver and kidney were reduced with the increase of applied dose and time, but in the heart tissue, they presented relative fluctuated changes, increasing at the 28th day and decreasing at the 42nd day. The body probably exhibited a compensatory phenomenon at the beginning of the damage. This change might be associated with the special function of the heart because Mg can resist arrhythmia [35]. Mg is the antagonist of many enzymes in the body; when Cr combines with certain enzymes, Mg is replaced [36]. The current research detected that, at the end of the experiment, the body was in a serious irreversible damaged situation. Therefore, the contents of the most elements were significantly reduced (P < 0.05). At the 42nd day of the experiment, the content of Cr in the kidney was essentially unchanged; it seemed that the dose had no obvious effects. The trace element selenium in the body would no longer be accumulated after it is deposited to a certain concentration [14, 37]; whether Cr would undergo this same phenomenon needs a further study.
In today’s scale and intensive production of animal feed, people commonly add excess trace elements to maximize productivity, but ignore the antagonistic effects that these substances incur, overlook a large amount of their deposition in poultry products, and discount the adverse influence that they induce on the environment. The absorption, metabolism, and biochemical function of Cr are clearly understood [38]; accordingly, the suitable additional amount of Cr in fodder must be determined, promoting the efficient absorption of such element in animal digestion and lowering the excretion of animals for reducing environmental pollution. In this study, CrCl3 was added in drinking water to investigate the contents of trace elements Ca, Mn, Mg, Fe, Cu, and Zn in tissues of Hyland chicken to provide certain theoretical reference about adding chromium reasonably in producing animal feed.
Conclusions
These findings suggest that Cr(III) exposure affects the Ca, Mg, Mn, Fe, Cu, and Zn uptake and accumulation in the heart, liver, and kidney of chickens in different degree and directions. Consequently, Cr(III) exposure increases the concentrations of Ca and Fe and decreases the contents of Mg, Mn, Cu, and Zn in the heart, liver, and kidney, possibly influencing the normal absorption and metabolism of elements.
References
Lushchak OV, Kubrak OI, Torous IM, Nazarchuk TY, Storey KB, Lushchak VI (2009) Trivalent chromium induces oxidative stress in goldfish brain. Chemosphere 75(1):56–62
Kumar S, Joshi UN, Sangwan S (2010) Chromium (VI) influenced nutritive value of forage sorghum (Sorghum bicolor L.). Anim Feed Sci Tech 160(3–4):121–127
Dkhil MA, Abdel-Baki AS, Al-Quraishy S, Abdel-Moneim AE (2013) Hepatic oxidative stress in Mongolian gerbils experimentally infected with Babesia divergens. Ticks Tick-borne Dis 4(4):346–351
Wang XF, Xing ML, Shen Y, Zhu X, Xu LH (2006) Oral administration of Cr(VI) induced oxidative stress, DNA damage and apoptotic cell death in mice. Toxicology 228(1):16–23
Davidson T, Kluz T, Burns F, Rossman T, Zhang Q, Uddin A, Nadas A, Costa M (2004) Exposure to chromium (VI) in the drinking water increases susceptibility to UV-induced skin tumors in hairless mice. Toxicol Appl Pharm 196(3):431–437
Lushchak OV, Kubrak OI, Lozinsky OV, Storey JM, Storey KB, Lushchak VI (2009) Chromium(III) induces oxidative stress in goldfish liver and kidney. Aquatic Toxicol 93(1):45–52
Scibior A, Zaporowska H, Ostrowski J, Banach A (2006) Combined effect of vanadium(V) and chromium(III) on lipid peroxidation in liver and kidney of rats. Chem Biol Interact 159(3):213–222
