Abstract
Lead (Pb) enhances the production of reactive oxygen species and depletes the antioxidant molecules that cause tissue damage. In the current study, we investigated the protective effect of Indigofera oblongifolia (hasr in Arabic) against lead acetate-induced reproductive toxicity in rats. Exposure of rats to lead acetate (PbAc; 20 mg/kg body weight; intraperitoneal injection) induced a significant change in both of body weight loss and the relative testis weight. Furthermore, a significant increase in lipid peroxidation and nitric oxide and a marked depletion of glutathione were evident in the testis of the PbAc group compared to the control group. Also, PbAc significantly reduced the activity of antioxidant enzymes. Pre-administration of I. oblongifolia leaves extract (IOLE; 100 mg/kg body weight) to the PbAc-treated rats restored most of the parameters mentioned above to near-normal levels. Additionally, pretreatment of animals with IOLE accompanied with a significant decrease in the toxic effects of PbAc as shown by caspase-3 and Bax expressions and prevented the histological injury in the testis. On the basis of the above results, I. oblongifolia appeared to be a promising agent for protection against lead-induced oxidative damage and apoptosis in the testis of rat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lead, an example of heavy metals, has being known for its destructive effects on various body tissues and organs such that their functions are compromised due to its interference with bioelements [1]. Over the past 30 years, the environmental levels of lead have increased more than 1000-fold as a result of human activity [2]. The toxic effect of lead on fertility is ubiquitous affecting all parts of the reproductive system [3]. It was reported that one of the important mechanisms associated with lead toxicity is the oxidative stress caused by prooxidant/antioxidant imbalance in the different cells [1]. However, the mechanisms of lead toxicity and also the protective measures against lead harmful effects still remain to be solved [4].
Due to the richness of the testicular tissue with polyunsaturated membrane lipids, it becomes one of the targets for lead toxicity [3]. Currently, the therapeutic strategy for lead toxicity is chelation therapy to encourage lead excretion. Chelators for lead toxicity such as CaNa2EDTA and meso-2,3-dimercaptosuccinic acid (DMSA) are themselves reported to have a number of adverse effects. Therefore, the supplementation with antioxidants could protect testicular functions damaged by lead [5]. Natural products from a variety of herbal sources contain a huge potential of pharmacologically active substances that might play a role in drug development [6]. Interest in natural products for drug development has waxed and waned, but there is no denying their importance in heavy metals chelators.
Indigofera oblongifolia (hasr in Arabic) is considered to be one of the medicinal plants (family Fabaceae). It is widely distributed throughout the tropical and subtropical regions. The leaves of hasr are generally used as an analgesic with anti-inflammatory activity in insect stings, snakebites, swellings, and peptic ulcer pain [7]. I. oblongifolia protects membrane lipids, nuclear DNA, and proteins from carbon tetrachloride-induced hepatotoxicity through its strong capacity to inhibit oxidative stress [8]. Therefore, the present study was aimed to investigate the ameliorative effect of I. oblongifolia in lead-induced testicular oxidative damage and apoptosis in adult rats.
Materials and Methods
Chemicals and Experimental Animals
Adult male Wistar albino rats (150–180 g) were purchased from the animal facility of the Holding Company for Biological Products and Vaccines (VACSERA, Cairo, Egypt). Rats were divided into four groups with seven rats per group. Animals were kept under standard condition of illumination with a 12-h light-dark cycle at 25 ± 2 °C. Animals got water and a balanced diet ad libitum. In all experiments, we followed the European Community Directive (86/609/EEC) and national rules on animal care that were carried out in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals 8th edition. This was approved by the committee of the Zoology and Entomology department, Helwan University, Egypt.
Preparation of Indigofera oblongifolia Extract
Indigofera oblongifolia (I. oblongifolia) leaves were collected from Jazan Province located in the southwest of Saudi Arabia. The collected I. oblongifolia leaves were air dried, powdered using a pulverizer, and then extracted with 70 % methanol. The crude extract was dissolved in distilled water [9].
