Abstract
Circulating concentration of the essential trace element selenium (Se) was significantly lower in inflammatory disorders. Although Se plays physiological roles mainly through the function of 25 selenoproteins, the response of the selenogenome in immune tissues during inflammatory reactions remains unclear. The objective of this study was to determine the Se retention and selenogenome expression in immune tissues during the lipopolysaccharide (LPS)-induced inflammatory response in porcine. A total of 12 male pigs were randomly divided into two groups and injected with LPS or saline. After 4 h postinjection, blood samples were collected and pigs were euthanized. Pigs challenged with LPS had 36.8 and 16.6 % lower (P < 0.05) Se concentrations in the serum and spleen, respectively, than those injected with saline. Moreover, the activities of GPX decreased (P < 0.05) by 23.4, 26.6, and 30.4 % in the serum, thymus, and lymph node, respectively, in the pigs injected with LPS. Furthermore, the LPS challenge altered (P < 0.05) the mRNA expression of 14, 16, 10, and 6 selenoprotein genes in the liver, spleen, thymus, and lymph node, respectively. Along with 10 previously reported selenoprotein genes, the response of Txnrd2, Txnrd3, Sep15, Selh, Seli, Seln, Selo, Selt, Selx, and Sephs2 to inflammatory reaction in immune tissues were newly illustrated in this study. In conclusion, the LPS-induced inflammatory response impaired Se metabolism and was associated with dysregulation of the selenogenome expression in immune tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) is an essential trace element for humans and animals. It plays pivotal roles in antioxidant defense, anticancer, detoxification, and improving immune function [1–5]. Numerous epidemiologic investigations and animal studies have shown inverse correlations between Se deficiency and inflammatory impairment, and lower serum Se status in inflammatory disorders with acute injury or trauma of tissues [6–10]. In contrast, supplementation of proper doses of Se can significantly alleviate and/or prevent the morbidity or mortality induced by inflammation in some clinical and animal studies [10–14]. In this regard, the retention and underlying molecular mechanism of Se in inflammatory response has recently gained increased attention.
A previous study has systematically investigated the retention of Se during the lipopolysaccharide (LPS)-induced acute phase response in rats, which found a significant decrease of Se content by 69.5 and 81.5 % in the plasma and liver, respectively [15]. While, the Se content was increased in the spleen, thymus, kidney, lung, heart, and muscle by LPS challenge, indicating that Se could play important roles in these tissues during an inflammatory response [15]. Since Se plays physiological roles mainly through the function of 25 selenoproteins that contain Se in the form of selenocysteine (Sec) [16, 17], understanding the response of selenogenome in those immune tissues during the inflammatory response would be an important aspect. A recent study showed that the serum Se and selenoprotein P (Sepp1) concentrations declined in parallel by 50 and 61 % in mice after injection of LPS, which was associated with downregulation of the hepatic selenoprotein biosynthesis machinery [9]. Another in vitro experiment showed that the amount of TXNRD1 was substantially increased, whereas GPX1 was modestly decreased in LPS-treated macrophages [18]. Moreover, LPS treatment or Fcγ receptor stimulation of mouse macrophages increased expression of two endoplasmic reticulum (ER) selenoproteins, Selk and Sels [19, 20]. In general, many of these past studies were focused on a single or a few selenoproteins in the response of inflammation in cells or single immune tissue. However, little information is available on the responses of the whole selenogenome during the inflammatory response in human or higher animals. Since pigs are an excellent model for human nutrition and medicine [21], we selected pigs to determine the Se retention and whole selenogenome expression in immune tissues, including the liver, spleen, thymus, and lymph node, during the LPS challenge induced inflammatory response.