Fan WT, Zhao XN, Cheng J, Liu YH, Liu JZ (2015) Oxidative stress and hepatocellular injury induced by oral administration of Cr(3+) in chicken. J Biochem Mol Toxic 29(6):280–287
Liu Y, Liu C, Cheng J, Fan W, Zhang X, Liu J (2015) Growth performance and oxidative damage in kidney induced by oral administration of Cr(III) in chicken. Chemosphere 139:365–371
Piva A, Meola E, Paolo Gatta P, Biagi G, Castellani G, Mordenti AL, Bernard Luchansky J, Silva S, Mordenti A (2003) The effect of dietary supplementation with trivalent chromium on production performance of laying hens and the chromium content in the yolk. Anim Feed Sci Tech 106(1–4):149–163
Haenlein GFW, Anke M (2011) Mineral and trace element research in goats: a review. Small Rumin Res 95(1):2–19
Aaseth J, Boivin G, Andersen O (2012) Osteoporosis and trace elements—an overview. J Trace Elem Med Biol 26(2–3):149–152
Lin CC, Huang HH, Hu CW, Chen BH, Chong IW, Chao YY, Huang YL (2014) Trace elements, oxidative stress and glycemic control in young people with type 1 diabetes mellitus. J Trace Elem Med Biol 28(1):18–22
Nriagu J, Boughanen M, Linder A, Howe A, Grant C, Rattray R, Vutchkov M, Lalor G (2009) Levels of As, Cd, Pb, Cu, Se and Zn in bovine kidneys and livers in Jamaica. Ecotoxicol Environ Safe 72(2):564–571
Wagner A, Boman J (2003) Biomonitoring of trace elements in muscle and liver tissue of freshwater fish. Spectrochim Acta B At Spectrosc 58(12):2215–2226
Kuriwaki J, Nishijo M, Honda R, Tawara K, Nakagawa H, Hori E, Nishijo H (2005) Effects of cadmium exposure during pregnancy on trace elements in fetal rat liver and kidney. Toxicol Lett 156(3):369–376
Ayhan D, Nurhan S, Mehmet T, Vijaya J, Cemal O, Muhittin O, James K, Kazim S (2009) The effects of chromium histidinate on mineral status of serum and tissue in fat-fed and streptozotocin-treated type II diabetic rats. Biol Trace Elem Res 131(2):124–132
Staniek H, Rhodes NR, Bona KR, Deng G, Love ST, Pledger LA, Blount J, Gomberg E, Grappe F, Cernosek C, Peoples B, Rasco JF, Krejpcio Z, Vincent JB (2013) Comparison of tissue metal concentrations in Zucker lean, Zucker obese, and Zucker diabetic fatty rats and the effects of chromium supplementation on tissue metal concentrations. Biol Trace Elem Res 151(3):373–383
Bittenbender H, Howell GS Jr (1974) Adaptation of the Spearman-Karber method for estimating the T50 of cold stressed flower buds. J Amer Soc Hort Sci 99:187–190
Cao J (2004) Effects of chromium(III) on humoral immune response, fecundity of chicks and bioavailability, residues, toxicity following oral adminisstration. Wuhan Huazhong Agricultural University. (in chinese)
Jarić I, Višnjić-Jeftić Ž, Cvijanović G, Gačić Z, Jovanović L, Skorić S, Lenhardt M (2011) Determination of differential heavy metal and trace element accumulation in liver, gills, intestine and muscle of sterlet (Acipenser ruthenus) from the Danube River in Serbia by ICP-OES. Microchem J 98(1):77–81
Rao SV, Prakash B, Kumari K, Raju MV, Panda AK (2013) Effect of supplementing different concentrations of organic trace minerals on performance, antioxidant activity, and bone mineralization in Vanaraja chickens developed for free range farming. Trop Anim Health Prod 45(6):1447–1451
Sahin K, Tuzcu M, Orhan C, Agca CA, Sahin N, Guvenc M, Krejpcio Z, Staniek H, Hayirli A (2011) The effects of chromium complex and level on glucose metabolism and memory acquisition in rats fed high-fat diet. Biol Trace Elem Res 143(2):1018–1030
Staniek H, Krejpcio Z (2009) The effects of tricentric chromium(III) propionate complex supplementation on pregnancy outcome and maternal and foetal mineral status in rat. Food Chem Toxicol 47(10):2673–2678