Experimental Design
To study the protective effects of I. oblongifolia on lead acetate (PbAc)-induced reproductive toxicity, the rats were randomly allocated into four groups of seven animals per group. The first group (Con group) is considered as the control group. Each rat in this group was orally received 300 μl saline, and after 1 h, they injected intraperitoneally (i.p.) with 100 μl of saline. Groups II (PbAc group) and IV (IOLE + PbAc group) received daily i.p. injection of PbAc (20 mg/kg bwt), and groups III (IOLE group) and IV were orally treated with IOLE (100 mg/kg bwt). Animals were daily administered their respective doses for 5 consecutive days. In IOLE + PbAc group, the treatment of IOLE was given before PbAc within an hour. IOLE was orally administered at a dose of 100 mg/kg bwt according to Lubbad et al., [9] while PbAc was i.p. injected at an acute toxic dose of 20 mg/kg bwt [4].
The animals were killed under mild ether anesthesia after 24 h from the last administration. The testes of the rats were removed, weighed, washed in ice-cold 50 mM Tris–HCl and then quickly homogenized to give a 10 % (w/v) homogenate in ice-cold medium that contained 50 mM Tris–HCl, pH 7.4. Finally, the testis homogenates was centrifuged at 2000×g at 4 °C for 10 min, and then, the collected supernatant was used for the biochemical determinations after determining the total protein.
Concentration of Lead
The concentration of lead in the rat testis was measured using standard method by using flame atomic absorption spectrophotometer (Perkin-Elmer, 3100) and expressed as μg lead/g wet tissue.
Changes in Testis Index of Rats
The relative testis weight was calculated as left testis weight / body weight × 100.
Blood Testosterone
According to the protocol provided with the rat serum testosterone ELISA kit (BioVendor, Gunma, Japan), we determine the testosterone level.
Oxidative Stress of Testes
According to Ohkawa et al. [10], the testis lipid peroxidation was determined and expressed as the amount of malondialdehyde (MDA) formed. The level of nitric oxide in testis homogenate was determined by the optimized acid reduction method of Green et al. [11]. Whereas, the testicular glutathione (GSH) was measured by the reduction of Elman’s reagent [5,5′ dithiobis (2-nitrobenzoic acid)] with GSH to produce a yellow compound and measured at 405 nm [12].
Antioxidant Activity
The antioxidant enzymes activities, such as superoxide dismutase (SOD) were determined by the reduction of nitroblue tetrazolium (NBT). Testicular catalase (CAT) was assayed by determining the rate of H2O2 disintegration at 340 nm. Glutathione reductase (GRd) was assayed indirectly by GRd catalysis of the reduction of glutathione in the presence of NADPH, which is oxidized to NADPH+. The decrease in absorbance at 340 nm was measured. Finally, glutathione peroxidase (GPx) activity in the testis homogenates was measured using the method of Paglia and Valentine [13].
Gene Expression Using Quantitative Real-Time PCR
RNeasy Plus Minikit (Qiagen, Valencia, CA) was used to isolate the total RNAs from the testis of rats. Also, RevertAid™ H Minus Reverse Transcriptase (Fermentas, Thermo Fisher Scientific Inc., Canada) was used to synthesis cDNA. The cDNA samples were run in triplicate for real-time PCR analysis. β-actin (accession number: NM_031144.3; sense: 5′-GGCATCCTGACCCTGAAGTA-3′; antisense: 5′-GGGGTGTTGAAGGTCTCAAA-3′) was used as the reference gene. SYBR® Green (Life Technologies, CA) was used for the real-time PCR using the Applied Biosystems 7500 system. The typical thermal profile for the PCR reaction was 95 °C for 4 min, followed by 40 cycles of 94 °C for 60 s and 55 °C for 60 s. For relative quantitation of gene expression, the log2 of 2−ΔΔCt was used based on the method of Pfaffl [14], where ΔCt was calculated by subtracting the β-actin cycle threshold value from each sample’s cycle threshold value (Ct). The PCR primers for the following genes were synthesized by Jena Bioscience GmbH (Jena, Germany) and designed using the Primer-Blast program from NCBI: SOD2 (accession number: NM_001270850.1; sense: 5′-AGCTGCACCACAGCAAGCAC-3′; antisense: 5′-TCCACCACCCTTAGGGCTCA-3′), CAT (accession number: NM_012520.2; sense: 5′-TCCGGGATCTTTTTAACGCCATTG-3′; antisense: 5′-TCGAGCACGGTAGGGACAGTTCAC-3′) and GPx (accession number: NM_017006.2; sense: 5′-CGGTTTCCCGTGCAATCAGT-3′; antisense: 5′-ACACCGGGGACCAAATGATG-3′).