Materials and Methods
Piglets, Treatments, and Sample Collection
All procedures for this experiment were approved by the Animal Care and Use Committee of Hubei Province, China. A total of 12 male pigs (Duroc × Large White × Landrace; body weight at 18.4 ± 1.2 kg) were randomly allotted to two groups. Each group of pigs were treated with saline or LPS. The challenged group received an intraperitoneal injection with E. coli LPS (E. coli serotype 055:B5, Sigma Chemical) at 100 μg/kg BW, and the unchallenged group received an equivalent amount of sterile saline. The dose of LPS (100 μg/kg BW) was chosen according to our previous studies [22, 23], which showed increased expression of proinflammatory cytokines in the immune tissues of weaned pigs. Many studies have shown that, within 1∼6 h postinjection, LPS activated the HPA axis and increased proinflammatory cytokine production in rats and pigs [22–25]. Thus, we used the time point of 4 h after LPS or saline treatment for experimental measurements in serum or tissues. Blood was collected from the anterior vena cava of pigs after 4 h of LPS injection. The serum was prepared by centrifugation at 3500×g for 10 min at 4 °C and stored at −80 °C until further analysis. After blood collection at 4 h, all pigs were humanely killed. The liver, spleen, thymus, and lymph node were collected, frozen immediately in liquid nitrogen, and then stored at −80 °C until analysis of mRNA abundance.
Se Concentrations and GPX Activity Analysis
The concentrations of Se in serum and tissues were measured by the hydride generation atomic fluorescence spectrometer (AF-610B, Beijing Rayleigh Analytical Instrument Corp., Beijing, China), against the standard reference of Se [GBW (E) 080441, National Research Center for Certified Reference Materials, Beijing, China] as previously described [14]. Total protein was extracted from 0.5 g liver tissue as previously described [26]. The activity of GPX was measured by a colorimetric method using specific assay kits (A005) from the Nanjing Jiancheng Bioengineering Institute of China. Protein concentrations were measured by the bicinchoninic acid assay [14].
Real-Time qPCR
Total RNA was prepared from the liver, spleen, thymus, and lymph node (20–50 mg tissue) using TRIzol (Invitrogen) and following the manufacturer’s instructions. The quality and quantity of RNA were analyzed by an Agilent Bioanalyzer 2100 using an RNA 6000 Labchip kit (Agilent Technologies, Amstelveen, Netherlands). The cDNA was synthesized from 1 μg total RNA by using Super Script III reverse transcriptase (Invitrogen) and following the manufacturer’s instructions. The mRNA levels of 25 selenoprotein genes were determined by qPCR (Bio-Rad CFX96 Touch) using the SYBRR Green PCR Master Mix (Bio-Rad, USA) and following the manufacturer’s instructions. Primers (Supplemental Table 1) for the selenoprotein genes and reference gene glyceraldehyde-3-phosphate dehydrogenase (GADPH) were referenced from previous studies [27] and/or designed using Primer Express 3.0 (Applied Biosystems, Foster City, CA, USA). The 2−ddCt method was used for the quantification with GADPH gene as a reference gene, and the relative abundance was normalized to the control (as 1).
Statistical Analysis
Data were analyzed using Student’s t test by SPSS version 13 (Chicago, IL, USA). Data are presented as mean ± SE, and significance was set at P < 0.05.
Results
Se Concentrations and GPX Activities
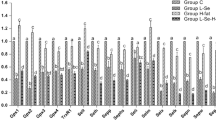
After 4 h of treatment with LPS, the Se concentrations in the serum, liver, spleen, thymus, and lymph node was measured; the results are presented in Fig. 1. Compared to pigs treated with saline, the concentration of Se decreased (P < 0.05) by 36.8 and 16.6 % in the serum and spleen, respectively, in the pigs injected with LPS. However, the concentrations of Se in the liver, thymus, and lymph node of pigs were not significantly affected (P > 0.05) by the challenge of LPS (Fig. 1). Meanwhile, the results of activities of GPX in the serum, liver, spleen, thymus, and lymph node are displayed in Fig. 2. Relative to pigs treated with saline, the pigs injected with LPS led to decreased (P < 0.05) activities of GPX by 23.4, 26.6, and 30.4 % in the serum, thymus, and lymph node, respectively. However, no changes in GPX activity (P > 0.05) were seen in the liver and spleen after challenge with LPS (Fig. 2).