Loguercio C, De Girolamo V, Federico AA, Feng SL, Cataldi V, Del Vecchio BC, Gialanella G (1997) Trace elements and chronic liver diseases. J Trace Elem Med Bio 11(3):158–161
Lal CS, Kumar S, Ranjan A, Rabidas VN, Verma N, Pandey K, Verma RB, Das S, Singh D, Das P (2013) Comparative analysis of serum zinc, copper, magnesium, calcium and iron level in acute and chronic patients of visceral leishmaniasis. J Trace Elem Med Bio 27(2):98–102
Zheng F, Zhang K, Wu S, Li N, Yu J, Gao X (2011) Hearing impairment in rats caused by chromium and the antagonism of copper or manganese. Acta Academiae Med Qingdao Univ 6:10–11
Vlad M, Caseanu E, Uza G, Petrescu M (1994) Concentration of copper, zinc, chromium, iron and nickel in the abdominal aorta of patients deceased with coronary heart disease. J Trace Elem Electrolytes Health Dis 8(2):111–114
Wang MQ, Xu ZR, Zha LY, Lindemann MD (2007) Effects of chromium nanocomposite supplementation on blood metabolites, endocrine parameters and immune traits in finishing pigs. Anim Feed Sci Tech 139(1–2):69–80
Liu HW, Liu DS, Zheng LX (2014) Study on Zn relative concentration and chemical state in broilers duodenum by micro-X-ray fluorescence and micro-X-ray absorption fine structure. Livest Sci 161:101–108
Taneja SK, Jain M, Mandal R, Megha K (2012) Excessive zinc in diet induces leptin resistance in Wistar rat through increased uptake of nutrients at intestinal level. J Trace Elem Med Bio 26(4):267–272
Eybl V, Kotyzova D, Bludovska M (2004) The effect of curcumin on cadmium-induced oxidative damage and trace elements level in the liver of rats and mice. Toxicol Lett 151(1):79–85
Plymate SR, Haugk KH, Sprenger CC, Nelson PS, Tennant MK, Zhang Y, Oberley LW, Zhong W, Drivdahl R, Oberley TD (2003) Increased manganese superoxide dismutase (SOD-2) is part of the mechanism for prostate tumor suppression by Mac25/insulin-like growth factor binding-protein-related protein-1. Oncogene 22(7):1024–1034
Liu X, Zuo N, Guan H, Han C, Xu SW (2013) Manganese-induced effects on cerebral trace element and nitric oxide of Hyline cocks. Bio Trace Elem Res 154(2):202–209
Sueta CA, Clarke SW, Dunlap SH, Jensen L, Blauwet MB, Koch G, Patterson JH, Adams K (1994) Effect of acute magnesium administration on the frequency of ventricular arrhythmia in patients with heart failure. Circulation 89(2):660–666
Garfinkel L, Garfinkel D (1984) Magnesium regulation of the glycolytic pathway and the enzymes involved. Magnesium 4(2–3):60–72
Hostetler CE, Kincaid RL, Mirando MA (2003) The role of essential trace elements in embryonic and fetal development in livestock. Vet J 166(2):125–139
Debski B, Zalewski W, Gralak MA, Kosla T (2004) Chromium-yeast supplementation of chicken broilers in an industrial farming system. J Trace Elem Med Biol 18(1):47–51
Acknowledgments
This work was supported by the Shandong Modern Agricultural Technology & Industry System (No. SDAIT-13-011-04).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All procedures related to this experiment were conducted under the protocols approved by the Institutional Animal Care and Use Committee of Shandong Agricultural University (SDAUA-2014-013).
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Yanhan Liu and Xiaona Zhao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, Y., Zhao, X., Zhang, X. et al. Effects of Oral Administration of CrCl3 on the Contents of Ca, Mg, Mn, Fe, Cu, and Zn in the Liver, Kidney, and Heart of Chicken. Biol Trace Elem Res 171, 459–467 (2016). https://doi.org/10.1007/s12011-015-0559-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0559-1