Histological Changes
Fixation of rat testis was carried out in 10 % neutral buffered formalin. After 24 h, samples were dehydrated in ethanol, cleared in xylene, mounted in paraplast, and then sectioned at a thickness of 4–5 μm. Sections were stained with hematoxylin and eosin.
Apoptotic Markers in Testes Tissue
Immunocytochemical reactions were performed using the peroxidase/anti-peroxidase (PAP) method [15]. Methanol containing 0.1 % H2O2 was used to block the non-specific peroxidase reactions, and the sections were also incubated with normal goat serum to avoid the non-specific reactions with the background once the samples were incubated with the specific antibody against caspase-3 or Bax (dilution, 1:2000, Santa Cruz CA, USA). Tissue sections were then washed with phosphate buffer and incubated with a secondary antibody (1:2000; Sigma, USA), before being washed in phosphate buffer again and, finally, incubated with the PAP complex (dilution, 1:200). After immunostaining, the sections were lightly counterstained with hematoxylin and observed under a light microscope.
Statistical Analysis
One-way analysis of variance (ANOVA) was used to analyze our data. Duncan’s test was performed as a post hoc test using the Statistical Package for the Social Sciences (SPSS version 20.0).
Results
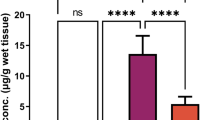
Data in Fig. 1 showed that Pb2+ concentration in the testes homogenate was significantly higher in PbAc group than in the control group (p < 0.05). Also, administration of IOLE 1 h before PbAc significantly decreased the concentration of testicular Pb2+ in IOLE + PbAc group compared to PbAc-treated group (P < 0.05).
Ameliorative effects of I. oblongifolia leaves extract (IOLE) on lead accumulation in testes tissue of rats treated with lead acetate (PbAc) for 5 days. Data are expressed as the mean ± SEM. a Significant change at p < 0.05 with respect to the Control group as negative control group. b Significant change at p < 0.05 with respect to the PbAc group as positive control group
The harmful effect of PbAc on testis weight and the relative testis weight were illustrated in Fig. 2. PbAc administration for 5 days induced significant elevations in testis weight and relative testis weight compared to the control group (p < 0.05). However, the pretreatment of IOLE to PbAc induced significant increases in both testis weight and relative testis weight compared to the control group (Fig. 2).
Ameliorative effects of I. oblongifolia leaves extract (IOLE) pre-administration on testis weight and the relative testis weight in rats exposed to lead acetate (PbAc) for 5 days. Data are expressed as the mean ± SEM. a Significant change at p < 0.05 with respect to the Control group as negative control group. b Significant change at p < 0.05 with respect to the PbAc group as positive control group
Lead acetate injected intraperitoneally to rats induced a marked decrease in serum testosterone (Fig. 3, p < 0.001). Also, a significant increase in serum testosterone level was observed in IOLE + PbAc group compared to that in the PbAc-alone-treated rat group.
Ameliorative effects of I. oblongifolia leaves extract (IOLE) on testosterone level in serum of rats treated with lead acetate (PbAc) for 5 days. Data are expressed as the mean ± SEM. a Significant change at p < 0.05 with respect to the Control group as negative control group. b Significant change at p < 0.05 with respect to the PbAc group as positive control group
PbAc administration for 5 days induced a marked significant increase (p ˂ 0.001) in the testes LPO and NO levels compared to the control rats (Fig. 4). Fortunately, IOLE previous to PbAc markedly attenuated the lipid peroxidation and also nitrosative stress in PbAc inoculated rats. Lead caused testicular oxidative damage that was evidenced by a significant depletion (p < 0.05) in the level of testes GSH. This depletion in the level of GSH was altered by IOLE pretreatment (Fig. 4).
Ameliorative effects of I. oblongifolia leaves extract (IOLE) pre-administration on oxidative stress markers in rats exposed to lead acetate (PbAc) for 5 days. Data are expressed as the mean ± SEM. a Significant change at p < 0.05 with respect to the Control group as negative control group. b Significant change at p < 0.05 with respect to the PbAc group as positive control group
To study the protective effects of I. oblongifolia in animals exposed to lead, alteration of the antioxidant defense system was investigated, including the activity of SOD, CAT, GPx, and GRd enzymes in the testes of rats. Lead could significantly modulate the antioxidant enzymes (Fig. 5). On day 5 post inoculation of rats with PbAc, levels of SOD, CAT, GPx, and GRd in rat testes were significantly inhibited (p < 0.001). However rats treated with IOLE could significantly increase levels of SOD, CAT, GPx, and GRd (Fig. 5). Constant with the biochemical findings, our quantitative PCR results showed that mRNA of SOD2, CAT, and GPx were downregulated after lead administration. However, IOLE was able to upregulate these genes (Fig. 6).