Liver mRNA Levels of Selenoprotein Genes
A total of 14 of the 25 assayed selenoprotein genes in the liver were affected by LPS challenge in pigs (Fig. 3). The results showed that the mRNA level of Dio3 (599 %) was the greatest increased (P < 0.05), followed by Dio2 (379 %), Sels (191 %), Txnrd1 (122 %), Seli (88 %), and Gpx1 (49 %) in the liver of pigs injected with LPS, when compared with those injected with saline. In contrast, pigs challenged with LPS had 45–77 % lower (P < 0.05) Sepp1, Gpx2, Dio1, Txnrd2, Sephs, Selo, Selx, and Seln mRNA abundance in the liver compared with those injected with saline.
Spleen mRNA Levels of Selenoprotein Genes
A total of 16 of the 25 assayed selenoprotein genes in the spleen were affected by LPS challenge in pigs (Fig. 4). The results showed that the mRNA level of Dio2 (19.6 times) was the greatest increased (P < 0.05), followed by Sels (246 %), Txnrd1 (183 %), Gpx3 (138 %), and Selt (110 %), along with other selenoprtoein genes (Selx, Dio1, Selk, Txnrd3, Seli, and Sep15) from 23 to 63 % in the spleen of pigs injected with LPS, when compared with those injected with saline. In contrast, pigs challenged with LPS showed the decrease of (P < 0.05) mRNA abundance of Sepp1, Gpx2, Dio3, Txnrd2, and Seln from 30 to 81 % in the spleen compared with those injected with saline.
Lymph Node mRNA Levels of Selenoprotein Genes
Among the 25 selenoprotein genes assayed, 10 genes in the lymph node were affected by LPS challenge in pigs (Fig. 5). Specifically, relative to pigs treated with saline, the pigs injected with LPS had higher (P < 0.05) Dio2 (140 %), Sels (135 %), Gpx3 (98 %), Dio1 (89 %), Txnrd1 (52 %), Selk (52 %), Selt (29 %), and Txnrd3 (28 %) mRNA abundance in lymph node. However, pigs challenged with LPS led to the increase of (P < 0.05) mRNA abundance of Sepp1 (25 %) and Txnrd2 (19 %) in the lymph node compared with those injected with saline.
Thymus mRNA Levels of Selenoprotein Genes
Among the 25 selenoprotein genes assayed, only 6 genes in the thymus were affected by LPS challenge in pigs (Fig. 6). Pigs challenged with LPS had higher (P < 0.05) Dio2 (193 %), Gpx3 (62 %), and Sels (52 %) mRNA abundance in the thymus compared with those injected with saline. However, pigs injected with LPS had lower (P < 0.05) Selh (36 %), Txnrd2 (24 %), and Selo(15 %) mRNA abundance in the thymus compared with those injected with saline.
Discussion
The status of Se in body has been identified as an important regulator of disease [28]. Particularly, the immune system seems to depend on a well-maintained Se status to combat bacterial and viral infections, oxidative damage from environmental-, nutritional-, and age-related noxae, as well as other inflammatory challenges [9, 29–31]. Moreover, many previous studies demonstrated circulating Se concentrations were significantly lower in inflammatory disorders [6–9]. However, the retention of Se and the responses of whole selenogenome in the immune tissues during the inflammatory response are not well understood. In an attempt to answer these questions, we have taken the advantage of the pig model of LPS-induced acute phase response that faithfully mimics some aspects of immune stress.
The concentration of Se was reduced in serum, while was not changed in the liver, thymus, and lymph node by LPS challenge in pigs, which are similar to those in previous studies [9, 15]. The Se concentration in spleen was decreased in our study, while Maehira et al. (2003) reported that the Se concentration in spleen was not affected by LPS challenge in rat [15]. These divergences could be due to the experimental condition, such as the LPS injection dose, animal species, and exposure times. Moreover, LPS challenge reduced GPX activity in the serum, thymus, and lymph node in pigs. This finding is in agreement with previous studies, which reported that serum GPX activity was lower in LPS-induced rodents and human subjects with severe systemic inflammatory response syndrome, sepsis, and septic shock [12, 32]. Taken together, these results are similar to previous reports, which indicated that the metabolism of Se in immune tissues was impaired in acute phase response.