Ameliorative effects of I. oblongifolia leaves extract (IOLE) pre-administration on superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and glutathione reductase (GRd) activities in rats exposed to lead acetate (PbAc) for 5 days. Data are expressed as the mean ± SEM. a Significant change at p < 0.05 with respect to the Control group as negative control group. b Significant change at p < 0.05 with respect to the PbAc group as positive control group
Ameliorative effects of I. oblongifolia leaves extract (IOLE) pre-administration on mRNA expression of SOD2, CAT, and GPx genes in the testes of rats exposed to lead acetate (PbAc) for 5 days. Data are expressed as the mean ± SEM. a Significant change at p < 0.05 with respect to the Control group as negative control group. b Significant change at p < 0.05 with respect to the PbAc group as positive control group
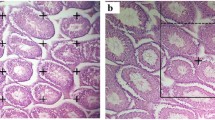
The seminiferous tubules lost their normal architecture after lead inoculation (Fig. 7). The epithelial elements of the testis became degenerated and some of their cells were necrotic. IOLE was able to improve the destructive changes in the seminiferous tubules (Fig. 7). In addition, immunohistochemical examination of control testis showed moderate positive reaction in Cas-3 and mild positive reaction in Bax immunostaining cells (Figs. 8 and 9, respectively). Meanwhile, in PbAc injected group marked increase in Cas-3 and Bax reactions were clearly shown in seminiferous tubules (Figs. 8 and 9, respectively). IOLE extract treated alone group showed a moderate positive staining of Cas-3 and mild positive affinity to Bax. Pretreatment with IOLE extract to PbAc group (IOLE + PbAc) showed improvement represented by mild positive reaction in Cas-3 and Bax in the seminiferous tubules (Figs. 8d and 9d, respectively). In this case, the numbers of Cas-3 and Bax immunostaining cells were decreased markedly (Table 1).
Light micrographs of rat testis. a normal histological architecture of testis seminiferous tubules. b PbAc-treated group with injured seminiferous tubules with deformed spermatozoa and absence of late stage germ cells. c normal histological structure of seminiferous tubules with well developed spermatozoa in IOLE group. d improved histological structure of seminiferous tubules with well developed spermatozoa in group of IOLE pre-administered to PbAc. Sections were stained with hematoxylin and eosin (400×)
Discussion
Environmental and occupational exposures to Pb are known to have adverse effects on the reproductive system, and fertility problems have attracted much public attention in the past years. Our results revealed that PbAc administration in rats disrupts hormonal balance and induces oxidative stress in the testes of rats. It has been proposed that Pb may initiate oxidative stress through two separate, although related pathways: (1) depletion antioxidant enzymes and (2) enhancing reactive oxygen species (ROS) production [16].
The effect of PbAc on testis weight of rats and relative testis weight were markedly higher than that of the normal control group. Lead poisoning is very well known to affect most of the body organ systems and is associated with a number of morphological, biochemical, and physiological changes that include reproductive dysfunction, disturbance in the biological trace elements, and impaired glucose metabolism. The increases in testis absolute and relative weights may be attributed to testis atrophy and to the interstitial edema that resulted from the fluid accumulation in the testis. The obtained results are in agreement with the results of Graca et al. [17] they observed that acute Pb administration caused significant increase in testis weight of adult mice followed 3 days of administration. Contradiction to our findings, Acharya et al. found a significant decline in testis weight in mice injected intraperitoneally with PbAc at a single dose, and they attributed the reduction in body weight after exposure to Pb may be due to ROS generation in testes [18].
The observed elevated level of testicular MDA, a biomarker of lipid peroxidation, implies that PbAc injection induces oxidative stress in the testes due to enhanced lipid peroxidation and, thus, directly indicating that Pd-intoxication induces a dropping down of the antioxidative defense concomitant with increase in free radicals generation, such as H2O2 and OH·. Indeed, lipid peroxidation is one of the indicators of oxidative injure, and it plays a very important role in xenobiotics toxicity, because it disrupts the physicochemical properties of the lipid bilayers of cellular membrane, thereby causing cellular dysfunction. This data supports our previous studies reporting that lead is capable of causing oxidative damages in the liver and kidney [1, 19].
Our results showed an elevation in the NO generation upon exposure to PbAc. This effect can be attributed to the ability to Pb to stimulate the inducible nitric oxide synthase (iNOS), thus resulting in an elevation in the rate of NO production. NO can combine with superoxide anion to form peroxynitrite anion (ONOO−). This highly reactive oxygenated species is able to induce membrane lipid peroxidation [20].
Furthermore, we observed significant depletion in testicular GSH content, following exposure to PbAc, which suggest disturbance of the redox homeostasis of testes. GSH is the most abundant –SH molecule in the organ tissues that could be either as a non-enzymatic antioxidant molecule by direct interaction of –SH group with ROS or it can be participated in the enzymatic detoxification reaction for ROS, as an essential cofactor or coenzyme [4, 21].
We observed a significant inhibition in SOD, CAT, GRd, and GPx activities in the testes in PbAc injected rats suggests an interaction between the accumulated free radicals and the active amino acids of these enzymes. During reproductive toxicity, these enzymes are structurally and functionally incapacitated by free radicals, resulting in testis damage. The observed PbAc-induced inhibition in antioxidant enzyme activities may be mainly due to the high affinity of Pb to SH groups or metal cofactors in these enzymes, as Pb competes and replaces Cu2+ and Zn2+ in their binding sites [22]. The inhibition of antioxidant enzymes may result in alteration in membrane integrity, thereby increasing the susceptibility of the membrane to lipid peroxidation [22].
Our results show that morphological changes in rat testis are associated with increased lipid peroxidation. Since Pb exposure may increase susceptibility of membranes to injure by altering the major constituents of biological membranes fatty acid that are good sites for lipid peroxidation reactions and are more sensitive to oxidative damage causing malfunction and eventually cell death. In the present study, lead concentration in testes is significantly correlated with the pathological features in the testis. Our data also revealed that exposure to PbAc reduced the testosterone levels in plasma, indicating interference with spermatogenesis and steroidogenesis.
Enhanced oxidative stress in the testes after PbAc exposure was reflected in the significant formation of apoptotic cell death in the testes of rats. The immunohistochemistry study of the lead intoxication revealed that control rat displayed the normal distribution of Bax and caspase-3 proteins. In contrast, a significant expression in caspase-3 and Bax proteins indicating that PbAc provokes apoptosis in the rat testes. However, excessive apoptosis could induce injuries of the testes and reduce male reproductive function [23]. Expression of caspase-3 and Bax proteins in testes tissue after exposure to PbAc in our study are in accordance with previous reports by Wang et al. [24] and Elgawish and Abdelrazek [23].
The observed decrease in testes Pb concentrations in I. oblongifolia with PbAc injection group reveals the possible chelating effect of I. oblongifolia. However, this action of I. oblongifolia extract requires further studies. Relatively few studies are available for the antioxidant effect of I. oblongifolia extract. However, the observed antioxidant activities of I. oblongifolia extract against PbAc-induced reproductive toxicity could be due to the antioxidant chemical compounds present in the plant extract. Phytochemical screening of I. oblongifolia revealed significant amounts of polyphenolic and flavonoid compounds in the extract (data not shown). The antioxidant compounds in I. oblongifolia could play an important role in scavenging the ROS induced by PbAc in the testes of rat. So, the protective effects of I. oblongifolia extract in keeping the GSH content towards control have increased the ability of antioxidant defense and increased the steady state of GSH and/or its rate of synthesis as well. This enhanced protection against Pb-induced oxidative stress.
From the data of the current study, PbAc exposure caused reproductive toxicity. This might be associated with decreased antioxidant molecules and enhancing reactive oxygen species production. The use of I. oblongifolia extract reduced PbAc-induced reproductive toxicity. I. oblongifolia extract might exert its acceptable mitigative protective effect in testis through antioxidative and antiapoptotic effects.