Another new dimension of the present study was the mRNA response profiles of 25 selenoprotein genes to the LPS challenge in the four immune tissues, including the liver, spleen, thymus, and lymph node. These profiles enabled us for the first time to systematically explore the selenoprotein genes that might be involved in the inflammatory reaction induced by LPS challenge. Among all the genes assayed, LPS challenge affected mRNA levels of 14, 16, 10, and 6 selenoprotein genes in the liver, spleen, thymus, and lymph node, respectively. Specifically, LPS challenge consistently downregulated the mRNA levels of Sepp1, Txnrd2, Gpx2, Seln, Selo, Selh, and Sephs2, but upregulated those of Sels, Dio2, Gpx3, Txnrd1, Txnrd3, Selk, Selt, Seli, Gpx1, and Sep15 in the liver, spleen, thymus, and/or lymph node. Whereas, there are three selenoprotein genes (Dio1, Dio3, and Selx) did not exhibit a common response to LPS challenge across the four tissues. Whereas Sepp1 [9, 18], Sels [33], Gpx1 [18], Gpx2 [34], Gpx3 [35], Txnrd1 [18], Selk [20, 36], Dio1 [37], Dio2 [37–39], and Dio3 [37] were shown to have protective functions against or involved in inflammation, the potential involvements of Txnrd2, Txnrd3, Sep15, Selh, Seli, Seln, Selo, Selt, Selx, and Sephs2 in this regard are newly illustrated by us in the present study. Coincidently, eight of these ten genes (Txnrd2, Txnrd3, Sep15, Selh, Seln, Selo, Selt, and Selx) code for proteins, which possess antioxidant capacity [5, 16, 17, 40–42]. Although these selenoproteins might cope with the oxidative stress induced by LPS [43, 44], it is hard for us to explain why some of them (Txnrd3, Sep15, Selt, and Selx) were upregulated and others (Txnrd2, Selh, Seln, Selo, and Selx) were downregulated in the immune tissues during the LPS challenge. Although the protein encoded by Seli possibly involved in phospholipid biosynthesis and Sephs2 plays a crucial role in synthesis of all selenoproteins [40], their exact role in inflammatory reaction remain to be determined.
Nevertheless, several seemingly conflicting or inconsistent scenarios were observed in the present study. Despite some selenoprtoein genes (Sepp1, Sels, Gpx3, Dio2, Txnrd1, and Txnrd2) exhibited a common response to LPS challenge across three to four immune tissues, other selenoprtoein genes (Dio1, Dio3, and Selx) even showed different responses to LPS challenge across immune tissues. Meanwhile, no single immune tissue showed any common response to LPS challenge across various selenoprotein genes. These divergences suggest that regulations of selenoprotein genes as well as Se metabolism by an LPS-induced inflammatory reaction may vary in various immune tissues. Meanwhile, the GPX activity in the thymus and lymph node were not in accordance with Gpx3 mRNA abundance. This paradoxic discrepancy may be interpreted by a complex feedback or posttranscriptional mechanism in regulating GPX3 synthesis [45, 46].
In conclusion, the present study indicated that a LPS-induced acute phase response impaired Se metabolism in immune tissues. This was demonstrated by decreasing Se concentrations and/or GPX activity in the serum, spleen, thymus, and/or lymph node. Moreover, the LPS-induced dysregulation of Se metabolism was associated with altered mRNA expression of 14, 16, 10, and 6 selenoprotein genes in the liver, spleen, thymus, and lymph node, respectively. These findings could help us to better understand the impairment of Se and selenogenome metabolism in immune tissues during an inflammatory reaction.
Reference
Tinggi U (2008) Selenium: its role as antioxidant in human health. Environ Health Prev Med 13(2):102–108
Sun LH, Li JG, Zhao H, Shi J, Huang JQ, Wang KN, Xia XJ, Li L, Lei XG (2013) Porcine serum can be biofortified with selenium to inhibit proliferation of three types of human cancer cells. J Nutr 143(7):1115–1122
Hoffmann PR, Berry MJ (2008) The influence of selenium on immune responses. Mol Nutr Food Res 52(11):1273–1280
Nuttall KL, Allen FS (1984) Selenium detoxification of heavy metals: a possible mechanism for the blood plasma. Inorg Chim Acta 92(3):187–189
Prabhu KS, Lei XG (2016) Selenium. Adv Nutr 7(2):415–417
Berger MM, Cavadini C, Bart A, Blondel A, Bartholdi I, Vandervale A, Krupp S, Chiolero R, Freeman J, Dirren H (1992) Selenium losses in 10 burned patients. Clin Nutr 11(2):75–82
Nichol C, Herdman J, Sattar N, O’Dwyer PJ, O’Reilly DJ, Littlejohn D, Fell G (1998) Changes in the concentrations of plasma selenium and selenoproteins following minor elective surgery: further evidence for a negative acute phase response? Clin Chem 44(8 Pt 1):1764–1766
Hawker FH, Stewart PM, Snitch PJ (1990) Effects of acute illness on selenium homeostasis. Crit Care Med 18(4):442–446
Renko K, Hofmann PJ, Stoedter M, Hollenbach B, Behrends T, Köhrle J, Schweizer U, Schomburg L (2009) Down-regulation of the hepatic selenoprotein biosynthesis machinery impairs selenium metabolism during the acute phase response in mice. FASEB J 23(6):1758–1765
Chen K, Peng X, Fang J, Cui H, Zuo Z, Deng J, Chen Z, Geng Y, Lai W, Tang L, Yang Q (2014) Effects of dietary selenium on histopathological changes and T cells of spleen in broilers exposed to aflatoxin B1. Int J Environ Res Public Health 11(2):1904–1913
Angstwurm MW, Schottdorf J, Schopohl J, Gaertner R (1999) Selenium replacement in patients with severe systemic inflammatory response syndrome improves clinical outcome. Crit Care Med 27(9):1807–1813
Angstwurm MW, Engelmann L, Zimmermann T, Lehmann C, Spes CH, Abel P, Strauss R, Meier-Hellmann A, Insel R, Radke J, Schüttler J, Gärtner R (2007) Selenium in intensive care (SIC): results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit Care Med 35(1):118–126
Kalantari P, Narayan V, Natarajan SK, Muralidhar K, Gandhi UH, Vunta H, Henderson AJ, Prabhu KS (2008) Thioredoxin reductase-1 negatively regulates HIV-1 transactivating protein Tat-dependent transcription in human macrophages. J Biol Chem 283(48):33183–33190
Sun LH, Zhang NY, Zhu MK, Zhao L, Zhou JC, Qi DS (2016) Prevention of alfatoxin B1 hepatoxicity by dietary selenium is associated with inhibition of cytochrome P450 isozymes and up-regulation of six selenoprotein genes in chick liver. J Nutr 146(4):655–661
Maehira F, Luyo GA, Miyagi I, Oshiro M, Yamane N, Kuba M, Nakazato Y (2002) Alterations of serum selenium concentrations in the acute phase of pathological conditions. Clin Chim Acta 316(1–2):137–146
Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, Gladyshev VN (2003) Characterization of mammalian selenoproteomes. Science 300(5624):1439–1443
Moghadaszadeh B, Beggs AH (2006) Selenoproteins and their impact on human health through diverse physiological pathways. Physiology (Bethesda) 21(5):307–315