References
Abdel-Moneim AE, Dkhil MA, Al-Quraishy S (2011) The redox status in rats treated with flaxseed oil and lead-induced hepatotoxicity. Biol Trace Elem Res 143(1):457–467
Elgawish RAR, Abdelrazek HMA (2014) Effects of lead acetate on testicular function and caspase-3 expression with respect to the protective effect of cinnamon in albino rats. Toxicology Reports 1 (0):795–801
Ayinde OC, Ogunnowo S, Ogedegbe RA (2012) Influence of vitamin C and vitamin E on testicular zinc content and testicular toxicity in lead exposed albino rats. BMC Pharmacol Toxicol 13:17
Abdel Moneim AE (2012) Flaxseed oil as a neuroprotective agent on lead acetate-induced monoamineric alterations and neurotoxicity in rats. Biol Trace Elem Res 148(3):363–370
Mabrouk A, Cheikh HB (2014) Thymoquinone supplementation ameliorates lead-induced testis function impairment in adult rats. Toxicol Ind Health. doi:0748233714548474
Newman DJ, Cragg GM (2012) Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 75(3):311–335
Ali NA, Julich WD, Kusnick C, Lindequist U (2001) Screening of Yemeni medicinal plants for antibacterial and cytotoxic activities. J Ethnopharmacol 74(2):173–179
Shahjahan M, Vani G, Devi CS (2005) Protective effect of Indigofera oblongifolia in CCl4-induced hepatotoxicity. J Med Food 8(2):261–265
Lubbad MY, Al-Quraishy S, Dkhil MA (2015) Antimalarial and antioxidant activities of Indigofera oblongifolia on Plasmodium chabaudi-induced spleen tissue injury in mice. Parasitol Res 114(9):3431–3438
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126(1):131–138
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Sternberger LA (1979) Immunocytochemistry. Wiley medical publication, 2d edn. Wiley, New York
Ponce-Canchihuaman JC, Perez-Mendez O, Hernandez-Munoz R, Torres-Duran PV, Juarez-Oropeza MA (2010) Protective effects of Spirulina maxima on hyperlipidemia and oxidative-stress induced by lead acetate in the liver and kidney. Lipids Health Dis 9:35
Graca A, Ramalho-Santos J, de Lourdes PM (2004) Effect of lead chloride on spermatogenesis and sperm parameters in mice. Asian J Androl 6(3):237–241
Acharya UR, Acharya S, Mishra M (2003) Lead acetate induced cytotoxicity in male germinal cells of Swiss mice. Ind Health 41(3):291–294
Abdel Moneim AE, Dkhil MA, Al-Quraishy S (2011) The protective effect of flaxseed oil on lead acetate-induced renal toxicity in rats. J Hazard Mater 194:250–255
Abdel Moneim AE (2015) The neuroprotective effect of berberine in mercury-induced neurotoxicity in rats. Metab Brain Dis. doi:10.1007/s11011-015-9652-6
Abdel Moneim AE, Dkhil MA, Al-Quraishy S (2011) The redox status in rats treated with flaxseed oil and lead-induced hepatotoxicity. Biol Trace Elem Res 143(1):457–467
Bazrgar M, Goudarzi I, Lashkarbolouki T, Elahdadi Salmani M (2015) Melatonin ameliorates oxidative damage induced by maternal lead exposure in rat pups. Physiol Behav 151:178–188
Elgawish RAR, Abdelrazek HMA (2014) Effects of lead acetate on testicular function and caspase-3 expression with respect to the protective effect of cinnamon in albino rats. Toxicology Reports 1:795–801
Wang C, Zhang Y, Liang J, Shan G, Wang Y, Shi Q (2006) Impacts of ascorbic acid and thiamine supplementation at different concentrations on lead toxicity in testis. Clin Chim Acta 370(1–2):82–88
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project no. RGP-198.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
In all experiments, we followed the European Community Directive (86/609/EEC) and national rules on animal care that were carried out in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals 8th edition. This was approved by the committee of the Zoology and Entomology department, Helwan University, Egypt.
Rights and permissions
About this article
Cite this article
Dkhil, M.A., Moneim, A.E.A. & Al-Quraishy, S. Indigofera oblongifolia Ameliorates Lead Acetate-Induced Testicular Oxidative Damage and Apoptosis in a Rat Model. Biol Trace Elem Res 173, 354–361 (2016). https://doi.org/10.1007/s12011-016-0689-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0689-0