Carlson BA, Yoo MH, Sano Y, Sengupta A, Kim JY, Irons R, Gladyshev VN, Hatfield DL, Park JM (2009) Selenoproteins regulate macrophage invasiveness and extracellular matrix-related gene expression. BMC Immunol 10:57
Stoedter M, Renko K, Hög A, Schomburg L (2010) Selenium controls the sex-specific immune response and selenoprotein expression during the acute-phase response in mice. Biochem J 429(1):43–51
Huang Z, Hoffmann FW, Norton RL, Hashimoto AC, Hoffmann PR (2011) Selenoprotein K is a novel target of m-calpain, and cleavage is regulated by Toll-like receptor-induced calpastatin in macrophages. J Biol Chem 286(40):34830–34838
Patterson JK, Lei XG, Miller DD (2008) The pig as an experimental model for elucidating the mechanisms governing dietary influence on mineral absorption. Exp Biol Med (Maywood) 233(6):651–664
Chen F, Liu YL, Zhu HL, Hong Y, Wu ZF, Hou YQ, Li Q, Ding BY, Yi D, Chen HB (2013) Fish oil attenuates liver injury caused by LPS in weaned pigs associated with inhibition of TLR4 and NOD signaling pathways. Innate Immun 19(5):504–515
Liu YL, Lu J, Shi JX, Hou YQ, Zhu HL, Zhao SJ, Liu HM, Ding BY, Yin YL, Yi GF (2008) Increased expression of the peroxisome proliferator-activated receptor γ in the immune system of weaned pigs after Escherichia coli lipopolysaccharide challenge. Vet Immunol Immunop 124(1–2):82–92
Liu YL, Shi JX, Lu J, Che ZQ, Zhu HL, Hou YQ, Yin YL, Zhao SJ, Ding BY, Liu HM (2010) Up-regulated expression of peroxisome proliferator-activated receptor gamma in the hypothalamic-pituitary-adrenal axis of weaned pigs after Escherichia coli lipopolysaccharide challenge. Vet J 184(2):230–235
Grinevich V, Ma XM, Herman JP, Jezova D, Akmayev I, Aguilera G (2001) Effect of repeated lipopolysaccharide administration on tissue cytokine expression and hypothalamic-pituitary-adrenal axis activity in rats. J Neuroendocrinol 13(8):711–723
Sun LH, Zhang NY, Zhai QH, Gao X, Li C, Zheng Q, Krumm CS, Qi DS (2014) Effects of dietary tin on growth performance, hematology, serum biochemistry, antioxidant status, and tin retention in broilers. Biol Trace Elem Res 162(1–3):302–308
Zhao H, Li K, Tang JY, Zhou JC, Wang KN, Xia XJ, Lei XG (2015) Expression of selenoprotein genes is affected by obesity of pigs fed a high-fat diet. J Nutr 145(7):1394–1401
Rayman MP (2002) The argument for increasing selenium intake. Proc Nutr Soc 61(2):203–215
Arthur JR, McKenzie RC, Beckett GJ (2003) Selenium in the immune system. J Nutr 133(5):1457S–1459S
Broome CS, McArdle F, Kyle JA, Andrews F, Lowe NM, Hart CA, Arthur JR, Jackson MJ (2004) An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr 80(1):154–162
Hurwitz BE, Klaus JR, Llabre MM, Gonzalez A, Lawrence PJ, Maher KJ, Greeson JM, Baum MK, Shor-Posner G, Skyler JS, Schneiderman N (2007) Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: a randomized controlled trial. Arch Intern Med 167(2):148–154
Valenca SS, Silva Bezerra F, Lopes AA, Romana-Souza B, Marinho Cavalcante MC, Lima AB, Gonçalves Koatz VL, Porto LC (2008) Oxidative stress in mouse plasma and lungs induced by cigarette smoke and lipopolysaccharide. Environ Res 108(2):199–204
Curran JE, Jowett JB, Elliott KS, Gao Y, Gluschenko K, Wang J, Abel Azim DM, Cai G, Mahaney MC, Comuzzie AG, Dyer TD, Walder KR, Zimmet P, MacCluer JW, Collier GR, Kissebah AH, Blangero J (2005) Genetic variation in selenoprotein S influences inflammatory response. Nat Genet 37(11):1234–1241
Esworthy RS, Yang L, Frankel PH, Chu FF (2005) Epithelium-specific glutathione peroxidase, Gpx2, is involved in the prevention of intestinal inflammation in selenium-deficient mice. J Nutr 135(4):740–745
Manzanares W, Biestro A, Galusso F, Torre MH, Mañay N, Pittini G, Facchin G, Hardy G (2009) Serum selenium and glutathione peroxidase-3 activity: biomarkers of systemic inflammation in the critically ill? Intensive Care Med 35(5):882–889
Verma S, Hoffmann FW, Kumar M, Huang Z, Roe K, Nguyen-Wu E, Hashimoto AS, Hoffmann PR (2011) Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses. J Immunol 186(4):2127–2137
Wajner SM, Goemann IM, Bueno AL, Larsen PR, Maia AL (2011) IL-6 promotes nonthyroidal illness syndrome by blocking thyroxine activation while promoting thyroid hormone inactivation in human cells. J Clin Invest 121(5):1834–1845
Cheng AW, Bolognesi M, Kraus VB (2012) Dio2 modifies inflammatory responses in chondrocytes. Osteoarthr Cartilage 20(5):440–445
Fekete C, Gereben B, Doleschall M, Harney JW, Dora JM, Bianco AC, Sarkar S, Liposits Z, Rand W, Emerson C, Kacskovics I, Larsen PR, Lechan RM (2004) Lipopolysaccharide induces type 2 iodothyronine deiodinase in the mediobasal hypothalamus: implications for the nonthyroidal illness syndrome. Endocrinology 145(4):1649–1655
Huang Z, Rose AH, Hoffmann PR (2012) The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 16(7):705–743
Han SJ, Lee BC, Yim SH, Gladyshev VN, Lee SR (2014) Characterization of mammalian selenoprotein O: a redox-active mitochondrial protein. PLoS One 9:e95518
Sengupta A, Carlson BA, Labunskyy VM, Gladyshev VN, Hatfield DL (2009) Selenoprotein T deficiency alters cell adhesion and elevates selenoprotein W expression in murine fibroblast cells. Biochem Cell Biol 87(6):953–961
Noworyta-Sokołowska K, Górska A, Gołembiowska K (2013) LPS-induced oxidative stress and inflammatory reaction in the rat striatum. Pharmacol Rep 65(4):863–869
Shah SA, Khan M, Jo MH, Jo MG, Amin FU, Kim MO (2016) Melatonin stimulates the SIRT1/Nrf2 signaling pathway counteracting lipopolysaccharide (LPS)-induced oxidative stress to rescue postnatal rat brain. CNS Neurosci Ther doi. doi:10.1111/cns.12588
Pepper MP, Vatamaniuk MZ, Yan X, Roneker CA, Lei XG (2011) Impacts of dietary selenium deficiency on metabolic phenotypes of diet-restricted GPX1-overexpressing mice. Antioxid Redox Signal 14(3):383–390
Chen G, Gharib TG, Huang CC, Taylor JM, Misek DE, Kardia SL, Giordano TJ, Iannettoni MD, Orringer MB, Hanash SM, Beer DG (2002) Discordant protein and mRNA expression in lung adenocarcinomas. Mol Cell Proteomics 1(4):304–313
Acknowledgments
We thank Dr. Christopher Steven Krumm for technical assistance in the editing of this article. This project was supported by the Chinese Natural Science Foundation Projects 31501987, 31422053, and 31372318.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Esm 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Sun, LH., Pi, DA., Zhao, L. et al. Response of Selenium and Selenogenome in Immune Tissues to LPS-Induced Inflammatory Reactions in Pigs. Biol Trace Elem Res 177, 90–96 (2017). https://doi.org/10.1007/s12011-016-0863-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0863-4